Abstract
The International Society for the Clinical Electrophysiology of Vision (ISCEV) standard for full-field electroretinography (ERG) describes a minimum set of tests, but encourages the use of additional protocols for clinical ERG testing. This extended protocol describes recording methods and derivations that will allow analysis of rod-driven components of the dark-adapted (DA) strong flash ERG a-wave, more closely related to rod phototransduction than ISCEV standard DA ERGs. The method involves recording ERGs to a flash strength equivalent to 30 cd s m2 under conditions of dark adaptation and additionally to the same stimulus following light adaptation (LA) and in the presence of a standard photopic background luminance of 30 cd m−2. The isolated rod-driven ERG a-wave is derived by subtracting the LA response from the DA ERG. The method is likely to be of value in the characterization of retinal disorders which affect rod quantal catch, diseases that affect the dynamics of any component of the activation phase of rod phototransduction, or those affecting total numbers of rod photoreceptors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The International Society for Clinical Electrophysiology of Vision (ISCEV) standard for full-field electroretinography (ERG) describes a minimum set of tests [1], but encourages the use of additional protocols for clinical ERG testing. This extended protocol describes extracting ERG components related to rod phototransduction from the a-wave response to a strong flash. The protocol was prepared by the authors in accordance with ISCEV procedures (http://www.iscev.org/standards/index.html).
Scope and applications
The normal human dark-adapted ERG to a strong brief flash is a mixed response with both rod- and cone-mediated contributions. The cone-driven contribution is not negligible; in diseases in which rod responses are selectively attenuated, the cone system is likely to contribute proportionately more to the response, making accurate assessment of rod photoreceptor signals more challenging. Rod-isolated ERG components may be obtained by subtracting cone-mediated responses as detailed below. Information about the dynamics of rod phototransduction can be derived from analysis of the initial portion of the rod-isolated ERG a-wave [2,3,4,5,6,7,8,9,10,11,12,13,14,15]. This extended protocol describes the recording of ERGs to the same strong flash under both dark-adapted and light-adapted (LA) or photopic rod-saturating conditions. The cone-driven LA ERG is then subtracted from the mixed response obtained in the dark.
The protocol is applicable to patients in whom diseases affecting the rod photoreceptors are suspected. Specifically, the protocol enables a more focused evaluation of the light responses of rods, not obtainable from the standard ISCEV protocol, and thus allows for more detailed phenotyping and understanding of disease mechanisms. This includes characterization of diseases which affect rod quantal catch (e.g. shortening of the outer segment), diseases that affect the dynamics of any component of the activation phase of rod phototransduction, or diseases that reduce the total number of rod photoreceptors. This protocol has thus far only been used as a research tool and is not currently used in a clinical setting for the diagnosis of retinal disease.
Patient population
Patients of all ages able to tolerate Ganzfeld stimulation referred for investigation of possible retinal or rod photoreceptor dysfunction, including those with reduced or delayed DA3 and DA10 ERG waves, consistent with abnormal rod function.
Technical issues
This protocol uses many of the recording parameters specified in the ISCEV protocol for the full-field ERG [1]. Additional considerations include the following:
- (a)
Methods of estimating the dark-adapted cone system contribution to the scotopic ERG. The rod-isolated a-wave can be obtained by removing the cone-mediated contribution to the scotopic ERG a-wave using one of the four methods ([4, 6, 7, 16,17,18,19,20]; see “Appendix 1”), outlined below.
- 1.
An identical flash may be delivered shortly after the DA very strong flash, at a time interval sufficient for the cones to recover from the first flash, but prior to the onset of rod recovery. A practical consideration is that the use of such paired bright flashes in quick succession can be difficult for some subjects to tolerate.
- 2.
A photopically matched red flash may be used to elicit an ERG mediated largely by L- and M-cones, to be subtracted from the mixed DA rod and cone system response. However, for strong flashes, it is likely the red flash would also stimulate the rods substantially, and so an estimation of the cone-derived component would be compromised [7]. It is possible to estimate the rod-derived component in the red flash response by delivering blue flashes that are scotopically matched to the red flashes [7, 9], but this increases the range of stimuli required and consequent testing time.
- 3.
