Abstract
Background
Bloating is a bothersome symptom in irritable bowel syndrome with constipation (IBS-C).
Aim
To evaluate plecanatide efficacy in patients with IBS-C stratified by bloating intensity.
Methods
Pooled phase 3 data (2 randomized, controlled IBS-C trials) from adults treated with plecanatide 3 mg or placebo for 12 weeks were analyzed. Patients were stratified post-hoc by baseline bloating severity (11-point scale: mild [≤ 5] and moderate-to-severe [> 5]). Assessments included change from baseline in bloating, abdominal pain, and complete spontaneous bowel movement (CSBM) frequency. Abdominal pain and bloating composite responders were defined as patients with ≥ 30% improvement from baseline in both bloating and abdominal pain at Week 12.
Results
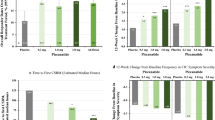
At baseline, 1104/1436 patients with IBS-C (76.9%) reported moderate-to-severe bloating. In the moderate-to-severe bloating subgroup, plecanatide significantly reduced bloating severity versus placebo (least-squares mean change [LSMC]: − 1.7 vs − 1.3; P = 0.002), reduced abdominal pain (− 1.7 vs − 1.3; P = 0.006), and increased CSBM frequency (1.4 vs 0.8; P < 0.0001). In the mild bloating subgroup, significant improvements were observed with plecanatide versus placebo for abdominal pain (LSMC: − 1.3 vs − 1.0; P = 0.046) and CSBM frequency (2.0 vs 1.2; P = 0.003) but not bloating (− 0.9 vs − 0.8; P = 0.28). A significantly greater percentage of patients were abdominal pain and bloating composite responders with plecanatide versus placebo (moderate-to-severe bloating: 33.6% vs 26.8% [P = 0.02]; mild bloating: 38.4% vs 27.2% [P = 0.03]).
Conclusion
Plecanatide treatment improved IBS-C abdominal and bowel symptoms, including in those who present with moderate-to-severe bloating.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Irritable bowel syndrome (IBS) with constipation (IBS-C) is a prevalent disorder of gut–brain interaction with overlapping abdominal symptoms (e.g. abdominal pain/discomfort, bloating) and bowel symptoms (e.g. constipation) [1,2,3]. Bloating is considered one of the most common and bothersome symptoms affecting patients with IBS-C [4, 5]. Bloating intensity correlates with the severity of abdominal pain and other abdominal symptoms in IBS [6] and is associated with reduced health-related quality of life [4, 7]. A statistically significantly higher prevalence of comorbid depression and fibromyalgia has been detected in IBS with bloating versus without bloating [6]. Clinicians often consider bloating a challenging symptom to treat [5] and, to date, no single therapy or combination of therapies has demonstrated universal efficacy for managing the various bowel and abdominal symptoms experienced by patients with IBS-C [5].
Plecanatide is a guanylate cyclase-C agonist indicated for the treatment of IBS-C and chronic idiopathic constipation in adults as a once-daily 3-mg dose [8]. Plecanatide acts on guanylate cyclase-C receptors to promote stool softening and gastrointestinal motility [9, 10]. Two phase 3 randomized, placebo-controlled clinical trials in IBS-C demonstrated that plecanatide 3 mg was efficacious and well tolerated [11]. During these trials, patients with IBS-C treated with plecanatide 3 mg had significant improvements from baseline in bloating, abdominal pain, and discomfort [11], symptoms often considered by patients to contribute to disease severity [12]. Given the relationship between bloating and other symptoms of IBS-C, the aim of this study was to further investigate the potential benefits of plecanatide treatment on individual and global symptoms in patients subgrouped by baseline bloating severity.
