Abstract
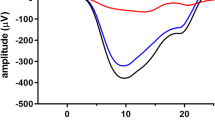
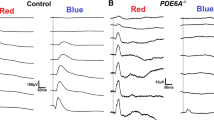
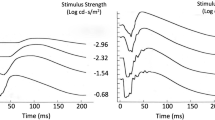
The impact of a disease on phototransduction can be assessed by fitting the leading edge of the rod a-wave to high-energy flashes with a quantitative expression. Two parameters of rod receptor activity are obtained, S (sensitivity) and Rm (maximum response). In this study, the meaning of these parameters and examples of conditions that change them were examined. In addition, a new protocol was developed for obtaining these parameters. A set of three to five white flashes were first presented in the dark and then on an adapting field (30 cd/m2). Subtracting the light-adapted responses from the dark-adapted responses yielded isolated rod a-wave responses. A clinical protocol was developed based on a single white flash energy. It is possible to determine whether a disease is producing a change in S and/or Rm with this single flash energy without the use of any equations.
Similar content being viewed by others
Abbreviations
- CRVO:
-
central retinal vein occlusion
- NVI:
-
neovascularization of the iris
- Rm:
-
maximum response
- S:
-
sensitivity
References
Granit R. The components of the retinal action potential in mammals and their relation to the discharge in the optic nerve. J Physiol 1933; 77: 207–39.
Penn RD, Hagins WA. Kinetics of the photocurrent of retinal rods. Biophys J 1972; 12: 1073–94.
Hood DC, Birch DG. A quantitative measure of the electrical activity of human rod photoreceptors using electroretinography. Vis Neurosci 1990; 5: 379–87.
Hood DC, Birch DG. The a-wave of the human ERG and rod receptor function. Invest Ophthalmol Vis Sci 1990; 31: 2070–81.
Hood DC, Birch DG. Light adaptation of human rod receptors: the leading edge of the human a-wave and models of rod receptor activity. Vision Res 1993; 33: 1605–18.
Cideciyan AV, Jacobson SG. Negative electroretinograms in retinitis pigmentosa. Invest Ophthalmol Vis Sci 1993; 34: 3253–63.
Breton M, Schueller A, Lamb T, Pugh EN Jr. Analysis of ERG a-wave amplification and kinetics in terms of the G-protein cascade of phototransduction. Invest Ophthalmol Vis Sci 1994; 35: 295–309.
Hood DC, Birch DG. Rod phototransduction in retinitis pigmentosa: estimation and interpretation of parameters derived from the rod a-wave. Invest Ophthalmol Vis Sci 1994; 35: 2948–61.
Hood DC, Birch DG. The b-wave of the scotopic (rod) ERG as a measure, of the activity of human on-bipolar cells. J Opt Soc Am 1996; 13: 623–33.
Bush R, Sieving P. A proximal retinal component in the primate photopic ERG a-wave. Invest Ophthalmol Vis Sci 1994; 35: 635–44.
Cideciyan AV, Jacobson SG. An alternative phototransduction model for the human rod and cone ERG a-wave; normal parameters and variations with age. Vision Res 1996; 36: 2609–21.
Lamb TD, Pugh EN. A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J Physiol 1992; 499: 719–58.
Johnson MA, Hood DC. Rod photoreceptor transduction is affected in central retinal vein occlusion associated with iris neovascularization. J Opt Soc Am 1996; 13: 572–6.
Holopigian K, Kelly R, Greenstein V, Seiple W, Hood DC. Rod and cone photoreceptor analysis in patients with diabetic retinopathy. Invest Ophthalmol Vis Sci 1995; 36(suppl): S480. Abstract.
Reynaud XR, Chunguang RM, Hansen RM, Fulton AB, Aouididi S, Dorey CK. Morphological and ERG evidence of retinal hypoxia in interrupted oxygen induced retinopathy in the neonatal rat. Invest Ophthalmol Vis Sci 1994; 35: 1378.
Fulton AB, Hansen RM. Photoreceptor function in infants and children with a history of mild retinopathy of prematurity. J Opt Soc Am 1996; 13: 566–71.
Fulton AB, Hansen RM. Electroretinogram responses and refractive errors in patients with a history of retinopathy of prematurity. Doc Ophthalmol 1996; 91: 87–100.
Reynaud X, Hansen RM, Fulton AB. Effect of prior oxygen exposure on the electroretinographic responses of infant rats. Invest Ophthalmol Vis Sci 1995; 36; 2071–2079.
Hood D, Birch D. Computational models of rod-driven retinal actitity. IEEE Eng Med Bio 1995; 14: 59–66.
