Abstract
Background
Our previous study indicated that Hcy exacerbated DSS-induced colitis by facilitating the differentiation of intestinal T helper cell 17 (Th17), but the precise mechanism remains unidentified. Therefore, our current research aims to elucidate the signaling pathway through which Hcy promotes the differentiation of Th17 cells.
Methods
BALb/c mice were randomly assigned into six groups. The model of mice colitis was induced using 3% DSS, while the model of Hyperhomocysteinemia was induced using 1.7% methionine. The concentrations of Hcy and prostaglandin E2 (PGE2) were measured using enzyme-linked immunosorbent assay (ELISA). The protein expressions of cytosolic phospholipase A2 (cPLA2), phosphorylated-cPLA2 (p-cPLA2), cyclooxygenase 2 (COX2), cyclic adenosine monophosphate (cAMP), signal transducer and activator of transcription 3 (STAT3), phosphorylated-STAT3 (p-STAT3), interleukin-17A (IL-17A), and retinoid-related orphan nuclear receptor-γt (RORγt) were assessed using western blot analysis.
Results
Compared to the DSS + HHcy group, the addition of the COX inhibitor did not significantly alter the protein expression of p-PLA2/PLA2, but led to significant decreases in serum PGE2 concentration, cAMP, and p-STAT3/STAT3 protein expression. The protein expressions of p-PLA2/PLA2, COX2, and cAMP upstream of STAT3 inhibitor addition did not exhibit significant changes. However, PGE2 concentration and p-STAT3/STAT3 protein expression were notably reduced. After the COX inhibitor and STAT3 inhibitor added, the protein expression of IL-17A and RORγt and the levels of IL-17A and IL-23R in CD4+ T cells were significantly reduced.
Conclusion
HHcy aggravated DSS-induced colitis by promoting the differentiation and proliferation of Th17 cells through the PGE2 / STAT3 signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammatory bowel disease (IBD) is a recurrent gastrointestinal disease based on inflammation, the pathogenesis of which is still poorly understood and may be closely related to genetic, environmental, and immune factors, so IBD is not only an autoimmune disease but also a complex systemic metabolic disease [1]. Homocysteine (Hcy), a metabolite of methionine, plays two primary physiological roles in the body, namely methylating and antioxidising. Hyperhomocysteinemia (HHcy) is defined as Hcy levels exceeding 15 mol/L and has been identified as a risk factor for various diseases. Previous studies have demonstrated that patients with IBD exhibited significantly elevated levels of Hcy in both their blood and intestinal mucosa [2]. These elevated Hcy levels have been found to be positively associated with the frequency and duration of relapses in both ulcerative colitis (UC) and Cohn’s disease (CD), as well as with markers of disease activity, such as acute phase reactive protein levels [3, 4], disease activity, and blood sedimentation [5, 6]. Hcy-induced cellular and vascular injury may be involved in the pathogenesis of IBD and its levels are highly correlated with the expression of intestinal inflammatory factors in IBD. For instance, elevated levels of homocysteine (Hcy) have been shown to induce the expression of vascular cell adhesion molecule-1 (VCAM-1) [2], monocyte chemoattractant protein-1 (MCP-1) [2], and activated matrix metalloproteinase-9 (MMP-9) [7] in intestinal mucosal microvascular endothelial cells under ex vivo conditions. This leads to increased permeability of the intestinal microvascular endothelial cells and the potential promotion of interleukin 1β (IL-1β), interleukin 6 (IL-6), tumor necrosis factor-α (TNF-α), and transforming growth factor-β (TGF-β) [8]. These findings are sufficient evidence that HHcy may play an important role in the development of IBD; however, its pathogenic mechanism is not clear.
Disturbances in intestinal metabolism leading to excessive activation of Th17 cells and continuous secretion of IL-17A are essential for the development of IBD. During the inflammatory state, CD4+T cells differentiate into Th17 effector cells in response to IL-6 and TGF-β secreted by macrophages [9]. Additionally, the abnormal microenvironment in the intestine, caused by metabolic disorders, such as high salt, high fat, or low oxygen, can further increase the expression of IL-23R on the surface of Th17 cells, causing them to continuously and over-secrete inflammatory factors such as IL-17A in response to IL-23 stimulation. Recruiting more pro-inflammatory cells contributes to the persistent inflammatory damage and progression of IBD. However, the specific metabolites responsible for the excessive activation of intestinal Th17 cells in IBD patients, as well as the underlying regulatory mechanisms, remain unidentified [10]. Investigating these metabolites and their regulatory mechanisms, which lead to the imbalance in Th17 cell response and secretion of inflammatory factors, may offer novel therapeutic strategies and potential clinical applications for the treatment of IBD.
