Abstract
Background
In addition to motor findings, non-motor findings including alterations in visual acuity, decrease in blink reflex, and pupil reactivity cause the impaired quality of life in idiopathic Parkinson’s disease (PD) and multiple system atrophy (MSA). Our study aimed to examine possible latency and amplitude changes in pattern visual evoked potentials (pVEP) along with retinal and macular changes in optical coherence tomography (OCT) in PD and MSA groups. We also intended to investigate whether any OCT parameters could be a biomarker for Parkinson's or MSA.
Methods
Our study included 50 patients with PD, 15 with MSA, and 50 healthy control subjects. All patients in the study underwent neurological and ophthalmological examination and investigations of OCT to measure the retinal and macular thickness and pVEP to assess visual pathways.
Results
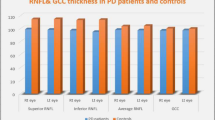
When PD, MSA, and control groups were compared, a significant difference was found in all retinal thickness values in average, nasal, and superior retinal nerve fiber thickness (pRNFL), and in all macular thickness values except nasal outer and inferior outer quadrants and in ganglion cell complex (GCC) thicknesses (p < 0.05). Moreover, a significant difference was found in N75, P100, and N145 latencies and N75-P100 amplitude (p < 0.05). The thickness of both pRNFL, inner and outer macular quadrants, was thinner in the MSA group than in PD but GCC thickness was thinner in PD group.
Conclusions
The present study compared pVEP and OCT parameters in PD and MSA groups. It was concluded that pVEP and OCT examinations were of importance in that they were easily accessible, affordable, noninvasive biomarkers that might be used in early periods and progression of the disease and in follow-up.



Similar content being viewed by others
Data availability
Not applicable.
References
Fahn S, Przedborski S (2000) Parkinsonizm. In: Rowland LP (ed) Merritt’s textbook of neurology. 10th edn, Lippincott Williams & Wilkins, Philadelphia, 9789752771819, pp 679–693
Rajput AH, Birdi S (1997) Epidemiology of Parkinson’s disease. Parkinsonism Relat Disord 3(4):175–186. https://doi.org/10.1016/S1353-8020(97)00029-1
Jellinger KA (2018) Multiple system atrophy: an oligodendroglioneural synucleinopathy. J Alzheimer’s Dis 62(3):1141–1179. https://doi.org/10.3233/JAD-170397
Armstrong RA (2014) Visual signs and symptoms of multiple system atrophy. Clin Exp Optom 97(6):483–491. https://doi.org/10.1111/cxo.12206
Armstrong RA (2008) Visual signs and symptoms of Parkinson’s disease: review. Clin Exp Optom 91(2):129–138. https://doi.org/10.1111/j.1444-0938.2007.00211.x
Alkabie S, Lange A, Manogaran P, Stoessl AJ, Costello F, Barton JJS (2020) Optical coherence tomography of patients with Parkinson ’ s disease and progressive supranuclear palsy. Clin Neurol Neurosurg 189:105635. https://doi.org/10.1016/j.clineuro.2019.105635
Albrecht P, Müller AK, Südmeyer M, Ferrea S, Ringelstein M, Cohn E, Aktas O, Dietlein T, Lappas A, Foerster A, Hartung HP, Schnitzler A, Methner A (2012) Optical coherence tomography in parkinsonian syndromes. PLoS ONE 7(4):e34891. https://doi.org/10.1371/journal.pone.0034891 (Epub 2012 Apr 13)
Pula JH, Towle VL, Staszak VM, Cao D, Bernard JT, Gomez CM (2011) Retinal nerve fibre layer and macular thinning in spinocerebellar ataxia and cerebellar multisystem atrophy. Neuro-Ophthalmology 35(3):108–114. https://doi.org/10.3109/01658107.2011.580898
Bodis-Wollner I, Yahr MD (1978) Measurements of visual evoked potentials in Parkinson’s disease. Brain 101(4):661–671. https://doi.org/10.1093/brain/101.4.661