Hood DC, Cideciyan AV, Halevy DA, Jacobson SG. Sites of disease action in a retinopathy with supernormal and delayed rod electroretinogram b-waves. Vision Res 1995; 36: 889–902.
Gouras P, Eggers M, MacKay C. Cone dystrophy, nyctalopia and supernormal rod responses. Arch Ophthalmol 1983; 101: 718–24.
Alexander K, Fishman G. Supernormal scotopic ERG in cone dystrophy. Br J Opthalmol 1984; 68: 69–78.
Yagasaki K, Miyake Y, Litao R, Ichikawa K. Two cases of retinal degeneration with an unusual form of electroretinogram. Doc Ophthalmol 1986; 63: 73–82.
Foerster M, Kellner U, Wessing A. Cone dystrophy and supernormal dark-adapted b-waves in the electroretinogram. Graefes Arch Clin Exp Ophthalmol 1990; 228: 116–9.
Sandberg M, Miller S, Berson E. Rod electroretinograms in an elevated cyclic guanosine monophosphate-type human retinal degeneration. Invest Ophthalmol Vis Sci 1990; 31: 2283–7.
Kato M, Kobayashi R, Watanabe I. Cone dysfunction and supernormal scotopic electroretinogram with a high-intensity stimulus. Doc Ophthalmol 1993; 84: 71–81.
Rosenberg T, Simonsen SE. Retinal dysfunction of supernormal rod ERG type. Acta Ophthalmol 1993; 71: 246–55.
Hood DC, Birch DG. Phototransduction in human cones measured using the a-wave of the ERG. Vision Res 1995; 35: 2801–10.
Hood DC, Birch DG. Human cone receptor activity: the leading edge of the a-wave and models of receptor activity. Vis Neurosci 1993; 10: 857–71.
Birch DG, Hood DC, Nusinowitz S, Pepperberg DR. Abnormal activation and deactivation mechanisms of rod transduction in patients with autosomal dominant retinitis pigmentosa and the pro-23-his mutation. Invest Ophthalmol Vis Sci 1995; 36: 1603–14.
Hood DC, Shady S, Birch DG. Heterogeneity in retinal disease and the computational model of the human rod response. J Opt Soc Am 1993; 10: 1624–30.
Shady S, Hood DC, Birch DG. Rod phototransduction in retinitis pigmentosa: distinguishing alternative mechanisms of degeneration. Invest Ophthalmol Vis Sci 1995; 36: 1027–37.
Jacobson S, Cideciyan A, Kemp CM, Sheffield VC, Stone EM. Photoreceptor function in heterozygotes with insertion or deletion mutations in the RDS gene. Invest Ophthalmol Vis Sci 1996; 37: 1662–74.
Birch DG, Hood DC. Abnormal rod photoreceptor function in retinitis pigmentosa. In: Anderson RE, La Vail MM, Hollyfield JG, eds. Degenerative diseases of the retina. 1995; 359–70. Plenum Press, NY.
Kedzierski W, Lloyd M, Birch DG, Bok D, Travis GH. Generation and analysis of transgenic mice expressing P216L-substituted rds/peripherin in rod photoreceptors. Invest Ophthalmol Vis Sci 1997; 38: 498–509.
Goto Y, Peachey N, Ziroli NE, Seiple WH, Gryczan C, Pepperberg DR, Naash MI. Rod phototransduction in transgenic mice expressing a mutant opsin gene. J Opt Soc Am 1996; 13: 577–85.
Hood DC, Birch DG. A computational model of the amplitude and implicit time of the b-wave of the human ERG. Vis Neurosci 1992; 8: 107–26.
Hood DC, Shady S, Birch DG. Understanding changes in the b-wave of the ERG caused by heterogeneous receptor damage. Invest Ophthalmol Vis Sci 1994; 35: 2477–88.
Baylor DA, Nunn BJ, Schnapf JL. The photocurrent, noise, and spectral sensitivity of rods of the monkey Macaca fascicularis. J Physiol 1984; 357: 575–607.
Robson JG, Frishman LJ. Photoreceptor and bipolar cell contributions to the cat electroretinogram: a kinetic model of the early part of the flash response. J Opt Soc Am 1996; 13: 613–22.
Pepperberg DR, Birch DG, Hood DC. Photoresponses of human rods in vivo derived from paired-flash electroretinograms. Vis Neurosci 1997; 14: 73–82.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hood, D.C., Birch, D.G. Assessing abnormal rod photoreceptor activity with the a-wave of the electroretinogram: Applications and methods. Doc Ophthalmol 92, 253–267 (1996). https://doi.org/10.1007/BF02584080
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02584080