Prior research has demonstrated that Hcy has the potential to enhance the release of interleukin-17A (IL-17A) by means of its lipid derivative, Prostaglandin E2 (PGE2), thereby aggravating the inflammatory response within the gastrointestinal tract of individuals with IBD [11]. High-throughput sequencing (HTS) of mice in the DSS and DSS + HHcy groups revealed significantly different proteins, further analysis of Kyoto Encyclopedia of Genes and Genomes (KEEG) revealed that these proteins were particularly significant in the expression of the lipid-metabolizing enzyme Phospholipase A2 (PLA2). Activated PLA2 has the capability to hydrolyze membrane phospholipids, resulting in the liberation of arachidonic acid. This liberated arachidonic acid is subsequently catalyzed by cyclooxygenase (COX2), leading to the production of PGE2 [12]. The binding of PGE2 to its ligands EP2 and EP4 facilitates the promotion of Th17 cells proliferation by activating cAMP and STAT3 to promote IL-23R expression in Th17 cells [13]. Previous research found that Hcy increased the levels of PLA2, PGE2, and IL-17A in the intestines of mice with DSS-induced colitis [11]. Additionally, CD4+T cells from the mouse intestines were isolated and cultured in vitro to induce their differentiation into Th17 cells, revealing a progressive increase in PGE2 and IL-17A levels as Hcy concentration rose [14].
However, the precise mechanism by which Hcy induces the upregulation of IL-17A via PGE2 remains uncertain. Consequently, this study aims to explore the regulatory mechanism of Hcy stimulating the excessive secretion of IL-17A by intestinal Th17 cells in patients with IBD.
Materials and Methods
Mice
Female BALB/c mice aged 6 to 8 weeks and weighing 18–22 g were procured from the Hubei Provincial Center for Disease Control and Prevention. The mice were housed in a specific pathogen-free (SPF) environment, maintaining a 12-h light/dark cycle with regulated temperature and humidity. They underwent a one-week acclimatization period with ad libitum access to food and water before the experiment commenced. The research followed the Regulations of the People’s Republic of China regarding Laboratory Animal Administration, and all procedures received approval from the Hubei Provincial Committee on the Management and Use of Laboratory Animals.
Construction of Colitis Model and Hyperhomocysteinemia Model in Mice
Thirty-six female BALB/c mice, aged 6–8 weeks and weighing approximately 18–22 g, were randomly allocated into six groups: (1) Control group; (2) DSS group; (3) Hyperhomocysteinemia group (HHcy); (4) DSS + HHcy group; (5) HHcy + DSS + COX inhibitor group; and (6) DSS + HHcy + STAT3 inhibitor group.
Free drinking water and feeding feed for 1 month were used as control group. Mice were drunk water freely with 3% (w/v) DSS [Sigma, USA] for 1 week to induce colitis and fed diet containing 1.7% methionine (Met) for the first 2 weeks to induce hyperhomocysteinemia. COX inhibitor was dissolved in saline and given to mice by gavage of indomethacin (3 mg/kg body weight) for 1 week during the DSS induction and twice daily during the third week. STAT3 inhibitor was dissolved in saline and given to mice by gavage of cryptotanshinone (30 mg/kg body weight) for 1 week during the DSS induction and once daily during the third week (Fig. 1).
Disease Activity Index and Histological Score
The weight of the mice was recorded every two days, and observations were made on fecal properties, food intake, mental status, and blood in the stool. These observations were used to calculate the disease active index [DAI] score, which served as a measure for evaluating the severity of intestinal inflammation, as depicted in Table 1. The colon tissues were extracted subsequent to the euthanization of the mice. Subsequently, sections with a thickness of 5 μm were meticulously prepared and subjected to hematoxylin and eosin [H&E] staining. The resulting stained sections were then utilized to assess and assign scores to the intestinal inflammation, employing the histopathological grading criteria delineated in Table 2.
Determination of Myeloperoxidase (MPO) Activity in Colon Tissue
Myeloperoxidase (MPO) is an enzyme present in neutrophils, which has the ability to reduce hydrogen peroxide (H2O2). This feature can be used to analyze the activity of the enzyme, quantitatively detect the number of neutrophils, and evaluate the degree of intestinal inflammation. The change in absorbance at 460 nm was detected using a UV spectrophotometer. 1 unit of enzyme activity was defined as the decomposition of 1-umol H2O2 per gram of tissue wet slice in the reaction system at 37 °C. MPO activity was measured according to the manufacturer’s instructions for the MPO activity assay kit [Jiancheng BioEngineering, Nanjing, China].
Enzyme-Linked Immunosorbent Assay [ELISA]
Blood samples and colon tissues were obtained from mice and the serum and colon tissues were assayed for the concentration of PGE2 and homocysteine in accordance with the instructions of ELISA kit strictly.
Western Blotting Analysis
The protein expressions of IL-17A, RORγt, cAMP, p-STAT3, STAT3, p-cPLA2, cPLA2 and COX2 in colon tissues from mice were assessed using Western blotting. Total protein was extracted from colon tissue using RIPA lysis buffer supplemented with protease inhibitors [ASPEN, USA]. Protein concentrations were determined using a Bradford protein assay kit [ASPEN, USA]. Subsequently, the proteins were separated by SDS-PAGE electrophoresis, followed by their transfer onto PVDF membranes. The membranes were then incubated with 5% skim milk powder at room temperature for aduration of 1 h. Primary antibodies was directly added and incubated overnight at 4 ℃. Afterward, they were removed and allowed to return to room temperature before washing thrice with TBST for 5 min each time. Secondary antibodies were added, followed by incubation at room temperature for 30 min. The membranes underwent four TBST washes, each for 5 min on a shaker at room temperature.
