Abstract
Introduction
Poor sleep quality has been associated with inflammatory bowel disease (IBD) activity, although studies incorporating actigraphy suggest that the perception of sleep differs rather than objective difference in sleep quality. Short sleep duration has been associated with increased pro-inflammatory cytokines that have been implicated in the pathogenesis of IBD.
Methods
An observational study incorporated home-based polysomnography that was conducted within twelve weeks of an objective assessment of IBD activity such as calprotectin, colonoscopy, or MRI. Participants completed a survey on subjective measures of sleep quality, clinical IBD activity, depression, and anxiety. Polysomnography results were normalized by standardized results for a healthy population matched by gender and age.
Results
Twenty participants were included in the final analysis. Those with objective evidence of active IBD had shorter stage 2 sleep duration, leading to shorter NREM sleep and total sleep time. Sleep latency was also longer in those with active IBD, leading to worse sleep efficiency—despite no difference in time available for sleep between the two groups. These changes persisted after normalization of polysomnography results by health population age and gender matched norms. Depression scores correlated with sleep latency and stage 2 sleep duration and were associated with objectively active IBD.
Conclusions
Objectively confirmed active IBD was associated with shorter sleep duration. Observed sleep changes may, in part, relate to coexistent depression. Further research should consider the utility of changes in sleep duration and quality as a means of longitudinally assessing objective IBD activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammatory bowel disease (IBD) is a chronic relapsing remitting immune-mediated disorder that involves a complex interplay of genetic and environmental factors [1]. Symptoms of active IBD, such as abdominal pain and diarrhea, may influence sleep quality and duration. Irritable bowel syndromes like symptoms are common in people with inflammatory bowel disease and can be misinterpreted as active IBD [2].
Short sleep duration, in the general population, has been associated with increased all-cause mortality [3], adverse health effects including cardiovascular disease [4], and metabolic syndrome [5], as well as economic consequences such as lower productivity and greater health care utilisation [6]. Upregulation of pro-inflammatory cytokines, such as IL-1β, IL-6, and TNFα, have been observed in short sleepers—with these same cytokines implicated in the pathogenesis of IBD [7]. Several previous studies have investigated the association between sleep and IBD [8,9,10] and postulated a bi-directional relationship between sleep and IBD activity [11].
Sleep can be assessed subjectively using a measure of perceived sleep via validated survey methods such as the Pittsburgh Sleep Quality Index (PSQI), with standardised cut off values to define likely significant sleep disturbance referred to as “poor sleep” [12]. Meta-analyses show that poor sleep is prevalent in people with IBD [13], worse in people with IBD than healthy controls [14], more common in those with subjectively active IBD [14], and worse in IBD in remission than in healthy controls [15].
However, there have also been several publications [16,17,18,19,20,21,22] suggesting no significant relationship between IBD disease activity and disturbed sleep, with some suggesting that only perceived sleep may be different. Studies incorporating objective sleep measurement have suggested that sleep efficiency is worse in subjectively active IBD and wake after sleep onset is longer in subjectively active IBD [19]. The lack of objective IBD assessment in these studies leads to the possibility of irritable bowel syndrome like symptoms being mistaken for clinically active IBD. The authors aim to address this gap in the literature and compare the gold standard of sleep assessment—polysomnography with objective assessments of IBD activity.
Methods
Ethics approval for this study was obtained from the Southern Adelaide Human Research Ethics Committee (203.20). Informed consent was obtained from all participants. Participants were recruited from a tertiary IBD unit and a private IBD service with the study advertised via email and flyer. Recruitment occurred from 2021 to the end of 2023. Participants were adults (age over 18) with a confirmed diagnosis of IBD from a gastroenterologist. Participants had a home sleep study (polysomnography) within 12 weeks of an objective assessment of IBD activity which was performed as per usual care. Accepted objective assessment(s) of IBD activity included magnetic resonance imaging enterography, colonoscopy, gastrointestinal ultrasound, and fecal calprotectin (> 150ug/g). Current medications including corticosteroids were recorded during the interval between the sleep study and the IBD assessment. Participants were offered $100 AUD for completion of the sleep study. Participants also completed a survey including demographic data, subjective IBD activity, depression, anxiety, and subjective sleep quality. The study aimed to recruit a minimum of twenty participants based on reported differences in polysomnography (sleep efficiency and wake after sleep onset) reported in other studies that incorporated subjective IBD assessment [19], with a goal of forty participants.