Methods
Post-hoc analyses of data from 2 identically designed, randomized, double-blind, placebo-controlled phase 3 trials in adults with IBS-C (ClinicalTrials.gov identifiers NCT02387359 and NCT02493452) were conducted [11]. The study designs and patient populations have been described previously [11]. The 2 trials included a screening period, a 12-week treatment period, and a 2-week treatment-free follow-up period [11]. In both trials, adults aged ≤ 85 years with IBS-C (Rome III criteria) and a body mass index of 18 to 40 kg/m2 were eligible for inclusion [11]. Patients were randomly assigned 1:1:1 (stratified by sex) to oral plecanatide 3 mg, plecanatide 6 mg, or placebo, administered once daily for 12 weeks [11]. Only patients treated with plecanatide 3 mg once daily, the recommended dosage for IBS-C [8, 13], or placebo were included in the current analyses.
Efficacy analyses were conducted in the intention-to-treat population, defined as all patients who were randomly assigned to treatment (excluding duplicate patients) and had baseline bloating data. Baseline values were determined during the last 2 weeks of the screening period, and patients were stratified post hoc by baseline bloating severity score. Mild bloating was rated as a score ≤ 5, and moderate-to-severe bloating was rated as a score > 5, using an 11-point scale (range, 0 [“none”] to 10 [“worst possible”]). Each trial received institutional review board approval and was conducted in accordance with the ethical principles of the Declaration of Helsinki, and all patients provided written informed consent [11].
Patient daily electronic diaries recorded information on abdominal pain and bloating severity (11-point scale; range, 0 [“none”] to 10 [“worst possible”]), frequency of spontaneous bowel movements (SBMs; defined as bowel movement occurring without laxative use within 24 h), and frequency of complete spontaneous bowel movements (CSBMs; defined as SBMs occurring with a sense of complete evacuation). Abdominal pain and bloating “composite responders” were defined as patients with ≥ 30% improvement from baseline in both bloating and abdominal pain at Week 12. Also, durable overall abdominal pain and CSBM responders were defined as patients who achieved a weekly response (i.e. ≥ 30% decrease from baseline in abdominal pain intensity and an increase from baseline of ≥ 1 CSBM in that same week) for ≥ 6 of the 12 treatment weeks, consistent with current US Food and Drug Administration (FDA) guidance [14].
Continuous variables were evaluated between treatment groups using a pairwise comparison of least-squares means and an analysis of covariance model with fixed effect for treatment and covariates of sex and corresponding baseline. For comparisons across the 12-week treatment period, pairwise comparisons of least-squares means between treatments used a linear mixed-effects model with fixed effects for sex (stratification variable), treatment, week, the interaction of treatment and week, corresponding baseline value, and a random intercept for patient. Consistent with an intention-to-treat approach, patients with missing data were considered nonresponders. Time to maximum improvement in bloating score was determined using Kaplan–Meier estimates. Responder rates between treatment groups were compared using a Cochran-Mantel–Haenszel test, stratified by sex. Spearman’s correlation coefficients were generated to assess the relationship between abdominal symptoms (baseline and change from baseline at Week 12). A coefficient (r) value of > 0.70 to 1.00 was considered a strong positive correlation (> 0.50‒0.70, “moderate” correlation; ≤ 0.50, “weak-to-negligible” correlation). All authors had access to the study data and reviewed and approved the final manuscript.
Results
Patients
A total of 1436 patients with IBS-C were included in the analyses, of whom 23.1% had a bloating score ≤ 5 (“mild” [11-point scale]) at baseline (plecanatide, n = 159; placebo, n = 173) and 76.9% had a bloating score of > 5 (“moderate/severe”) at baseline (plecanatide, n = 560; placebo, n = 544; Table 1). The majority of patients in both bloating severity cohorts and treatment groups completed the studies (Supplementary Figure; Patient Disposition). Demographic characteristics were comparable between treatment groups within the bloating severity cohorts for IBS-C (Table 1). The mean baseline bloating score for the mild bloating subgroup was 4.1 for both treatment groups; for the moderate-to-severe bloating group, the mean score was 7.2 for both treatment groups.