The protein side of the membrane was exposed to a freshly prepared ECL mixture (A:B = 1:1) in a dark room. Exposure conditions were adjusted based on varying light intensities, followed by development, fixation, scanning, and archiving of the film. The optical density values of the target bands were analyzed using the AlphaEaseFC software processing system.
Flow Cytometry
Th17 cells and IL-23R were characterized using flow cytometry. Colon tissue-derived lymphocytes from mice were stimulated by adding 10 ng/mL of PMA [Sigma, USA], 1 μM of ionomycin [MCE, USA], and Golgistop [BD Biosciences, USA] before the experiment. Subsequently, the cells were washed with phosphate-buffered saline (PBS).
The lymphocytes were stained using anti-CD4 FITC [Biolegend, USA] and anti-IL-17 PE antibodies [Biolegend, USA] to identify Th17 cells. Further, antibodies including anti-IFN-γ PE [Biolegend, USA], anti-IL-17A APC [Biolegend, USA], and anti-IL-23R PE [Biolegend, USA] were separately added to stain lymphocytes for specific markers. Flow cytometry was utilized to determine the percentages of CD4+IL-17A+ (Th17) and CD4+IL-17A+IL-23R+ (IL-23R) cells. Analysis of the obtained data was performed using FlowJo V.10 software.
Statistical Analysis
GraphPad Prism 8.0.2 graphing software were used for graphing and analysis. Experimental data were presented as mean ± standard deviation (SD). Normality and homogeneity of variance were assessed using Levene’s method and chi-square tests. One-way analysis of variance (ANOVA) was employed for individual group analysis. For statistically significant differences determined by chi-square tests among groups, post hoc LSD tests were conducted. In cases where variances were not statistically significant among groups based on chi-square, the Tamhane T2 test was utilized for comparison. Statistical significance was set at P < 0.05.
Results
Changes in Intestinal Inflammation After the Addition of COX and STAT3 Inhibitors
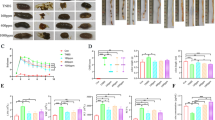
Levels of Hcy in serum and colon tissues of mice were measured to validate the hyperhomocysteinemia (HHcy) model. Compared to the control group, both serum and colon tissue exhibited elevated Hcy levels in the HHcy group [HHcy: 782.09 ± 182.39 vs. Control: 411.78 ± 72.17 ng/mL, P = 0.004; HHcy: 277.94 ± 50.58 vs. Control: 143.73 ± 24.07 ng/mg, P = 0.004] [Fig. 2A and B]. Additionally, in comparison to the DSS group, the HHcy + DSS group showed significantly higher Hcy concentrations in serum and Hcy content in colon tissues [DSS + HHcy: 755.55 ± 164.56 vs. DSS: 424.75 ± 58.68 ng/mL, P = 0.008; DSS + HHcy: 285.11 ± 57.30 vs. DSS: 149.79 ± 18.47 ng/mg, P = 0.008] (Fig. 2A and B).
Homocysteine regulates intestinal inflammation in DSS mice. A Concentration of homocysteine in serum; B content of homocysteine in colon tissues; C body weight; D DAI score; E histological scores; F the activity of MPO in colon tissues; G colon histopathological features (HE X 200). *DSS vs. Control, P < 0.05; # DSS + HHcy vs. DSS, P < 0.05; ^DSS + HHcy + COX inhibitor vs. DSS + HHcy, P < 0.05; +DSS + HHcy + STAT3 inhibitor vs. DSS + HHcy, P < 0.05; ⊙HHcy vs. Control, P < 0.05
Following DSS treatment, mice exhibited weight loss from day 2 onward in comparison to blank controls (Fig. 2C). The Disease Activity Index (DAI) score increased progressively with DSS treatment duration (Fig. 2D). Evaluating the degree of inflammation in DSS-induced colitis, histological scores and MPO activity were employed. Histological scores were significantly higher in the DSS group than the control group [DSS: 1.78 ± 0.39 vs. Control: 0.22 ± 0.19, P < 0.001] (Fig. 2E), indicating evident inflammatory changes. Similarly, MPO activity was notably elevated in the DSS group compared to controls [DSS: 0.86 ± 0.08 vs. Control: 0.46 ± 0.06, P < 0.001] (Fig. 2F). Colon histology of the control group showed no signs of inflammation, whereas DSS treatment led to pathological changes, such as epithelial and cupular cell loss, inflammatory cell infiltration, and crypt damage (Fig. 2G).
Further examination of Hcy’s impact on colonic inflammation revealed more pronounced weight loss in the HHcy + DSS group compared to the DSS group (Fig. 2C). Additionally, the HHcy + DSS group displayed a significantly higher DAI score (Fig. 2D). Histological scores demonstrated more severe inflammatory infiltration and crypt damage in colon tissue induced by HHcy-mediated colitis [DSS + HHcy: 2.78 ± 0.39 vs. DSS: 1.78 ± 0.39, P = 0.004] (Fig. 2E and G). Furthermore, HHcy intensified MPO activity in mice within the DSS group [DSS + HHcy: 1.32 ± 0.11 vs. DSS: 0.86 ± 0.08, P < 0.001] (Fig. 2F).