Participants were excluded if they had an uncontrolled psychiatric disorder, substance abuse disorder, heavy vehicle license or a known sleep disorder such as obstructive sleep apnea.
Sleep Quality Assessment
Polysomnography consists of the recording of multiple variables during sleep and can be performed in sleep laboratory or home setting. Variables include—electrocardiogram, electromyography to capture muscle movement, electroencephalography to capture brain activity, electrooculogram to capture eye movement, respiratory airflow channels to capture apnoeas or hypopnoeas, and pulse oximetry monitoring and respiratory effort channels to measure movement of chest and abdomen [23]. Sleep staging is divided into REM sleep (rapid eye movement) and NREM sleep—(non-rapid eye movement) which is further divided into stage 1, stage 2, and slow wave sleep (previously stage 3 and 4 sleep). Variables of interest in polysomnography are seen in Table 1. Given the well-established sleep differences due to age and gender [24, 25], polysomnography results were normalized by published values based on age and gender [26].
Polysomnography was performed using the Compumedics Somte PSG and Somte PSG 2 devices (Compumedicas Limited, Victoria, Australia). All participants were manually setup by a trained technician. The device used does not show impedance values; however, all traces were visually checked via a staff member prior to leaving the sleep laboratory. All sleep studies have electroencephalography, electrooculogram, electromyography, respiratory, ECG, and limb recordings and are directly comparable to gold standard polysomnography. All of the data were manually scored with the exception of limb movements which was predominantly scored by auto analysis by Profusion software (Compumedicas Limited, Victoria, Australia). All the sleep studies were scored by a trained sleep technician with a minimum of 5 years’ experience in sleep scoring. The sleep laboratory used regularly participates in QSleep quality assurance testing.
Questionnaire Data
Clinical IBD activity was assessed using the Harvey Bradshaw Index (HBI) in participants with Crohn’s disease; with HBI > 5 considered active disease [27]. The patient-reported version of the HBI was used in the survey, although a decision was made to maintain the general well-being and abdominal pain score similar to the physician HBI rather than using a ten-point Likert scale [28]. The Simple Clinical Colitis Activity Index (SCCAI) was used in participants with ulcerative colitis; with an SCCAI > 5 considered active disease [29]. The patient-reported form of the SCCAI was used. The use of a self-reported SCCAI has been previously validated with good agreement with physician reported SCCAI [30].
Anxiety was assessed using the generalized anxiety disorder 7-item scale (GAD-7) [31]. The Patient Health Questionnaire 9 (PHQ-9) was used to assess depression [32]. Subjective sleep quality was measured using the Pittsburgh Sleep Quality Index (PSQI), a validated tool to assess perceived sleep quality [12].
Statistical Analysis
Statistical analysis was performed using Stata SE 16 (StataCorp, College Station, TX, USA). For normally distributed variables, mean and standard deviation (SD) were reported with comparisons made using the student t-test or paired t-test when appropriate. For non-normally distributed variables, median and interquartile range (IQR) were reported, with comparisons made using the Mann- Whitney U test. For categorical data, Pearsons χ2 test was used or Fisher’s exact test when appropriate. No incomplete survey responses were included in the analysis. Pearson’s or Spearman’s correlation was used as appropriate, with interpretation of coefficients as follows: very weak < 0.19, weak 0.2–0.3, moderate 0.3–0.5, strong 0.5–0.79 and very strong > 0.80 [33]. Any missing survey data result in exclusion of that instrument from analysis.
Individual polysomnography results were matched by age and gender to standardized health adult polysomnography results generated via an online calculator based on a meta-analysis of healthy population values from 169 studies [26] (https://omc.ohri.ca/psgnorms/). Individual polysomnography results were then normalized by matched age and gender polysomnography results. Polysomnography results were reported according to the American Academy of Sleep Medicine manual [23, 34].