A flash delivered immediately (300 ms) after extinction of a rod-saturating background [18]. Such stimulus delivery may not be readily achievable with standard equipment and also increases testing time, especially if averaging is needed.
- 4.
An identical flash to that used under DA conditions, delivered in the presence of a steady rod-saturating background. Such stimuli may be conveniently integrated into the ISCEV standard ERG method, and this technique forms the basis of the extended protocol specified below.
- (b)
Sequence and timing of scotopic and photopic ERGs.
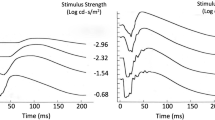
Suitable scotopic strong flash responses for a-wave analysis may be recorded after 20 min DA or after the ISCEV standard DA ERG protocol. The ISCEV standard LA ERGs are recorded after 10 min light adaptation, to allow the cone-mediated responses to reach a steady-state amplitude. During the 10 min of light adaptation, the cone-mediated ERG grows in amplitude [21,22,23], and so a larger a-wave may be recorded. Thus, it could be argued that for estimation of the cone component in the dark, the strong flash should be delivered immediately after onset of the photopic background rather than following adaptation to this background as the latter may lead to over-estimation. However, there is evidence that the ERG following a period of light adaptation may be closer to the dark-adapted cone-driven ERG than that obtained immediately following onset of the rod-saturating background [18]. Strong flashes presented immediately after onset of the photopic background can also be difficult to tolerate.
- (c)
Spectral characteristics of scotopic flash.
Shorter wavelength (blue) flashes preferentially stimulate the rods while minimizing stimulation of L- and M-cones and have been used in a number of studies (e.g. [2,3,4,5,6,7,8,9]). However, white flashes are consistent with ISCEV standard ERG stimuli and have also been widely used to derive the rod-isolated ERG a-wave (e.g. [10, 12,13,14]).
- (d)
Scotopic stimulus flash strengths.
Studies isolating and fitting models to the rod-mediated ERG a-wave have used a series of strong flashes [2,3,4,5,6,7,8,9,10,11,12,13,14,15]. However, shortened protocols based on one or two flashes have been proposed for clinical use [7, 10]. The stimulus should be strong enough to saturate the rods and suitable to probe changes in rod sensitivity. The flashes should be well-tolerated by most patients and chosen to avoid the flash artefacts that may be associated with the strongest flash stimuli.
- (e)
Flash duration.
The trough of the ERG a-wave to a 3.6 log sc Td-s flash occurs at approximately 10 ms. The duration of the flash must be considerably less than the photoreceptor integration time. Xenon flashes typically have flash durations of less than 1 ms and are ideal. Longer duration flashes produced by LED sources should be not more than 4 ms (i.e. at least 2 times shorter than a-wave peak time).
- (f)
Scotopic inter-stimulus interval.
The interval between flashes should be a minimum of 30 s which allows for complete recovery of the rod ERG a-wave to a 3.6 log sc Td s flash [17, 19].
- (g)
Photopic background.
The photopic background is used to saturate rods, enabling recording of cone-mediated ERGs. A white background of 3.3 log sc Td (15 ph cd m2) eliminates the rod a-wave and has a small effect on the cone a-wave [4, 19]. To minimize cone desensitization, a blue background which has a high scotopic, but low photopic luminance, has also been used [18, 20]. The ISCEV standard for photopic ERGs stipulates a 30 ph cd m−2 background which will produce a reasonable estimate of the cone-isolated ERG over the rising phase of the ERG a-wave (although there may be some desensitization of the cone photoreceptors).
- (h)
Photopic flash strength.
Photopic ERGs in this protocol are intended to estimate the cone-mediated contribution to the mixed DA strong flash ERG. To achieve this, stimulus strength should be unchanged from that used under scotopic conditions (see protocol specification below).
- (i)
Photopic inter-stimulus interval.
Cones recover within 2 s even in response to strong flashes [20]. Thus, an interval of ≥ 3 s between flashes is sufficient when averaging photopic responses. Flashes of this strength delivered at shorter intervals are less likely to be comfortable for subjects.
- (j)
Signal averaging.
Averaging may be used to improve the signal/noise ratio, and typically 3–10 responses have been averaged [5,6,7,8,9,10,11,12,13,14,15,16,17,18].
- (k)
Response analysis.