In this investigation, mice received treatment with the COX inhibitor indomethacin and the STAT3 inhibitor cryptotanshinone. It was observed that the COX inhibitor reduced both colon histological score and MPO activity in the HHcy + DSS group [DSS + HHcy + COX inhibitor: 2.00 ± 0.33 vs. DSS + HHcy: 2.78 ± 0.39, p = 0.019; DSS + HHcy + COX inhibitor: 1.10 ± 0.07 vs. DSS + HHcy: 1.32 ± 0.11, P = 0.005] (Fig. 2E and F). Notably, this treatment ameliorated pathological changes such as inflammatory cell infiltration and crypt damage in colon tissue compared to the preceding conditions (Fig. 2G). Moreover, the addition of the STAT3 inhibitor also yielded reductions in colon histological score and MPO activity in the HHcy + DSS group [DSS + HHcy + STAT3 inhibitor: 2.78 ± 0.39 vs. DSS + HHcy: 1.89 ± 0.51, P < 0.001; DSS + HHcy + STAT3 inhibitor: 1.32 ± 0.11 vs. DSS + HHcy: 0.99 ± 0.09, P < 0.001] (Fig. 2E and F). This intervention corresponded with a mitigation of inflammatory cell infiltration in colon tissue.
Changes in Expression of PLA2, PGE2, cAMP, and STAT3 After Addition of COX and STAT3 Inhibitors
Protein expressions of COX2 (Fig. 3A and C) and p-STAT3/STAT3 (Fig. 3A and F) were notably higher in the DSS group compared to the control group [DSS: 0.53 ± 0.13 vs. Control: 0.12 ± 0.07, P < 0.001; DSS: 0.30 ± 0.04 vs. Control: 0.10 ± 0.01, P < 0.001]. Further investigation into Hcy’s impact on intestine-related signaling molecules in DSS-induced mice revealed significantly elevated protein expressions of COX2 (Fig. 3A and C) and p-STAT3/STAT3 (Fig. 3A and F) in the Hcy + DSS group compared to the DSS group [DSS + HHcy: 1.03 ± 0.055 vs. 0.53 ± 0.13, P < 0.001; DSS + HHcy: 0.60 ± 0.06 vs. DSS: 0.29 ± 0.04, P < 0.001].
Signaling pathway of homocysteine regulating intestinal inflammation in DSS mice. A Immunoblotting analysis of the cPLA2, phosphorylated-cPLA2, COX2, cAMP, STAT3, and phosphorylated-STAT3 protein in the colon of mice; B quantitative analysis of the protein level of p-cPLA2/cPLA2; C quantitative analysis of the protein level of COX2; D quantitative detection concentration of PGE2 in serum by ELISA; E quantitative analysis of the protein level of cAMP; D quantitative analysis of the protein level of p-STAT3/STAT3. *DSS vs. Control, P < 0.05; #DSS + HHcy vs. DSS, P < 0.05; ^DSS + HHcy + COX inhibitor vs. DSS + HHcy, P < 0.05; +DSS + HHcy + STAT3 inhibitor vs. DSS + HHcy, P < 0.05. a: Control group, b: DSS, c: HHcy group, d: DSS + HHcy group, e: DSS + HHcy + COX inhibitor group, f: DSS + HHcy + STAT3 inhibitor group
Continuing to explore Hcy’s promotion of intestinal inflammatory responses via the PGE2/COX2 signaling pathway, mice received ongoing treatment with the COX inhibitor indomethacin and the STAT3 inhibitor cryptotanshinone. Post-treatment, the protein level of COX2 (Fig. 3A and C) significantly decreased compared to DSS + HHcy [DSS + HHcy + COX inhibitor: 0.74 ± 0.06 vs. DSS + HHcy: 1.03 ± 0.055, P = 0.001]. Moreover, the addition of the STAT3 inhibitor notably reduced the protein level of p-STAT3/STAT3 [DSS + HHcy + STAT3 inhibitor: 0.42 ± 0.04 vs. DSS + HHcy: 0.60 ± 0.06, P < 0.001] (Fig. 3A and F).
Notably, the addition of the COX inhibitor did not significantly alter the protein expression of p-PLA2/PLA2 [DSS + HHcy + COX inhibitor: 0.74 ± 0.06 vs. DSS + HHcy: 0.81 ± 0.04, P = 0.105] (Fig. 3A and B), but led to significant decreases in serum PGE2 concentration (Fig. 3D), cAMP, and p-STAT3/STAT3 protein expression (Fig. 3A, E and F) [DSS + HHcy + COX inhibitor: 101.65 ± 8.77 pg/mL vs. DSS + HHcy: 131.36 ± 11.63 pg/mL, P = 0.001; DSS + HHcy + COX inhibitor: 0.46 ± 0.08 vs. DSS + HHcy: 0.70 ± 0.07, P = 0.002; DSS + HHcy + COX inhibitor: 0.46 ± 0.08 vs. DSS + HHcy: 0.70 ± 0.07, P = 0.002].