Bloating and Abdominal Pain
Patients with IBS-C and moderate-to-severe bloating at baseline experienced a significant improvement in the individual symptom of bloating across 12 weeks of treatment (mean difference vs placebo: − 0.4; 95% CI − 0.6, − 0.1; P = 0.002; Table 2). The median time to maximum improvement in bloating score was 7 weeks in both treatment groups, for both the overall population and the mild and moderate-to-severe bloating subgroups. For the individual symptom of abdominal pain, a significant improvement from baseline in severity was observed in both the moderate-to-severe (P = 0.006) and mild (P = 0.046) bloating subgroups across the 12-week treatment period (Table 2). In an analysis of bloating and abdominal pain composite response at Week 12, a significantly greater percentage of patients with IBS-C with moderate-to-severe bloating were responders (≥ 30% improvement from baseline in bloating and abdominal pain) after treatment with plecanatide (33.6% [188/560]) compared with placebo (26.8% [146/544]; P = 0.02; Fig. 1). Similarly, a significantly greater percentage of responders in the mild bloating subgroup was observed for plecanatide (38.4% [61/159]) compared with placebo (27.2% [47/173]; P = 0.03; Fig. 1). An additional composite analysis of abdominal pain and bloating (≥ 30% decrease from baseline in abdominal pain and bloating scores in the same week for ≥ 6 of 12 weeks) revealed a significant difference favoring plecanatide versus placebo only in the moderate-to-severe bloating subgroup (Supplementary Table; Response for ≥ 6 of 12 Weeks).
Bowel Movement Frequency and Responders
Patients with IBS-C experienced a significant improvement from baseline in the number of weekly CSBMs and SBMs during 12 weeks of plecanatide treatment compared with placebo, regardless of baseline bloating severity (P ≤ 0.003 for all vs placebo; Table 2). A significantly greater percentage of patients with IBS-C were durable overall responders with plecanatide compared with placebo (30.8% vs 18.5% [baseline bloating score ≤ 5]; 24.3% vs 15.6% [baseline bloating score > 5]; P ≤ 0.009 for both vs placebo; Fig. 2). Additionally, a composite analysis of CSBM frequency and bloating (increase from baseline of ≥ 1 CSBM/week and ≥ 30% decrease from baseline in bloating score in the same week, for ≥ 6 of the 12 weeks of treatment) showed a significant difference favoring plecanatide versus placebo for both bloating severity subgroups (Supplementary Table; Response for ≥ 6 of 12 Weeks).
Durable overall abdominal pain and CSBM responders*, subgrouped by baseline bloating severity. *Patients who were weekly responders (i.e. ≥ 30% decrease from baseline for abdominal pain intensity and an increase from baseline ≥ 1 CSBM in same week) for ≥ 6 of the 12 treatment weeks. CSBM complete spontaneous bowel movement
Symptom Correlation Analysis
The relationships between bloating and abdominal pain or weekly CSBM frequency at baseline and changes from baseline after 12 weeks of treatment were examined by a correlation analysis. Baseline bloating severity and abdominal pain were strongly and positively correlated (r = 0.87; P < 0.0001). Week 12 changes from baseline in bloating and abdominal pain also were strongly positively correlated (r = 0.85; P < 0.0001). However, baseline bloating severity was weakly negatively correlated with baseline CSBMs/week (r = − 0.15; P < 0.0001) and remained weakly negatively correlated at Week 12 (r = − 0.42; P < 0.0001).
Safety
Safety data for the individual trials have been published previously [11]. The adverse event (AE) profiles of plecanatide in patients subgrouped by baseline bloating severity were comparable, with most patients experiencing AEs of mild or moderate intensity (Table 3). Diarrhea was reported in ≤ 5.0% of patients with IBS-C treated with plecanatide in any subgroup.