Once the LA (cone-driven) response has been subtracted from the DA (rod- and cone-driven) response to obtain the isolated rod-driven a-wave, the conventional measurements of a-wave amplitude and peak time can be applied to the derived response. In addition, the time taken by the response to proceed from 10% to 90% of the peak amplitude has been proposed as an indicator of rod photoreceptor sensitivity [15]. When LED flashes are used, the timing of the delay to the a-wave peak should be determined from the mid-time of the stimulus.
A large number of studies have used mathematical models to fit the leading edge of the rod-isolated a-wave to extract parameters relating to rod phototransduction [2,3,4,5,6,7,8,9,10,11,12,13,14]. However, as there are some important limitations [15] to such analysis (discussed in “Appendix 2”), this protocol does not endorse the use of a particular mathematical model at this time.
Calibration
The stimulus parameters are specified in scotopic trolands with conversion to photopic units for a white spectral light source with spectral composition similar to a xenon flash. If a source with different spectral composition is used, scotopic and photopic stimulus strength should be measured directly using appropriate filters. All other calibration issues are identical to those specified in the ISCEV ERG standard [26].
Protocol specifications
Patient preparation follows that for the current ISCEV standard ERG [1]. This extended protocol has the following additional specifications.
- (a)
Sequence and timing of scotopic and photopic ERGs.
Suitable scotopic strong flash responses for a-wave analysis are recorded after 20 min DA or following the standard DA ERG protocol. Photopic responses are recorded after 10 min light adaptation after the standard LA ERG protocol.
- (b)
Scotopic stimulus flash strengths.
This protocol specifies a flash strength equivalent to 75 scotopic cd s m−2 or 30 photopic cd s m−2. Assuming a pupil diameter of 8 mm and a broad-spectrum white (7000 k) light source, this equates to a flash of 3.6 log sc Td s. To be consistent with ISCEV stimulus notation, this stimulus should be referred to as DA30. If the pupils are smaller, a higher flash strength may be needed. If a range of flash strengths is used, the DA30 ERG should be included.
- (c)
Flash duration.
This should be a brief flash not longer than 4 ms.
- (d)
Scotopic inter-stimulus interval.
For the DA30 ERG, a minimum inter-stimulus interval of 30 s is specified.
- (e)
Photopic background.
The photopic background luminance is 30 cd m−2, as for the ISCEV standard ERG protocol.
- (f)
Photopic flash strength.
The same flash strength should correspond to that used to elicit the DA strong flash ERG. The stimulus in this context is referred to as LA30, assuming it is delivered on the same background and after at least 10 min LA or after the ISCEV standard LA ERG protocol.
- (g)
Photopic inter-stimulus interval.
The inter-stimulus interval is a minimum of 3 s. This allows cone recovery between flashes and is comfortable for most patients, and an interval of 3 s allows efficient averaging if needed.
- (h)
Averaging may be used to improve the signal/noise ratio, and typically 3–10 responses have been averaged in previous studies [5,6,7,8,9,10,11,12,13,14,15,16,17,18].
Reporting
The dark-adapted (DA30) mixed response, the light-adapted (cone-mediated) response (LA30) and the rod-isolated ERG should be shown. The time axis should be chosen so that the ascending portion of the a-wave can be clearly visualized as well as the pre-stimulus baseline (Fig. 1). Trough amplitude and peak time of the isolated rod-driven a-wave together with normative ranges (if available) should be reported. Stimulus and recording parameters should be specified as for the ISCEV full-field ERG. Pupil diameter should be reported. Mathematical modelling of the a-wave is not included in this extended protocol (see “Appendix 2” for discussion of previous studies).