The protein expressions of p-PLA2/PLA2, COX2, and cAMP upstream of STAT3 inhibitor addition did not exhibit significant changes [DSS + HHcy + STAT3 inhibitor: 0.74 ± 0.04 vs. DSS + HHcy: 0.81 ± 0.04, P = 0.077; DSS + HHcy + STAT3 inhibitor: 0.96 ± 0.05 vs. DSS + HHcy: 1.03 ± 0.055, P = 0.329; DSS + HHcy + STAT3 inhibitor: 0.63 ± 0.10 vs. DSS + HHcy: 0.70 ± 0.07, P = 0.28] (Fig. 3A-C and E). However, serum PGE2 concentration significantly decreased (Fig. 3D) and p-STAT3/STAT3 protein expression was notably reduced (Fig. 3A and F) [DSS + HHcy + STAT3 inhibitor: 93.24 ± 6.77 pg/mL vs. DSS + HHcy: 131.36 ± 11.63 pg/mL, P < 0.001; DSS + HHcy + STAT3 inhibitor: 0.42 ± 0.04 vs. DSS + HHcy: 0.60 ± 0.06, P < 0.001].
The above findings confirm that PGE2/STAT3 is the signaling pathway that may be responsible for the exacerbation of intestinal inflammatory responses by homocysteine.
Changes in the Expression of Th17 Differentiation and Proliferation-related Molecules After the Addition of COX and STAT3 Inhibitors
Th17 cells are central cells in the regulation of intestinal inflammation in IBD, and RORγt is not only a specific transcription factor in the differentiation of Th17 cells, but also a powerful target molecule for the regulation of the transcription factor STAT3 [15]. We investigated whether Hcy aggravates the intestinal inflammatory response by promoting the expression of IL-17A and its specific transcription factor RORγt in colonic tissue colon tissues through the PGE2/STAT3 signaling pathway.
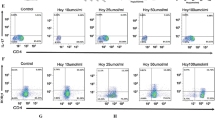
Western blotting showed that the protein expression of IL-17A and RORγt in colon tissue was significantly higher in the Hcy + DSS group compared with the DSS group [Hcy + DSS: 0.87 ± 0.11 vs. DSS: 0.47 ± 0.03, P < 0.001; Hcy + DSS: 0.92 ± 0.08 vs. DSS: 0.40 ± 0.06, P < 0.001] (Fig. 4A and B). Flow cytometry revealed that the level of CD4+17A+T cell was higher in the Hcy + DSS group than in the DSS group [Hcy + DSS: 7.95 ± 0.20 vs. DSS: 5.78 ± 0.21, P < 0.001] (Fig. 4D and F). IL-23R plays a crucial role in Th17-mediated inflammation, maintains the Th17 phenotype and promotes its proliferation, and is essential for the terminal differentiation of Th17 [16]. The expression of IL-23R was significantly higher in the Hcy + DSS group compared with the DSS group by flow cytometry [Hcy + DSS: 13.87 ± 1.55 vs. DSS: 8.80 ± 0.68, P < 0.001] (Fig. 4E and G).
Homocysteine promotes the differentiation of Th17 cells in DSS-induced colitis tissue through the PGE2/STAT3 signaling pathway. A Immunoblotting analysis of the IL-17A and RORγt protein in the colon of mice; B quantitative analysis of the protein level of IL-17A; C quantitative analysis of the protein level of RORγt; D and E IL-17A and IL-23R in CD4+T cells analyzed by flow cytometry; F and G quantitative analysis of the T cell level of CD4 + IL-17A + and IL-23R. *DSS vs. Control, P < 0.05; #DSS + HHcy vs. DSS, P < 0.05; ^DSS + HHcy + COX inhibitor vs. DSS + HHcy, P < 0.05; +DSS + HHcy + STAT3 inhibitor vs. DSS + HHcy, P < 0.05. a: Control group, b: DSS, c: HHcy group, d: DSS + HHcy group, e: DSS + HHcy + COX inhibitor group, f: DSS + HHcy + STAT3 inhibitor
To further investigate whether Hcy promotes Th17 cells differentiation in the colon tissue of DSS-induced colitis mice through the PGE2/STAT3 signaling pathway, we added COX inhibitor to the experimental mice and found that the protein expression of IL-17A and RORγt (Fig. 4A-C) and the level of CD4+IL-17A+T and IL-23R in CD4+T cells (Fig. 4D-G) were significantly reduced after the inhibitor added [DSS + HHcy + COX inhibitor: 0.52 ± 0.05 vs. DSS + HHcy: 0.87 ± 0.11, P < 0.001; DSS + HHcy + COX inhibitor: 0.52 ± 0.05 vs. DSS + HHcy 0.92 ± 0.08, P < 0.006; DSS + HHcy + COX inhibitor: 6.06 ± 0.34 vs. DSS + HHcy 7.95 ± 0.20, P < 0.001; DSS + HHcy + COX inhibitor: 10.17 ± 0.45 vs. DSS + HHcy: 13.87 ± 1.55, P < 0.001].