Delalande I, Hache JC, Forzy G, Bughin M, Benhadjali J, Destée A (1998) Do visual-evoked potentials and spatiotemporal contrast sensitivity help to distinguish idiopathic Parkinson’s disease and multiple system atrophy? Mov Disord 13(3):446–452. https://doi.org/10.1002/mds.870130312
Dinner DS, Luders H, Hanson M, Lesser RP, Klem G (1995) Pattern evoked potentials (PEPs) in Parkinson’s disease. Neurology 35(4):610–610. https://doi.org/10.1212/WNL.35.4.610
Ehle AL, Malcolm Stewart R, Lellelid NE, Arthur Leventhal N (1982) Normal checkerboard pattern reversal evoked potentials in Parkinsonism. Electroencephalogr Clin Neurophysiol 54(3):336–338. https://doi.org/10.1016/0013-4694(82)90182-1
Liu C, Zhang Y, Tang W, Wang B, Wang B, He S (2017) Evoked potential changes in patients with Parkinson’s disease. Brain Behav 7(5):1–8. https://doi.org/10.1002/brb3.703
Tartaglione A, Pizio N, Bino G, Spadavecchia L, Favale E (1984) VEP changes in Parkinson’s disease are stimulus dependent. J Neurol Neurosurg Psychiatry 47(3):305–307. https://doi.org/10.1136/jnnp.47.3.305
Kaur M, Saxena R, Singh D, Behari M (2015) Correlation Between Structural and Functional Retinal Changes in Parkinson Disease Published online 254–258. doi:https://doi.org/10.1097/WNO.0000000000000240
Kupersmith MJ, Shakin E, Siegel IM, Lieberman A (1982) Visual system abnormalities in patients with Parkinson’s disease. Arch Neurol 39(5):284–286. https://doi.org/10.1001/archneur.1982.00510170026007
P J Delwaide, B Mesraoua VDP (1980) Les potentiels évoqués visuels dans la maladie de Parkinson. Rev d&’apos; Electroencéphalographie Neurophysiol Clin 10(4):338–342. doi:https://doi.org/10.1016/S0370-4475(80)80031-1
Gawel MJ, Das P, Vincent S, Rose FC (1981) Visual and auditory evoked responses in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 44(3):227–232. https://doi.org/10.1136/jnnp.44.3.227
Ahn J, Lee J, Kim TW (2016) Retinal thinning correlates with clinical severity in multiple system atrophy. J Neurol 263(10):2039–2047. https://doi.org/10.1007/s00415-016-8230-0
Mendoza-Santiesteban CE, Gabilondo I, Palma JA, Norcliffe-Kaufmann L, Kaufmann H (2017) The retina in multiple system atrophy: systematic review and meta-analysis. Front Neurol 24(8):206. https://doi.org/10.3389/fneur.2017.00206
La Morgia C, Di Vito L, Carelli V, Carbonelli M (2017) Patterns of retinal ganglion cell damage in neurodegenerative disorders: parvocellular vs magnocellular degeneration in optical coherence tomography studies. Front Neurol 22(8):710. https://doi.org/10.3389/fneur.2017.00710
Abele M, Schulz JB, Bürk K, Topka H, Dichgans J, Klockgether T (2000) Evoked potentials in multiple system atrophy (MSA). Acta Neurol Scand 101(2):111–115. https://doi.org/10.1034/j.1600-0404.2000.101002111.x
Respondek G, Roeber S, Kretzschmar H, Troakes C, Al-Sarraj S, Gelpi E, Gaig C, Chiu WZ, van Swieten JC, Oertel WH, Höglinger GU (2013) Accuracy of the National Institute for Neurological Disorders and Stroke/Society for Progressive Supranuclear Palsy and neuroprotection and natural history in Parkinson plus syndromes criteria for the diagnosis of progressive supranuclear palsy. Mov Disord 28(4):504–509. https://doi.org/10.1002/mds.25327 (Epub 2013 Feb 21)
Inzelberg R, Ramirez JA, Nisipeanu P, Ophir A (2004) Retinal nerve fiber layer thinning in Parkinson disease. Vision Res 44(24):2793–2797. https://doi.org/10.1016/j.visres.2004.06.009
Altintaş O, Işeri P, Ozkan B, Cağlar Y (2008) Correlation between retinal morphological and functional findings and clinical severity in Parkinson’s disease. Doc Ophthalmol 116(2):137–146. https://doi.org/10.1007/s10633-007-9091-8 (Epub 2007 Oct 26)