Discussion
Patients with IBS-C have reported bloating to be one of the most bothersome symptoms they experience [4, 5]. Indeed, bloating often drives patients with constipation to seek medical care [15]. In this post-hoc analysis of 2 IBS-C trials, more than 75% of individuals with IBS-C had moderate-to-severe bloating at baseline. Patients with IBS-C with moderate-to-severe baseline bloating who were treated with plecanatide 3 mg once daily for 12 weeks experienced a significant improvement in bloating and in abdominal pain compared with patients treated with placebo. This finding mirrored those of the primary and secondary endpoints, particularly in this subgroup of patients with moderate-to-severe bloating [11], further validating the effects of plecanatide across a spectrum of symptoms-based endpoints in patients with IBS-C. The durable overall abdominal pain and CSBM responder definitions used in this analysis are consistent with guidance from the FDA regarding clinical trials of patients with IBS-C [14]. As well, the ≥ 30% improvement cutoff in the responder definition used herein is consistent with other studies on bloating in patients with IBS-C [16]. For IBS-C and mild baseline bloating, significant improvement with plecanatide compared with placebo was observed for abdominal pain, but not bloating, after 12 weeks of treatment. Overall, the minimal improvements in bloating observed versus placebo in patients with mild baseline bloating may be related to a floor (basement) effect, limitations in observation of significant improvement between the active and placebo treatments [17], and/or persistent symptom reporting and heightened awareness of symptoms [18]. In an evaluation of the potential impact of baseline bloating on bowel movement response, the percentage of durable overall CSBM responders was significantly greater with plecanatide versus placebo in patients with IBS-C, regardless of baseline bloating severity.
Exploratory analyses presented here indicated a weak negative correlation between bloating and CSBM frequency in patients with IBS-C. This observed inverse relationship between constipation and bloating was anticipated, as it would be expected that fewer CSBMs (i.e. more severe constipation) might lead to more severe bloating symptoms. At the same time, the weak strength of this relationship is illustrative of the complexity of the mechanistic underpinnings of bloating symptoms in the constipated patient. Improvement of stool frequency and consistency alone is likely insufficient to improve bloating symptoms in many patients suffering from IBS-C. Bloating severity has been previously reported to correlate with abdominal pain severity and other abdominal symptoms [6]. A strong positive correlation between bloating and abdominal pain was observed in patients with IBS-C in the current study. These results are consistent with the 2023 findings of Ballou, et al. [19] indicating a potential shared pathophysiology between the 2 abdominal sensory symptoms. Guanylate cyclase-C agonists, including plecanatide, improve abdominal symptoms (eg, abdominal pain and bloating) through a decrease in activation of visceral nociceptive neurons and reverse altered afferent gut–brain signalling [20,21,22,23]. These findings further underscore the central role of abdominal symptoms in the IBS-C patient experience.
Plecanatide was well tolerated in patients with IBS-C, regardless of baseline bloating severity. The incidence of diarrhea, the most common AE reported with plecanatide, was low (≤ 5%) across the baseline bloating subgroups and consistent with previous reports of guanylate cyclase-C agonists and the prosecretory mechanism of action of plecanatide [21].
The strengths of the current analysis included the large number of patients, use of Rome Foundation criteria to standardize patient inclusion, utilization of rigorous FDA-defined endpoints, and the correlational analysis included to assess the relationships between abdominal and bowel symptoms (e.g. thresholds for correlation). Furthermore, subanalyses stratified by bloating severity provided insight into the efficacy and safety of plecanatide in patients with this common symptom. Limitations included the use of a post-hoc data evaluation, the possibility of floor effects impacting outcomes in patients with mild baseline bloating, and reduced generalizability of findings to the broader population of individuals with IBS-C due to the limited demographics of those included in the trials (i.e. predominantly female and white patients).
Finally, it is important to comment upon the subjective nature of the term bloating and to recognize that many patients use this term synonymously to describe abdominal distention. Whereas the latter is an objective physiological and potentially quantifiable change, bloating represents a visceral sensation [24]. Thus, the identification and severity of bloating in clinical trials can be complex. While visual analog and numeric rating scales represent the current assessment standard in clinical trials, further validation studies are warranted to determine the best ways to measure this symptom and to establish clinically meaningful difference thresholds. This may require the use of different tools including questionnaires, pictograms, or even biosensors which could be directly compared to determine the most appropriate assessment.