After the STAT3 inhibitor was added, we also observed the protein expression of IL-17A and RORγt in colon tissues (Fig. 4A–C), and the level of CD4+IL-17A+T cell and IL-23R in CD4+ T cells was also significantly decreased [DSS + HHcy + STAT3 inhibitor: 0.50 ± 0.02 vs. DSS + HHcy: 0.87 ± 0.11, P < 0.001; DSS + HHcy + STAT3 inhibitor: 0.50 ± 0.02 vs. DSS + HHcy: 0.92 ± 0.08, P < 0.001; DSS + HHcy + STAT3 inhibitor: 7.95 ± 0.20 vs. DSS + HHcy: 5.93 ± 0.27, P < 0.001; DSS + HHcy + STAT3 inhibitor: 13.87 ± 1.55 vs. DSS + HHcy: 9.65 ± 0.64, P < 0.001] (Fig. 4D–G).
These results suggest that Hcy may exacerbate intestinal inflammation by promoting the expression of IL-17A, IL-23R, and RORγt in colonic tissues via the PGE2/STAT3 signaling pathway.
Discussion
The metabolic disorders of Hcy in humans serve as the underlying mechanism for the development of cardiovascular and inflammatory diseases [17], IBD is a chronic inflammation affecting the intestinal tract, and the impact of Hcy on the intestinal tract of IBD patients has been extensively investigated [2, 18]. It has been demonstrated that Hcy levels were significantly elevated in IBD patients and could exacerbate the intestinal inflammation [2]. Furthermore, Hcy promotes inflammatory infiltration, thereby intensifying the severity of intestinal inflammation [2, 7]. In vitro experiments have also clearly demonstrated the role of Hcy in regulating the intestinal inflammatory response of mice, and studies have found that in the TNBS-induced rat colitis model, the injection of Hcy can aggravate the inflammatory response of colitis rats [19]. This study revealed that following the administration of DSS, mice experienced a decrease in weight and an increase in MPO activity and colon histological score and exhibited pathological changes, such as inflammatory cell infiltration and crypt damage in the colon tissue. Moreover, the weight loss observed in DSS mice treated with Hcy was more pronounced, and the extent of colon tissue damage was more severe, aligning with previous research [11]. These findings provide evidence that Hcy exacerbates intestinal inflammation in DSS mice.
Following the confirmation of Hcy’s role in exacerbating intestinal inflammation, we delved deeper into its mechanisms. Hcy, possessing pro-inflammatory traits, can impair endothelial cell function by stimulating the expression of inflammatory substances. It disrupts the endothelial barrier, elevates intestinal microvascular endothelial cell permeability, and incites inflammatory responses [20]. Previous studies reported that Hcy upregulates VCAM-1 and MCP-1 expression on human intestinal microvascular endothelial cells, intensifying inflammatory infiltration and worsening intestinal inflammation [2]. Through the ERK/MLCK pathway, Hcy activates MMP-9, enhancing intestinal microvascular endothelial cell permeability and undermining intestinal mucosal barrier function during IBD-associated inflammation [7, 21]. Moreover, Hcy prompts the release of pro-inflammatory factors like TNF-α, IL-6, and IFN-γ, disrupting tight intercellular junctions and increasing intestinal mucosal permeability, thus exacerbating inflammation [19]. Additionally, Hcy triggers inflammatory responses via oxidative stress, contributing to intestinal mucosal endothelial dysfunction, a significant factor in IBD development [2, 18]. Studies reported that high Hcy levels upregulate nitric oxide synthase (iNOS), promoting Nitrogen monoxide (NO) production, leading to neutrophil infiltration in the intestine, resulting in tissue damage and inflammation in IBD patients [6, 22]. Hcy has been implicated in the pathogenesis of intestinal inflammation by activating nuclear factor kappa-B (NF-κB) signaling, leading to the generation of reactive oxygen species (ROS) [23]. The elevated levels of intestinal ROS, in turn, contribute to the progression of intestinal inflammation by promoting the degradation of the inhibitor of NF-κB (IκB) and subsequent activation of NF-κB [24]. It has been documented that the activation of NF-κB signaling plays a significant role in the development of IBD, the production of pro-inflammatory cytokines TNF-α and IL-6 through the NF-κB signaling pathway has been shown to induce UC [25], and a recent study has demonstrated that NaHSO3, primarily derived from Hcy, can enhance the ROS-mediated NF-κB pathway, thereby promoting intestinal inflammation in mice [26]. Furthermore, it has been observed that Hcy possesses the ability to induce intestinal inflammation in patients with IBD through the activation of the p38 MAPK signaling pathway. Notably, in rats with colitis induced by DSS, there was a notable elevation in the levels of phosphorylated p38 MAPK, and the expression of p38 MAPK was further augmented upon the addition of Hcy. Additionally, the secretion of inflammatory mediators, namely PGE2 and IL-17A, was also enhanced, providing evidence that Hcy exacerbates DSS-induced colitis in rats [11]. Building upon our prior research, we conducted a more comprehensive investigation into the involvement and molecular mechanism of Hcy in the development of DSS-induced intestinal inflammation in mice. In comparison to mice with DSS-induced colitis, the levels of p-PLA2, COX2, cAMP, PGE2, and p-STAT3 were found to be elevated in the colon tissues of mice with Hcy-treated colitis. Subsequent administration of a COX inhibitor and STAT3 inhibitor did not significantly alter the levels of upstream signaling molecules, but effectively suppressed the levels of downstream signaling molecules. Furthermore, the incorporation of inhibitors exhibited a notable reduction in the inflammation induced by Hcy in mice. Consequently, our investigation has determined that Hcy exacerbates colon inflammation induced by DSS in mice via the PGE2/STAT3 signaling pathway.