Recordings from a healthy volunteer using a Burian–Allen electrode in response to identical flash strengths (30 cd s m2) under dark-adapted (DA30; black trace) and light-adapted conditions (LA30; red trace). The blue trace is the result of subtracting the LA30 response from the DA30 response and represents the rod contribution to the mixed response
References
McCulloch DL, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R et al (2015) ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol 130(1):1–12
Hood DC, Birch DG (1990) A quantitative measure of the electrical activity of human rod photoreceptors using electroretinography. Vis Neurosci 5(4):379–387
Hood DC, Birch DG (1990) The A-wave of the human electroretinogram and rod receptor function. Invest Ophthalmol Vis Sci 31(10):2070–2081
Hood DC, Birch DG (1993) Light adaptation of human rod receptors: the leading edge of the human a-wave and models of rod receptor activity. Vision Res 33(12):1605–1618
Breton ME, Schueller AW, Lamb TD, Pugh EN Jr (1994) Analysis of ERG a-wave amplification and kinetics in terms of the G-protein cascade of phototransduction. Invest Ophthalmol Vis Sci 35(1):295–309
Hood DC, Birch DG (1994) Rod phototransduction in retinitis pigmentosa: estimation and interpretation of parameters derived from the rod a-wave. Invest Ophthalmol Vis Sci 35(7):2948–2961
Cideciyan AV, Jacobson SG (1996) An alternative phototransduction model for the human rod and cone ERG a-wave; normal parameters and variations with age. Vision Res 36:2609–2621
Fulton AB, Hansen RM (1996) Photoreceptor function in infants and children with a history of mild retinopathy of prematurity. J Opt Soc Am A 13:566–571
Smith NP, Lamb TD (1997) The a-wave of the human electroretinogram recorded with a minimally invasive technique. Vision Res 37(21):2943–2952
Hood DC, Birch DG (1996–1997) Assessing abnormal rod photoreceptor activity with the a-wave of the electroretinogram: applications and methods. Doc Ophthalmol 92(4):253–267
Fulton AB, Hansen RM, Petersen RA, Vanderveen DK (2001) The rod photoreceptors in retinopathy of prematurity: an electroretinographic study. Arch Ophthalmol 119:499–505
Birch DG, Hood DC, Locke KG, Hoffman DR, Tzekov RT (2002) Quantitative electroretinogram measures of phototransduction in cone and rod photoreceptors: normal aging, progression with disease, and test–retest variability. Arch Ophthalmol 120(8):1045–1051
Tzekov RT, Locke KG, Hood DC, Birch DG (2003) Cone and rod ERG phototransduction parameters in retinitis pigmentosa. Invest Ophthalmol Vis Sci 44:3993–4000
Dimopoulos IS, Tennant M, Johnson A, Fisher S, Freund PR, Sauvé Y (2013) Subjects with unilateral neovascular AMD have bilateral delays in rod-mediated phototransduction activation kinetics and in dark adaptation recovery. Invest Ophthalmol Vis Sci 54(8):5186–5195
Robson JG, Frishman LJ (2014) The rod-driven a-wave of the dark-adapted mammalian electroretinogram. Prog Retina Eye Res 39:1–22
Verdon WA, Schneck ME, Haegerstrom-Portnoy G (2003) A comparison of three techniques to estimate the human dark-adapted cone electroretinogram. Vision Res 43(19):2089–2099
Jeffrey BG, Mitchell DC, Gibson RA, Neuringer M (2002) n − 3 fatty acid deficiency alters recovery of the rod photoresponse in rhesus monkeys. Invest Ophthalmol Vis Sci 43(8):2806–2814
Robson JG, Saszik SM, Ahmed J, Frishman LJ (2003) Rod and cone contributions to the a-wave of the electroretinogram of the macaque. J Physiol 547(Pt 2):509–530
Birch DG, Hood DC, Nusinowitz S, Pepperberg D (1995) Abnormal activation and inactivation mechanisms of rod transduction in patients with autosomal dominant retinitis pigmentosa and the Pro-23His mutation. Invest Ophthalmol Vis Sci 36:1603–1614
Friedburg C, Thomas MM, Lamb TD (2001) Time course of the flash response of dark- and light-adapted human rod photoreceptors derived from the electroretinogram. J Physiol 534(1):217–242
Armington JC, Biersdorf WR (1958) Long-term light adaptation of the human electroretinogram. J Comp Physiol Psychol 51(1):1–5
Peachey NS, Alexander KR, Fishman GA, Derlacki DJ (1989) Properties of the human cone system electroretinogram during light adaptation. Appl Opt 28(6):1145–1150
Gouras P, MacKay CJ (1989) Growth in amplitude of the human cone electroretinogram with light adaptation. Invest Ophthalmol Vis Sci 30(4):625–630
Hood DC, Birch DG (2006) Measuring the health of the human photoreceptors with the leading edge of the a-wave, Chapter 35. In: Heckenlively JR, Arden GB (eds) Principles and practice of clinical electrophysiology of vision. MIT Press, Cambridge, pp 487–501