In conclusion, this analysis revealed that, in patients with IBS-C who reported bloating, both individual and composite abdominal and bowel symptoms significantly improved with plecanatide compared with placebo. These results support the efficacy of plecanatide as a potential option for bloating in addition to its more established and approved treatments for symptoms related to constipation and abdominal pain in IBS-C. The clinical importance of these observations is underscored by the reality that, at present, there are no regulatory agency–approved treatments for bloating. This creates a strong need, on behalf of clinicians and patients, to enhance our informed approach to the management of bloating symptoms in IBS-C.
Data Availability
Qualified researchers interested in obtaining access to trial data should submit a detailed research proposal and data access request to datasharing@bauschhealth.com. For more information, please see https://www.bauschhealth.com/ESG/access-to-clinical-study-data/.
References
Heidelbaugh JJ, Stelwagon M, Miller SA, Shea EP, Chey WD. The spectrum of constipation-predominant irritable bowel syndrome and chronic idiopathic constipation: US survey assessing symptoms, care seeking, and disease burden. Am J Gastroenterol 2015;110:580–587.
Palsson OS, Whitehead W, Törnblom H, Sperber AD, Simren M. Prevalence of Rome IV functional bowel disorders among adults in the United States, Canada, and the United Kingdom. Gastroenterology 2020;158:1262–1273.
Sharma A, Rao SSC, Kearns K, Orleck KD, Waldman SA. Review article: diagnosis, management and patient perspectives of the spectrum of constipation disorders. Aliment Pharmacol Ther 2021;53:1250–1267.
Ringel Y, Williams RE, Kalilani L, Cook SF. Prevalence, characteristics, and impact of bloating symptoms in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 2009;7:68–72.
Lacy BE, Cangemi D, Vazquez-Roque M. Management of chronic abdominal distension and bloating. Clin Gastroenterol Hepatol 2021;19:219–231.
Hod K, Ringel Y, van Tilburg MAL, Ringel-Kulka T. Bloating in irritable bowel syndrome is associated with symptoms severity, psychological factors, and comorbidities. Dig Dis Sci 2019;64:1288–1295.
Neri L, Iovino P, Laxative Inadequate Relief Survey (LIRS) Group. Bloating is associated with worse quality of life, treatment satisfaction, and treatment responsiveness among patients with constipation-predominant irritable bowel syndrome and functional constipation. Neurogastroenterol Motil 2016;28:581–591.
Trulance tablets, for oral use [package insert]. Salix Pharmaceuticals; 2021.
Rao SSC. Plecanatide: a new guanylate cyclase agonist for the treatment of chronic idiopathic constipation. Therap Adv Gastroenterol 2018;11:1756284818777945.
Shailubhai K, Comiskey S, Foss JA et al. Plecanatide, an oral guanylate cyclase C agonist acting locally in the gastrointestinal tract, is safe and well-tolerated in single doses. Dig Dis Sci 2013;58:2580–2586.
Brenner DM, Fogel R, Dorn SD et al. Efficacy, safety, and tolerability of plecanatide in patients with irritable bowel syndrome with constipation: results of two phase 3 randomized clinical trials. Am J Gastroenterol 2018;113:735–745.
Drossman DA, Morris CB, Schneck S et al. International survey of patients with IBS: symptom features and their severity, health status, treatments, and risk taking to achieve clinical benefit. J Clin Gastroenterol 2009;43:541–550.
Bausch Health Canada Inc. Trulance Product Monograph, Including Patient Medication Information. Laval, Canada: Bausch Health Canada Inc.; 2021.
Guidance for industry: irritable bowel syndrome — clinical evaluation of drugs for treatment. Available at: http://www.fda.gov/downloads/Drugs/Guidances/UCM205269.pdf. Accessed May 12, 2022.
Jiang X, Locke GR, Zinsmeister AR, Schleck CD, Talley NJ. Health care seeking for abdominal bloating and visible distention. Aliment Pharmacol Ther 2009;30:775–783.