Hajee ME (2009) Inner retinal layer thinning in Parkinson disease. Arch Ophthalmol 127(6):737. https://doi.org/10.1001/archophthalmol.2009.106
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson ’ s disease : a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55(3):181–4
Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Dürr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K, Vidailhet M (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71(9):670–6. https://doi.org/10.1212/01.wnl.0000324625.00404.15
Odom JV, Bach M, Brigell M, Holder GE, McCulloch DL, Mizota A, Tormene AP, International Society for Clinical Electrophysiology of Vision (2016) ISCEV standard for clinical visual evoked potentials: (2016 update). Doc Ophthalmol 133(1):1–9. doi:https://doi.org/10.1007/s10633-016-9553-y. Epub 2016 Jul 21
Moschos MM, Tagaris G, Markopoulos I, Margetis I, Tsapakis S, Kanakis M, Koutsandrea C (2011) Morphologic changes and functional retinal impairment in patients with Parkinson disease without visual loss. Eur J Ophthalmol 21(1):24–29. https://doi.org/10.5301/ejo.2010.1318
Yu JG, Feng YF, Xiang Y, Huang JH, Savini G, Parisi V, Yang WJ, Fu XA (2014) Retinal nerve fiber layer thickness changes in Parkinson disease: a meta-analysis. PLoS One 9(1):e85718. https://doi.org/10.1371/journal.pone.0085718
Aaker GD, Myung JS, Ehrlich JR, Mohammed M, Henchcliffe C, Kiss S (2010) Detection of retinal changes in Parkinson’s disease with spectral-domain optical coherence tomography. Clin Ophthalmol 4(1):1427–1432. https://doi.org/10.2147/OPTH.S15136
Benedek G, Horváth G, Kéri S, Braunitzer G, Janáky M (2016). The development and aging of the magnocellular and parvocellular visual pathways as indicated by VEP recordings between 5 and 84 years of age. Vision (Basel) 1(1):7. doi:https://doi.org/10.3390/vision1010007.
Bodis-Wollner I, Marx MS, Mitra S, Bobak P, Mylin L, Yahr M (1987) Visual dysfunction in Parkinson's disease: loss in spatiotemporal contrast sensitivity. Brain 110(Pt 6):1675–1698. doi:https://doi.org/10.1093/brain/110.6.1675
Schneider M, Müller HP, Lauda F, Tumani H, Ludolph AC, Kassubek J, Pinkhardt EH (2014) Retinal single-layer analysis in Parkinsonian syndromes: an optical coherence tomography study. J Neural Transm (Vienna) 121(1):41–47. https://doi.org/10.1007/s00702-013-1072-3 (Epub 2013 Aug 2)
Mendoza-Santiesteban CE, Palma JA, Martinez J, Norcliffe-Kaufmann L, Hedges TR, Kaufmann H (2015) Progressive retinal structure abnormalities in multiple system atrophy. Mov Disord 30(14):1944–1953. https://doi.org/10.1002/mds.26360
Yavas GF, Yilmaz O, Kusbeci T, Ozturk F (2007) The effect of levodopa and dopamine agonists on optic nerve head in Parkinson disease. Eur J Ophthalmol 17:812–816
Matlach J, Wagner M, Malzahn U, Schmidtmann I, Steigerwald F, Musacchio T, Volkmann J, Grehn F, Göbel W, Klebe S (2018) Retinal changes in Parkinson’s disease and glaucoma. Parkinsonism Relat Disord 56:41–46. https://doi.org/10.1016/j.parkreldis.2018.06.016 (Epub 2018 Jun 21)
Archibald NK, Clarke MP, Mosimann UP, Burn DJ (2011) Retinal thickness in Parkinson’s disease. Parkinsonism Relat Disord 17(6):431–436. https://doi.org/10.1016/j.parkreldis.2011.03.004 (Epub 2011 Mar 31)
Chrysou A, Jansonius NM, van Laar T (2019) Retinal layers in Parkinson’s disease: a meta-analysis of spectral-domain optical coherence tomography studies. Parkinsonism Relat Disord 64:40–49. https://doi.org/10.1016/j.parkreldis.2019.04.023
