Abstract
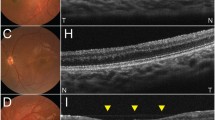
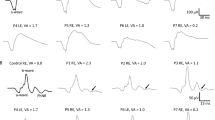
The purpose of the study is to characterise retinal function using light-adapted electroretinograms (ERGs) in a series of young children with ONH, congenital dysplasia of retinal ganglion cells. ERGs were recorded with chloral hydrate sedation in 27 children with ONH (18 with bilateral and 9 with unilateral ONH, age 4–35 months) and an adult reference population (n = 12). Stimuli included ISCEV standard flash, oscillatory potentials (OPs) and standard flicker as well as a light-adapted luminance–response series (photopic hill). The disc diameter to disc macula (DD:DM) ratio was measured from fundus photographs. The results are eyes with ONH, classified by DD:DM, were severe (≤0.15, n = 22), moderate (0.16–0.30, n = 22), mild (0.31–0.35, n = 1), and fellow eyes (>0.35, n = 9), all had prolonged ERG implicit times and smaller i-waves than those of adults. Eyes with moderate or severe ONH also had smaller amplitudes for OPs and flicker ERGs and required stronger flashes to obtain the peak b-wave amplitude. Abnormalities of the photopic hill were a common but inconsistent feature of ONH and were not indicative of ONH severity. Abnormalities of the photopic hill of the ERG suggest that some cases of ONH may have retinal dysfunction with specific deficits in the ON or OFF pathways of the retina. ONH is a complex and heterogeneous condition that may involve dysfunction distal to the retinal ganglion cells.




Similar content being viewed by others
Notes
This stimulus complies with the ISCEV standards for electroretinography (2004 update) and earlier standards but not the 2008 update which specifies the standard flash as 3.0 cd·s/m2 (±10%) [30]. Our stimulus is slightly weaker than the new standard.
References
Lambert SR, Hoyt GS, Narahara MH (1987) Optic nerve hypoplasia. Surv Ophthalmol 32:1–9
Zeki SM, Hollman AS, Dutton GN (1992) Neuroradiological features of patients with optic nerve hypoplasia. J Pediatr Ophthalmol Strabismus 29(2):107–112
Tornqvist K, Ericsson A, Kallen B (2002) Optic nerve hypoplasia: risk factors and epidemiology. Acta Ophthalmol Scand 80(3):300–304
Oster SF, Deiner M, Birgbauer E, Sretavan DW (2004) Ganglion cell axon pathfinding in the retina and optic nerve. Semin Cell Dev Biol 15(1):125–136
Dutton GN (2004) Congenital disorders of the optic nerve: excavations and hypoplasia. Eye 18:1038–1048
Billock VA, Vingrys AJ, King-Smith PE (1994) Opponent-colour detection threshold asymmetries may result from reduction of ganglion cell subpopulations. Vis Neurosci 11:99–109
Heron G, Dutton GN, McCulloch DL, Stanger S Pulfrich’s phenomenon in optic nerve hypoplasia. Greafe’s archive for clinical and experimental ophthalmology, online
Phillips PH, Spear C, Brodsky MC (2001) Magnetic resonance diagnosis of congenital hypopituitarism in children with optic nerve hypoplasia. J AAPOS 5(5):275–280
Ahmad T, Garcia-Filion P, Borchert M, Kaufman F, Burkett L, Geffner M (2006) Endocrinological and auxological abnormalities in young children with optic nerve hypoplasia: a prospective study. J Pediatr 148(1):78–84
Garcia-Filion P, Nelson M, Azen C, Geffner M, Fink C, Borchert M (2008) Neuroradiographic, endocrinological, and ophthalmic correlates of adverse developmental outcomes in children with optic nerve hypoplasia. Pediatrics 121(3):e653–e659
Lempert P (2000) Optic nerve hypoplasia and small eyes in presumed amblyopia. J AAPOS 4(5):258–266
Borchert M, Garcia-Filion P The syndrome of optic nerve hypoplasia Current neurology and neuroscience reports 8 5 395–403 Sep 2008
Taylor D (2007) Developmental abnormalities of the optic nerve and chiasm. Eye 21(10):1271–1284
Alvarez E, Wakakura M, Khan Z, Dutton GN (1988) The disc-macula distance to disc diameter ratio: a new test for confirming optic nerve hypoplasia in young children. J Pediatr Ophthalmol Strabismus 25(3):151–154
Barr DB, Weir CR, Purdie AT (1999) An appraisal of the disc-macula distance to disc diameter ratio in the assessment of optic disc size. Ophthalmic Physiol Opt 19(5):365–375
Zeki SM, Dutton GN (1990) Optic nerve hypoplasia in children. Br J Ophthalmol 74:300–304
Borchert MS, McCulloch DL, Rother C, Stout AU (1995) Clinical assessment, optic disk measurements, and visual-evoked potential in optic nerve hypoplasia. Am J Ophthalmol 120:605–612
Francois J, Rouck AD (1976) Electroretinographical study of the hypoplasia of the optic nerve. Ophthalmologica 172:308–330