The relationship between Th17 cells and their cytokine IL-17A with the pathogenesis of IBD has been established. Growing evidence suggests that Hcy can facilitate the differentiation of Th17 cells, thereby contributing to the inflammatory response in the intestines of individuals with IBD [27]. Nevertheless, the mechanism through which Hcy promotes Th17 cells differentiation requires further investigation. In vitro research has demonstrated that Hcy can dose dependently induce the differentiation of CD4+T cells into Th17 cells, as well as enhance the expression of RORγt and IL-17 in mice [14]. RORγt, a crucial transcription factor, plays a significant role in driving the differentiation of naive CD4+T cells into Th17 cells and facilitating the expression of IL-17A [28]. Previous investigations have demonstrated that Hcy can enhance the differentiation of Th17 cells through the p38/cPLA2/COX2/PGE2 signaling pathway, leading to increased expression of IL-17 and RORγt in the intestinal tract of rats [11]. Furthermore, Hcy can stimulate T cell activation by inducing ER stress, ER-mitochondrial coupling, and mitochondrial metabolism [29], while also up-regulating the expression of IL-17A through methylation of IL-17A mRNA mediated by methyltransferase (NSun2) in T cells [30]. IL-23 acts on members of the innate immune system and promotes the growth and maintenance of Th17 cells [31]. IL-23R serves as a crucial surface marker for Th17 cells, and binding of IL-23 to it promotes the proliferation and differentiation of Th17 cells [32]. IL-23-activated Th17 cells produce a range of cytokines that includes IL-17A, IL-17F, IL-21, and IL-22 [33, 34]. Previous studies have shown that IL-21 is upregulated in DSS-treated mice and that IL-21-deficient mice have attenuated intestinal inflammation, which correlates with a marked downregulation in the expression of Th17-related molecules [35]. IL-22 has a dual function in that it can have both pro-inflammatory and protective effects on tissues depending on the degree of inflammation. A previous study showed that IL-22 protects mice from IBD [36]. The findings of our study indicate that Hcy has the ability to enhance the differentiation of Th17 cells and the secretion of IL-17A in the colon tissues of DSS-induced colitis. This effect is mediated through the PGE2/STAT3 signaling pathway. Additionally, Hcy also promotes the expression of IL-23R on the surface of Th17 cells.
In conclusion, these findings provide evidence for the regulation of Hcy during the DSS-induced intestinal inflammatory response and further clarify that Hcy may promote intestinal Th17 cell differentiation and IL-17A expression through the regulation of PGE2/STAT3 signaling pathway, thereby promoting intestinal histopathological changes and exacerbating intestinal inflammation in mice. Therefore, comprehending the underlying mechanisms outlined in this study presents novel avenues for the prevention and treatment of IBD.
Data availability
No datasets were generated or analyzed during the current study.
References
Lavelle A, Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 2020;17:223–237.
Danese S, Sgambato A, Papa A et al. Homocysteine triggers mucosal microvascular activation in inflammatory bowel disease. Am J Gastroenterol 2005;100:886–895.
Kallel L, Feki M, Sekri W et al. Prevalence and risk factors of hyperhomocysteinemia in Tunisian patients with Crohn’s disease. J Crohns Colitis 2011;5:110–114.
Drzewoski J, Gasiorowska A, Malecka-Panas E et al. Plasma total homocysteine in the active stage of ulcerative colitis. J Gastroenterol Hepatol 2006;21:739–743.
Bjerregaard LT, Nederby NJ, Fredholm L et al. Hyperhomocysteinaemia, coagulation pathway activation and thrombophilia in patients with inflammatory bowel disease. Scand J Gastroenterol 2002;37:62–67.
Erzin Y, Uzun H, Celik AF et al. Hyperhomocysteinemia in inflammatory bowel disease patients without past intestinal resections: correlations with cobalamin, pyridoxine, folate concentrations, acute phase reactants, disease activity, and prior thromboembolic complications. J Clin Gastroenterol 2008;42:481–486.
Munjal C, Tyagi N, Lominadze D et al. Matrix metalloproteinase-9 in homocysteine-induced intestinal microvascular endothelial paracellular and transcellular permeability. J Cell Biochem 2012;113:1159–1169.
Ding H, Gan HZ, Fan WJ et al. Homocysteine promotes intestinal fibrosis in rats with trinitrobenzene sulfonic acid-induced colitis. Dig Dis Sci 2015;60:375–381.
Harbour SN, DiToro DF, Witte SJ et al. T(H)17 cells require ongoing classic IL-6 receptor signaling to retain transcriptional and functional identity. Sci Immunol. 2020;5.
Stockinger B, Omenetti S. The dichotomous nature of T helper 17 cells. Nat Rev Immunol 2017;17:535–544.
Zhu S, Li J, Bing Y et al. Diet-induced hyperhomocysteinaemia increases intestinal inflammation in an animal model of colitis. J Crohns Colitis 2015;9:708–719.
Dong M, Guda K, Nambiar PR et al. Inverse association between phospholipase A2 and COX-2 expression during mouse colon tumorigenesis. Carcinogenesis 2003;24:307–315.