Mahroo OA, Ban VS, Bussmann BM, Copley HC, Hammond CJ, Lamb TD (2012) Modelling the initial phase of the human rod photoreceptor response to the onset of steady illumination. Doc Ophthalmol 124(2):125–131
McCulloch DL, Hamilton R (2010) Essentials of photometry for clinical electrophysiology of vision. Doc Ophthalmol 121:77–84
Lamb TD, Pugh EN Jr (1992) A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J Physiol 449:719–758
Jeffrey BG, Neuringer M (2009) Age-related decline in rod phototransduction sensitivity in rhesus monkeys fed an n − 3 fatty acid-deficient diet. Invest Ophthalmol Vis Sci 50(9):4360–4367
Jamison JA, Bush RA, Lei B, Sieving PA (2001) Characterization of the rod photoresponse isolated from the dark-adapted primate ERG. Vis Neurosci 18(3):445–455
Kang Derwent JJ, Linsenmeier RA (2001) Intraretinal analysis of the a-wave of the electroretinogram (ERG) in dark-adapted intact cat retina. Vis Neurosci 18(3):353–363
Lamb TD, Pugh EN Jr (2006) Phototransduction, dark adaptation, and rhodopsin regeneration the proctor lecture. Invest Ophthalmol Vis Sci 47(12):5137–5152
Acknowledgements
Valuable feedback was provided by David Birch, Donald Hood and Trevor Lamb, Anthony Robson and John Robson. This protocol was available for review by the ISCEV membership and was approved by the ISCEV Board on 6 November 2019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Statement of human rights
This article does not contain any studies with human participants performed by any of the author.
Statement on the welfare of animals
This article does not contain any studies with animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1: Literature search and justification of method of rod isolation
A literature search was performed using the PubMed database (search date 4 October 2018) using search terms including “electroretinogram”, “a-wave” and “phototransduction”. Studies relating to the rod-derived human ERG were primarily considered, although relevant findings from animal studies (including, for example, [17, 18, 24, 27,28,29,30]) were also taken into account as these studies provide experimental evidence for origin of a-wave components. The search yielded a large number of studies, and selected key or exemplary references are included in the References section and in Table 1. Four main methods of isolating the rod-driven response were found, and these are discussed within the protocol, with justification of the choices made (see “Technical issues” section).
Appendix 2: Use of mathematical models of phototransduction
The literature search also revealed that many authors have applied mathematical modelling to the rod-isolated a-wave, with the most prevalent model in use in different laboratories for fitting the human rod a-wave based on the derivation of Lamb and Pugh [27, 31], usually as formulated by Hood and Birch [4, 10]. Lamb and Pugh considered the kinetics of the individual activation stages of phototransduction and developed a mathematical expression that provided a close fit to photocurrent responses of single rods (recorded in vitro) to a wide range of stimulus energies, with a single set of parameters. Modified versions of the same model have been applied to the ERG a-wave.
However, assuming the photoreceptor outer segment current is directly reflected in the massed voltage measurements of the ERG may not be necessarily valid. Robson and Frishman [15] have proposed that the a-wave is influenced also by currents flowing in the photoreceptor inner segments and axons. Explicitly taking these into account enables a close fit that includes the a-wave trough (including the last 10% of the downward slope). This model has the potential to be more precise and applicable to a wider set of clinical conditions, but it cannot be easily summarized by a single equation, and is not presently in a form that can be widely applied.
Mathematical modelling to derive parameters of phototransduction has also been applied in a number of studies to the cone-driven ERG [7, 9, 12, 13]. The cone-driven a-wave is known to contain a substantial contribution from OFF-bipolar cells in addition to photoreceptors [18], making direct estimation of parameters relating to cone phototransduction less straightforward. Methods relating to cone-driven a-wave analysis were therefore excluded from this protocol.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brigell, M., Jeffrey, B.G., Mahroo, O. et al. ISCEV extended protocol for derivation and analysis of the strong flash rod-isolated ERG a-wave. Doc Ophthalmol 140, 5–12 (2020). https://doi.org/10.1007/s10633-019-09740-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-019-09740-4