Bayer AU, Keller ON, Ferrari F, Maag KP (2002) Association of glaucoma with neurodegenerative diseases with apoptotic cell death: Alzheimer’s disease and Parkinson’s disease. Am J Ophthalmol 133(1):135–137. https://doi.org/10.1016/s0002-9394(01)01196-5
Nowacka B, Lubinski W, Honczarenko K, Potemkowski A, Safranow K (2014) Ophthalmological features of Parkinson disease. Med Sci Monit 11(20):2243–2249. https://doi.org/10.12659/MSM.890861
Fischer MD, Synofzik M, Kernstock C, Dietzsch J, Heidlauf R, Schicks J, Srulijes K, Wiethoff S, Menn O, Berg D, Schöls L, Schiefer U (2013) Decreased retinal sensitivity and loss of retinal nerve fibers in multiple system atrophy. Graefes Arch Clin Exp Ophthalmol 251(1):235–241. https://doi.org/10.1007/s00417-012-2118-1 (Epub 2012 Aug 10)
Eraslan M, Balci SY, Cerman E, Temel A, Suer D, Elmaci NT (2016) Comparison of optical coherence tomography findings in patients with primary open-angle glaucoma and Parkinson disease. J Glaucoma 25(7):e639–e646. https://doi.org/10.1097/IJG.0000000000000239
Garcia-Martin ES, Rojas B, Ramirez AI, de Hoz R, Salazar JJ, Yubero R, Gil P, Triviño A, Ramirez JM (2014) Macular thickness as a potential biomarker of mild Alzheimer’s disease. Ophthalmology 121(5):1149–1151. https://doi.org/10.1016/j.ophtha.2013.12.023 (Epub 2014 Mar 18)
Bayhan HA, Aslan Bayhan S, Tanık N, Gürdal C (2014) The Association of spectral-domain optical coherence tomography determined ganglion cell complex parameters and disease severity in Parkinson’s disease. Curr Eye Res 39(11):1117–1122. https://doi.org/10.3109/02713683.2014.894080
Spund B, Ding Y, Liu T, Selesnick I, Glazman S, Shrier EM, Bodis-Wollner I (2013) Remodeling of the fovea in Parkinson disease. J Neural Transm (Vienna) 120(5):745–53. doi: https://doi.org/10.1007/s00702-012-0909-5. Epub 2012 Dec 23
Shrier EM, Adam CR, Spund B, Glazman S, Bodis-Wollner I (2012) Interocular asymmetry of foveal thickness in Parkinson disease. J Ophthalmol 2012:728457. https://doi.org/10.1155/2012/728457 (Epub 2012 Aug 1)
Mailankody P, Battu R, Khanna A, Lenka A, Yadav R, Pal PK (2015) Optical coherence tomography as a tool to evaluate retinal changes in Parkinson’s disease. Parkinsonism Relat Disord 21(10):1164–1169. https://doi.org/10.1016/j.parkreldis.2015.08.002
Uchida A, Pillai JA, Bermel R, Bonner-Jackson A, Rae-Grant A, Fernandez H, Bena J, Jones SE, Leverenz JB, Srivastava SK, Ehlers JP (2018) Outer retinal assessment using spectral-domain optical coherence tomography in patients with alzheimer’s and Parkinson’s disease. Invest Ophthalmol Vis Sci 59(7):2768–2777. https://doi.org/10.1167/iovs.17-23240
QuagLiato LB (2014) Applications of visual evoked potentials and Fourier-domain optical coherence tomography in Parkinson’s disease : a controlled study 77(4):238–242
Cubo E, López Peña MJ, Diez Feijo Varela E, Pérez Gil O, Garcia Gutierrez P, Araus González E, Prieto Tedejo R, Mariscal Pérez N, Armesto D (2014) Lack of association of morphologic and functional retinal changes with motor and non-motor symptoms severity in Parkinson’s disease. J Neural Transm (Vienna) 121(2):139–145. https://doi.org/10.1007/s00702-013-1093-y
Frederick JM, Rayborn ME, Laties AM, Lam DMK, Hollyfield JG (1982) Dopaminergic neurons in the human retina. J Comput Neurol 210(1):65–79. https://doi.org/10.1002/cne.902100108
Bodis-Wollner I, Kozlowski PB, Glazman S, Miri S (2014) α-synuclein in the inner retina in Parkinson disease. Ann Neurol 75(6):964–966. https://doi.org/10.1002/ana.24182
Ma X, Wang Y, Wang N, Zhang R (2021). Retina thickness in atypical parkinsonism: a systematic review and meta-analysis. J Neurol. doi: https://doi.org/10.1007/s00415-021-10703-6. Epub ahead of print