Brecelj J, Stirn-Kranjc B (2004) Visual electrophysiology screening in diagnosing infants with congenital nystagmus. Clin Neurophysiol 115(2):461–470
McCulloch DL, Garcia-Fillion P, van Boemel GB, Borchert MS (2007) Retinal function in children with optic nerve hypoplasia: electroretinograms to large patterns and photopic flash. Eye 21(6):712–720
Sprague JB, Wilson WB (1981) Electrophysiologic findings in bilateral optic nerve hypoplasia. Arch Ophthal 1029(99):1028
Kriss A, Russell-Eggitt I (1992) Electrophysiological assessment of visual pathway function in infants. Eye 6:145–153
Cibis GW, Fitzgerald KM (1994) Optic nerve hypoplasia in association with brain anomalies and an abnormal electroretinogram. Doc Ophthalmol 86:11–22
Janaky M, Deak A, Pelle Z, Benedek G (1994) Electrophysiologic alterations in patients with optic nerve hypoplasia. Doc Ophthalmol 86:247–257
Sieving PA, Murayama K, Naarendorp F (1994) Push-pull model of the primate photopic electroretinogram: a role for hyperpolarizing neurons in shaping the b-wave. Vis Neurosci 11:519–532
Ueno S, Kondo M, Niwa Y, Terasaki H, Miyake Y (2004) Luminance dependence of neural components that underlies the primate electroretinogram. Invest Ophthalmol Vis Sci 45:1033–1040
Wali N, Leguire LE (1992) The photopic hill: A new phenomenon of the light adapted electroretinogram. Doc Ophthalmol 80(4):1573–2622
McCulloch DL, van Boemel GB, Borchert MS (1998) Comparison of corneal, conjunctival and skin electrodes for pattern electroretinograms. Doc Ophthalmol 94:327–340
Marmor MF, Holder GE, Seeliger MW, Yamamoto S (2004) Standard for clinical electroretinography (2004 update). Doc Ophthalmol 108:107–114
Marmor MF, Fulton AB, Holder GE, Miyake Y, Brigell M, Bach M (2009) Standard for clinical electroretinography (2008 update). Doc Ophthalmol 118:69–77
Hamilton R, Bees MA, Chaplin CA, McCulloch DL (2007) The luminance-response function of the human photopic electroretinogram: a mathematical model. Vis Res 47(23):2968–2972
Rufiange M, Dassa J, Dembinska O, Koenekoop RK, Little JM, Polomeno RC, Dumont M, Chemtob S, Lachapelle P (2003) The photopic ERG luminance-response function (photopic hill): method of analysis and clinical application. Vision Res 43(12):1405–1412
Westall CA, Panton CM, Levin AV (1999) Time courses for maturation of electroretinogram responses from infancy to adulthood. Doc Ophthalmol 96(4):355–379
Fulton AB, Hansen RM, Westall CA (2003) Development of ERG responses: the ISCEV standard rod, maximal and cone responses in normal subjects. Doc Ophthalmol 107:235–241
Hansen RM, Fulton AB (2005) Development of the cone ERG in infants. Invest Ophthalmol Vis Sci 46(9):3458–3462
Viswanathan S, Frishman LJ, Robson JG, Harwerth RS, Smith ELIII (1999) The photopic negative response of the macaque electroretinogram: reduction by experimental glaucoma. Invest Ophthalmol Vis Sci 40(6):1124–1136
Rangaswamy NV, Shirato S, Kaneko M, Digby BI, Robson JG, Frishman LJ (2007) Effects of spectral characteristics of Ganzfeld stimuli on the photopic negative response (PhNR) of the ERG. Invest Ophthalmol Vis Sci 48(10):4818–4828
Viswanathan S, Frishman LJ, Robson JG (2002) Inner-retinal contributions to the photopic sinusoidal flicker electroretinogram of macaques. Doc Ophthalmol 105:223–242
Bush RA, Sieving PA (1996) Inner retinal contributions to the primate photopic fast flicker electroretinogram. J Opt Soc Am 13(3):557–565
Peachey NS, Alexander KR, Derlacki DJ, Fishman GA (1992) Light adaptation, rods, and the human cone flicker ERG. Vis Neurosci 8(2):145–150
Usui T, Kremers J, Sharpe LT, Zrenner E (1998) Response phase of the flicker electroretinogram (ERG) is influenced by cone excitation strength. Vis Res 38:3247–3251
Kondo M, Sieving PA (2001) Primate photopic sine-wave flicker ERG: vector modelling analysis of component origins using glutamate analogs. Invest Ophthalmol Vis Sci 42(1):305–312
Moskowitz A, Hansen RM, Fulton AB (2005) ERG oscillatory potentials in infants. Doc Ophthalmol 110:265–270
Bui BV, Armitage JA, Vingrys AJ (2002) Extraction and modelling of oscillatory potentials. Doc Ophthalmol 104(1):7–36
Acknowledgments
This research was supported in part by The One Small Voice Foundation, by NIH General Research Center (GCRG) grant (M01 RR00043) and by the Carnegie Trust for the Universities of Scotland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chaplin, C., Borchert, M.S., Fink, C. et al. Light-adapted electroretinograms in optic nerve hypoplasia. Doc Ophthalmol 119, 123–132 (2009). https://doi.org/10.1007/s10633-009-9188-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-009-9188-3