This study is reported as per the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline [35].
Results
Twenty-nine participants were screened for inclusion. Two were excluded due to an unstable psychiatric disorder. Five were excluded due to a known sleep disorder (insomnia in one case and obstructive sleep apnea in the others). Following screening, 22 participants were included; however, two participants did not undergo the sleep study within twelve weeks of objective IBD assessment and were subsequently excluded. The majority underwent polysomnography within a month of objective IBD activity measurement (65%) (for further details see supplementary figures). Polysomnography was performed prior to the objective IBD assessment in 60%. Objective IBD assessment took the form of calprotectin in 30%, magnetic resonance imaging in 40%, and colonoscopy in 50%. Evidence of active inflammation on any objective assessment was considered as objectively active IBD and was present in 40%.
The median age was 41 years (33–48), 55% male, and the majority had Crohn’s disease (85%) (see Table 2). Previous IBD surgery was reported by 40%, and over half were on advanced therapies such as biologics (65%) with none on small molecules. For all participants, no medication changes, including no steroid use, occurred during the period between the sleep study and IBD assessment. No participant was admitted to hospital during the period between sleep study and IBD assessment. No participant had an ileostomy or colostomy.
Polysomnography
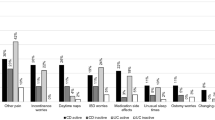
There was similar time available for sleep in both objectively inactive IBD and active IBD groups (see Table 3, Fig. 1). However, total sleep time was less in those with active IBD (p = 0.049) – primarily as decreased NREM sleep duration due to decreased stage 2 sleep time (p = 0.045). No difference in the percentage of time in each sleep stage was seen. Sleep latency was longer in the active IBD group (p = 0.015); i.e., it took a longer time for them to fall asleep. As a result of this, sleep efficiency was lower in the active IBD group (p = 0.014). No difference in wake after sleep onset was seen (p = 0.41). The polysomnography results were normalized by healthy population results matched by age and gender (see Table 3) with similar differences between inactive and active IBD seen—that is those with active IBD had longer sleep latency, shorter total sleep time due to stage 2 sleep, and worse sleep efficiency, although percentage of time in sleep stage was similar.
Questionnaire
Survey results were available for 75% of the cohort within one month of the sleep study. Subjective symptoms of active IBD were present in 45%, with mean HBI 5.6 (4.1) and mean SCCAI 3.3 (2.9). Objective evidence of active IBD was present in 40% of the cohort and was associated with higher depression scores (see supplementary Table 1) but no difference in anxiety or subjective sleep quality scores. Depression scores moderately correlated with stage 2 sleep duration (ρ 0.53 p = 0.046) and strongly correlated with sleep latency (ρ 0.75 p = 0.0013) (see supplementary Table 2). There were no significant correlations between other polysomnography measures and subjective IBD activity, depression scores, or anxiety scores. Subjective sleep quality (PSQI) moderately correlated with depression scores (PHQ9) (ρ 0.57, p = 0.026). A modified PHQ9 score was considered excluding the PHQ9 question concerning sleep with correlations between this score and sleep latency (ρ 0.69, p = 0.0059), and PSQI remaining significant (ρ 0.59, 0.025), with stage 2 sleep duration no longer significant (ρ 0.47, p = 0.088).
Discussion
Herein, we report novel evidence of associations between objective, but not subjective, IBD activity and objectively assessed sleep quality. Prolonged sleep latency in the active IBD group and shortened stage 2 sleep duration led to lower sleep efficiency and shorter total sleep time in the active IBD group. Unlikely previous actigraphy studies, no difference in wake after sleep onset was seen [36, 37]. This may be consistent—noting that actigraphy cannot accurately determine wakefulness while lying still compared to light sleep (unlike EEG measurement as with polysomnography) and therefore may misclassify wake after sleep onset among other parameters [38].