Lee J, Aoki T, Thumkeo D et al. T cell-intrinsic prostaglandin E(2)-EP2/EP4 signaling is critical in pathogenic T(H)17 cell-driven inflammation. J Allergy Clin Immunol 2019;143:631–643.
Gao X, Li J, Chen M. Effect of homocysteine on the differentiation of CD4(+) T cells into Th17 cells. Dig Dis Sci 2018;63:3339–3347.
Zhao J, Lu Q, Liu Y et al. Th17 cells in inflammatory bowel disease: cytokines, plasticity, and therapies. J Immunol Res. 2021;8816041.
Gaffen SL, Jain R, Garg AV et al. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol 2014;14:585–600.
Chiocchetti A, Prodam F, Dianzani U. Homocysteine and folate in inflammatory bowel disease: can reducing sulfur reduce suffering? Dig Dis Sci 2018;63:3161–3163.
Wang S, Huang Z, Li W et al. IL-37 expression is decreased in patients with hyperhomocysteinemia and protects cells from inflammatory injury by homocysteine. Mol Med Rep 2020;21:371–378.
Ding H, Mei Q, Gan HZ et al. Effect of homocysteine on intestinal permeability in rats with experimental colitis, and its mechanism. Gastroenterol Rep (Oxf) 2014;2:215–220.
Liang S, Liu S, Liu H et al. Homocysteine aggravates intestinal epithelial barrier dysfunction in rats with experimental uremia. Kidney Blood Press Res 2018;43:1516–1528.
Tyagi N, Gillespie W, Vacek JC et al. Activation of GABA-A receptor ameliorates homocysteine-induced MMP-9 activation by ERK pathway. J Cell Physiol 2009;220:257–266.
Wang Y, Shou Z, Fan H et al. Protective effects of oxymatrine against DSS-induced acute intestinal inflammation in mice via blocking the RhoA/ROCK signaling pathway. Biosci Rep 2019. https://doi.org/10.1042/BSR20182297.
Chu J, Yan B, Zhang J et al. Casticin attenuates osteoarthritis-related cartilage degeneration by inhibiting the ROS-mediated NF-kappaB signaling pathway in vitro and in vivo. Inflammation 2020;43:810–820.
Akanda MR, Nam HH, Tian W et al. Regulation of JAK2/STAT3 and NF-kappaB signal transduction pathways; Veronica polita alleviates dextran sulfate sodium-induced murine colitis. Biomed Pharmacother 2018;100:296–303.
Tatiya-Aphiradee N, Chatuphonprasert W, Jarukamjorn K. Immune response and inflammatory pathway of ulcerative colitis. J Basic Clin Physiol Pharmacol 2018;30:1–10.
Wu W, Fu G, Xuan R et al. Food additive sodium bisulfite induces intracellular imbalance of biothiols levels in NCM460 colonic cells to trigger intestinal inflammation in mice. Toxicol Lett 2022;359:73–83.
Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol 2014;14:329–342.
Ivanov II, McKenzie BS, Zhou L et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006;126:1121–1133.
Feng J, Lu S, Ding Y et al. Homocysteine activates T cells by enhancing endoplasmic reticulum-mitochondria coupling and increasing mitochondrial respiration. Protein Cell 2016;7:91–402.
Wang N, Tang H, Wang X et al. Homocysteine upregulates interleukin-17A expression via NSun2-mediated RNA methylation in T lymphocytes. Biochem Biophys Res Commun 2017;493:94–99.
Korta A, Kula J, Gomułka K. The role of IL-23 in the pathogenesis and therapy of inflammatory bowel disease. Int J Mol Sci 2023;24:10172.
Jacobse J, Brown RE, Li J et al. Interleukin-23 receptor signaling impairs the stability and function of colonic regulatory T cells. Cell Rep 2023;42:112128.
Moschen AR, Tilg H, Raine T. IL-12, IL-23 and IL-17 in IBD: immunobiology and therapeutic targeting. Nat. Rev. Gastroenterol. Hepatol. 2019;16:185–196.
Harper EG, Guo C, Rizzo H et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J. Investig. Dermatol. 2009;129:2175–2183.
Fina D, Sarra M, Fantini MC et al. Regulation of gut inflammation and th17 cell response by interleukin-21. Gastroenterology 2008;134:1038–1048.
Zenewicz LA, Yancopoulos GD, Valenzuela DM et al. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957.
Acknowledgments
Project supported by the National Natural Science Foundation of China (No. 8227033272).
Author information
Authors and Affiliations
Contributions
All authors made significant contributions to the study. Conceptualized and designed the experiments: AS, QZ, and MC; performed the experiments: AS and MC; acquire and analyzed the data: AS; wrote the manuscript: AS, QZ, and MC.
Corresponding author
Ethics declarations
Conflict of interest
There is no financial conflict of interest to declare for any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Shao, A., Zhao, Q. & Chen, M. Homocysteine Promotes Intestinal Inflammation in Colitis Mice Through the PGE2/STAT3 Signaling Pathway. Dig Dis Sci (2024). https://doi.org/10.1007/s10620-024-08588-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10620-024-08588-2