Nelson AD, Black CJ, Houghton LA, Lugo-Fagundo NS, Lacy BE, Ford AC. Systematic review and network meta-analysis: efficacy of licensed drugs for abdominal bloating in irritable bowel syndrome with constipation. Aliment Pharmacol Ther 2021;54:98–108.
Lembo AJ. Understanding the placebo and nocebo effects in patients with irritable bowel syndrome. Gastroenterol Hepatol 2020;16:374–376.
Katon W, Sullivan M, Walker E. Medical symptoms without identified pathology: relationship to psychiatric disorders, childhood and adult trauma, and personality traits. Ann Intern Med 2001;134:917–925.
Ballou S, Singh P, Nee J et al. Prevalence and associated factors of bloating: results from the Rome Foundation Global Epidemiology Study. Gastroenterology 2023;165:647–655.e4.
Brierley SM, Grundy L, Castro J, Harrington AM, Hannig G, Camilleri M. Guanylate cyclase-C agonists as peripherally acting treatments of chronic visceral pain. Trends Pharmacol Sci 2022;43:110–122.
Waldman SA, Camilleri M. Guanylate cyclase-C as a therapeutic target in gastrointestinal disorders. Gut 2018;67:1543–1552.
Sharma A, Herekar AA, Bhagatwala J, Rao SSC. Profile of plecanatide in the treatment of chronic idiopathic constipation: design, development, and place in therapy. Clin Exp Gastroenterol 2019;12:31–36.
Rao SSC, Xiang X, Yan Y et al. Randomised clinical trial: linaclotide vs placebo—a study of bi-directional gut and brain axis. Aliment Pharmacol Ther 2020;51:1332–1341.
Moshiree B, Drossman D, Shaukat A. AGA clinical practice update on evaluation and management of belching, abdominal bloating, and distention: expert review. Gastroenterology 2023;165:791–800.
Acknowledgments
Technical editorial assistance was provided, under direction of the authors, by Mary Beth Moncrief, PhD, and Sophie Bolick, PhD, Synchrony Medical Communications LLC, West Chester, PA.
Funding
The clinical trials, current analyses, and technical editorial assistance were supported by Salix Pharmaceuticals, Bridgewater, NJ.
Author information
Authors and Affiliations
Contributions
DMB, AS, SSCR, and GSS contributed to the post hoc methodology, and all authors were involved in data analysis and interpretation. DMB and SGG developed the first draft of the manuscript. All authors critically reviewed and edited the manuscript and approved the final version of the manuscript for submission.
Corresponding author
Ethics declarations
Conflict of interest
DMB is a consultant and speaker for Salix Pharmaceuticals and is supported in research by an unrestricted gift from the IDP Foundation. AS served on advisory boards for Ironwood Pharmaceuticals, Phathom Pharmaceuticals, Salix Pharmaceuticals, and Takeda Pharmaceuticals, and is supported in research by a grant from Vibrant Ltd. SSCR has served on the advisory boards for Ironwood Pharmaceuticals, Medtronic, Salix Pharmaceuticals, Sanofi Pharmaceuticals, Takeda Pharmaceuticals, and Vibrant Ltd. APL, ZH, and CA are employees of Salix Pharmaceuticals. GSS is a consultant and speaker for AbbVie/Ironwood Pharmaceuticals and Salix Pharmaceuticals and is a consultant for the GI Health Foundation
Ethical approval
Each trial received institutional review board approval and was conducted in accordance with the ethical principles of the Declaration of Helsinki, and all patients provided written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
An invited commentary on this article is available at https://doi.org/10.1007/s10620-024-08332-w
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Brenner, D.M., Sharma, A., Rao, S.S.C. et al. Plecanatide Improves Abdominal Bloating and Bowel Symptoms of Irritable Bowel Syndrome with Constipation. Dig Dis Sci 69, 1731–1738 (2024). https://doi.org/10.1007/s10620-024-08330-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-024-08330-y