Subjective sleep quality in IBD has been previously associated with depression with similar results obtained in this study [20, 22, 39]. Aspects of polysomnography did correlate with depression scores and not surprisingly depression scores were higher in those with objectively active IBD. Depression is associated with sleep abnormalities such as prolonged sleep latency that was observed in this study, but also other abnormalities such as decreased slow wave sleep and REM sleep abnormalities [40, 41]—which were not seen in the objective active IBD group here. Consequently, we suggest that not all polysomnography differences demonstrated are explained by depression scores alone and may represent an additional influence from active IBD.
Objectively active IBD has been associated with elevated pro-inflammatory cytokines such as TNF-a and IL-6—which are also produced via sleep restriction. In animal studies, elevated pro-inflammatory cytokines drive the need to sleep and correlate with the duration of recovery sleep [42]. The understanding of inflammatory cytokines and sleep in human is less well understood [43]. In rheumatoid arthritis, a small study demonstrated a reciprocal relationship between sleep efficiency and TNFa production [44]. Other studies in rheumatoid arthritis have not demonstrated any differences in polysomnography findings between active and inactive rheumatoid arthritis [45]. A meta-analysis of studies incorporating markers of inflammation and measurement of sleep showed an association between elevated markers of inflammation and sleep disturbance and long sleep duration [46]. Our findings are more consistent with sleep restriction rather than inflammatory cytokine-mediated sleep recovery.
Limitations to this study include the limited sample size which is typically for studies incorporating polysomnography and limits the generalisability of these results. While our use of objective IBD activity allowed a variety of different measures of IBD activity that likely introduces heterogeneity, it did allow for verification of active inflammatory disease. There was some difficulty in arranging sleep studies within an acceptable time of the IBD assessment with two participants not completing the sleep study within the allowed time. This was due to repeated cancelations or non-attendance by the participant. As a post hoc verification of date, we incorporated results from these two further participants and confirmed that it did not change the study outcomes. Also note that the study utilized a single night of polysomnography with others arguing for utilization of multiple nights of polysomnography to increase validity of results and avoid a first night effect [47]. Recruitment for this study proved challenging as with other polysomnography studies because of the time needed for overnight polysomnography.
Future research should consider the prognostic value of objective measurement of sleep as a marker of active IBD with less intrusive methods to measure sleep. Consideration should also be given to performing polysomnography in people with clinical remission but ongoing endoscopic activity in order to observe changes in sleep due to inflammatory cytokines and not IBD-related symptoms.
Conclusion
Specific changes in sleep stages were demonstrated via polysomnography in active IBD compared to inactive IBD. Depression was associated with polysomnography differences along with IBD activity and may explain some but not all of the observed polysomnography changes. Further research should consider quantifying polysomnography differences associated with IBD-related pro-inflammatory cytokines as well as considering the utility of changes in sleep quality as a means of longitudinally assessing objective IBD activity.
Data availability
The data underlying this work is available upon a reasonable request to the corresponding author.
References
Ananthakrishnan AN. Environmental risk factors for inflammatory bowel diseases: a review. Digestive Diseases and Sciences 2015;60:290–298.
Halpin SJ, Ford AC. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2012;107:1474–1482.
Cappuccio F, D’Elia L, Strazzullo P, Miller M. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep 2010;33:585–592.
Daghlas I, Dashti H, Lane J et al. Sleep Duration and Myocardial Infarction. Journal of the American College of Cardiology. 2019;74:1304.
Deng H, Tam T, Zee B et al. Short Sleep Duration Increases Metabolic Impact in Healthy Adults: A Population-Based Cohort Study. Sleep. 2017. https://doi.org/10.1093/sleep/zsx130.
Hillman D, Mitchell S, Streatfeild J, Burns C, Bruck D, Pezzullo L. The economic cost of inadequate sleep. Sleep. 2018. https://doi.org/10.1093/sleep/zsy083.
Mullington J, Simpson N, Meier-Ewert H. Sleep loss and inflammation. Best Practice & Research Clinical Endocrinology & Metabolism. 2010;24:775–784.
Ali T. Sleep and Inflammatory Bowel Disease. Inflammatory bowel diseases. 2014;10:1986–1995.