Yaar I (1980) The effect of levodopa treatment on the visual evoked potentials in Parkinsonian patients. Electroencephalogr Clin Neurophysiol 50(3–4):267–274. https://doi.org/10.1016/0013-4694(80)90154-6
Onofrj M, Ghilardi MF, Basciani M, Gambi D (1986) Visual evoked potentials in parkinsonism and dopamine blockade reveal a stimulus-dependent dopamine function in humans. J Neurol Neurosurg Psychiatry 49(10):1150–1159. doi:https://doi.org/10.1136/jnnp.49.10.1150
Barbato L, Rinalduzzi S, Laurenti M, Ruggieri S, Accornero N (1994) Color VEPs in Parkinson’s disease. Electroencephalogr Clin Neurophysiol Potentials Sect 92(2):169–172. https://doi.org/10.1016/0168-5597(94)90057-4
Hammond EJ, Wilder BJ (1983) Evoked potentials in olivopontocerebellar atrophy. Arch Neurol 40(6):366–369. https://doi.org/10.1001/archneur.1983.04050060066012
Nuwer MR, Perlman SL, Packwood JW, Kark RAP (1983) Evoked potential abnormalities in the various inherited ataxias. Ann Neurol 13(1):20–27. https://doi.org/10.1002/ana.410130106
Wessekl K, Huss GP, Brückmann H, Kömpf D (1993) Follow-up of neurophysiological tests and CT in late-onset cerebellar ataxia and multiple system atrophy. J Neurol 240(3):168–176. https://doi.org/10.1007/BF00857523
Okuda B, Tachibana H, Kawabatd K, Takeda M, Sugita M (1995) Visual evoked potentials (VEPs) in Parkinson’s disease: correlation of pattern VEPs abnormality with dementia. Alzheimer Dis Assoc Disord 9:68–72
Schafer EWP, McKean CM (1975) Evidence that monoamines influence human evoked potentials. Brain Res 99(1):49–58. https://doi.org/10.1016/0006-8993(75)90607-1
Kangasniemi P, Falck B, Langvik V-A, Hyyppa MT (1978) Levotryptophan treatment in migraine. Headache J Head Face Pain 18(3):161–166. https://doi.org/10.1111/j.1526-4610.1978.hed1803161.x
Kolb H, Fernández E, Ammermüller J, Cuenca N (1995) Substance P: a neurotransmitter of amacrine and ganglion cells in the vertebrate retina. Histol Histopathol 10(4):947–968
Funding
This study was funded by Celal Bayar University Scientific Research Commission.
Author information
Authors and Affiliations
Contributions
MB and AKA contributed to study conception and design, data analysis, and manuscript preparation. MB contributed to pVEP recordings, data analysis, manuscript preparation, final approval, and submission. MSA contributed to data input. HM and EK contributed to ophthalmic evaluations. DS contributed to data analysis and interpretation, manuscript preparation, and revision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the Celal Bayar University Medical Faculty Ethics Committee (Approval Date: 14/11/2018 Approval Number: 20.478.486) and with Helsinki declaration (1964) and its later amendments or comparable ethical standards.
Informed consent
All subjects freely consented to participate in this study, and an informed consent was obtained from all participants included in the study.
Statements of human rights
All procedures performed on human participants were done so in accordance with the ethical standards of the Celal Bayar University Medical Faculty Ethics Committee and in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Statement on the welfare of animals
This article does not contain any studies with animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Batum, M., Ak, A.K., Arı, M.S. et al. Evaluation of the visual system with visual evoked potential and optical coherence tomography in patients with idiopathic Parkinson's disease and with multiple system atrophy. Doc Ophthalmol 145, 99–112 (2022). https://doi.org/10.1007/s10633-022-09887-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-022-09887-7