Swanson G, Burgess H, Keshavarzian A. Sleep disturbances and inflammatory bowel disease: a potential trigger for disease flare? Expert review of Clinical Immunology 2011;7:36.
Eissa N, Mujawar Q, Alabdoulsalam T, Zohni S, El-Matary W. The immune-sleep crosstalk in inflammatory bowel disease. Sleep Medicine 2020;73:38–46.
Qazi T, Farraye F. Sleep and Inflammatory Bowel Disease: An Important Bi-Directional Relationship. Inflammatory Bowel Diseases 2019;25:843–852.
Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Pyschiatry Research 1989;28:193–213.
Barnes A, Mountifield R, Baker J et al. A systematic review and meta-analysis of the prevalence of poor sleep in inflammatory bowel disease. SLEEP Advances. 2022. https://doi.org/10.1093/sleepadvances/zpac025.
Ballesioa A, Zagariaa A, Baccinib F, Michelib F, Di Nardoc G, Lombardoa C. A meta-analysis on sleep quality in inflammatory bowel disease. Sleep Medicine Reviews 2021;60:301–308.
Barnes A, Mountifield R, Baker J, Spizzo P, Bampton P, Mukherjee S. Systematic review and meta-analysis of sleep quality in inactive inflammatory bowel disease. JGH Open. 2022. https://doi.org/10.1093/sleepadvances/zpac025.
Marinelli C, Savarino E, Marsilio I et al. Sleep disturbance in Inflammatory Bowel Disease: prevalence and risk factors—A cross-sectional study. Scientific Reports 2020;10:507.
Sofia M, Lipowska A, Zmeter N, Perez E, Kavitt R, Rubin D. Poor Sleep Quality in Crohn’s Disease Is Associated With Disease Activity and Risk for Hospitalization or Surgery. Inflammatory Bowel Diseases 2020;26:1251–1259.
Sochal M, Małecka-Panas E, Gabryelska A et al. Determinants of Sleep Quality in Inflammatory Bowel Diseases. Journal of Clinical Medicine 2020;9:2921.
Zhang Y, Pi B, Xu X et al. Sleep Characteristics and Influencing Factors of Sleep Quality in Patients With Inflammatory Bowel Disease-Peripheral Arthritis. Frontiers in Medicine 2019;6:190.
Gîlc-Blanariu G, Ștefnescu G, Trifan A et al. Sleep Impairment and Psychological Distress among Patients with Inflammatory Bowel Disease-beyond the Obvious. Journal of Clinical Medicine 2020;9:2304.
Michalopoulos G, Vrakas S, Makris K, Tzathas C. Association of sleep quality and mucosal healing in patients with inflammatory bowel disease in clinical remission. Annals of Gastroenterology 2018;31:211–216.
Hood M, Wilson R, Gorenz A et al. Sleep Quality in Ulcerative Colitis: Associations with Inflammation. Psychological Distress, and Quality of Life 2018;25:517–525.
Berry R, Quan S, Abreu A et al. ASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. American Academy of Sleep Medicine 2020;16:1753–1760.
Luca G, Haba Rubio J, Andries D et al. Age and gender variations of sleep in subjects without sleep disorders. Ann Med. 2015;47:482–491.
Ohayon M, Carskadon M, Guilleminault C, Vitiello M. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255.
Boulos MI, Jairam T, Kendzerska T, Im J, Mekhael A, Murray BJ. Normal polysomnography parameters in healthy adults: a systematic review and meta-analysis. Lancet Respir Med. 2019;7:533–543.
Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet 1980;8:514.
Bennebroek Evertsz F, Hoeks CC, Nieuwkerk PT et al. Development of the patient Harvey Bradshaw index and a comparison with a clinician-based Harvey Bradshaw index assessment of Crohn’s disease activity. J Clin Gastroenterol. 2013;47:850–856.
Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut. 1998;43:29–32.
Marín-Jiménez I, Nos P, Domènech E et al. Diagnostic Performance of the Simple Clinical Colitis Activity Index Self-Administered Online at Home by Patients With Ulcerative Colitis: CRONICA-UC Study. Official journal of the American College of Gastroenterology ACG. 2016;111:261–268.
Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092–1097.
Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613.
Schober P, Boer C, Schwarte LA. Correlation Coefficients: Appropriate Use and Interpretation. Anesth Analg 2018;126:1763–1768.
Berry RB, Budhiraja R, Gottlieb DJ et al. Rules for scoring respiratory events in sleep : update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619.
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Journal of clinical epidemiology. 2008;61:344–349.
Qazi T, Verma R, Hamilton M, Kaplan E, Redline S, Burakoff R. The Use of Actigraphy Differentiates Sleep Disturbances in Active and Inactive Crohn’s Disease. Inflammatory Bowel Diseases 2019;25:1044–1053.
van Langenberg D, Papandony M, Gibson P. Sleep and physical activity measured by accelerometry in Crohn’s disease. Alimentary Pharmacology and Therapeutics. 2015;41:991–1004.
Eiman MN, Pomeroy JML, Weinstein AA. Relationship of actigraphy-assessed sleep efficiency and sleep duration to reactivity to stress. Sleep Sci 2019;12:257–264.
Wilson R, Stevens B, Guo A et al. High C-Reactive Protein Is Associated with Poor Sleep Quality Independent of Nocturnal Symptoms in Patients with Inflammatory Bowel Disease. Digestive Diseases and Sciences 2015;60:43.
Ilanković A, Damjanović A, Ilanković V, Filipović B, Janković S, Ilanković N. Polysomnographic sleep patterns in depressive, schizophrenic and healthy subjects. Psychiatr Danub 2014;26:20–26.
Riemann D, Krone LB, Wulff K, Nissen C. Sleep, insomnia, and depression. Neuropsychopharmacology 2020;45:74–89.
Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci 2009;10:199–210.
Irwin MR. Sleep and inflammation: partners in sickness and in health. Nat Rev Immunol 2019;19:702–715.
Bjurström MF, Olmstead R, Irwin MR. Reciprocal Relationship Between Sleep Macrostructure and Evening and Morning Cellular Inflammation in Rheumatoid Arthritis. Psychosom Med 2017;79:24–33.
Hirsch M, Carlander B, Vergé M et al. Objective and subjective sleep disturbances in patients with rheumatoid arthritis. A reappraisal. Arthritis Rheum 1994;37:41–49.
Irwin MR, Olmstead R, Carroll JE. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol Psychiatry 2016;80:40–52.
Salwen-Deremer JK, Reid MJ, Westvold SJ, Siegel CA, Smith MT. People with IBD evidence more microarousals during sleep architecture assessments. BMJ Open Gastroenterol. 2023. https://doi.org/10.1136/bmjgast-2023-001249.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This work received funding from Ferring Pharmaceuticals, and IBD SA.
Author information
Authors and Affiliations
Contributions
Alex Barnes is responsible for study concept and design, data acquisition, analysis, and data interpretation, drafting of manuscript, and critical revision of the manuscript. Sutapa Mukherjee is responsible for study concept and design and responsible for critical revision of the manuscript. Jane Andrews is responsible for critical revision of the manuscript. Paul Spizzo is responsible for critical revision of the manuscript. Réme Mountifield is responsible for study concept and design, and responsible for critical revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Speakers fees, and Ad Boards from Abbott, AbbVie, Allergan, Anatara, AstraZeneca, Bayer, BMS 2020, Celegene, Celltrion, Falk, Ferring, Gilead, Hospira, Immuninc, ImmunsanT, Janssen, MSD, Nestle, Novartis, Progenity, Pfizer, Sandoz, Shire, Takeda, Vifor, RAH research Fund, The Hospital Research Fund 2020–2022, The Helmsley Trust 2020–2023.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Barnes, A., Mukherjee, S., Andrews, J.M. et al. Active Inflammatory Bowel Disease Is Associated with Short Sleep Duration via Objective Measures. Dig Dis Sci 69, 2937–2943 (2024). https://doi.org/10.1007/s10620-024-08485-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-024-08485-8