Abstract
Objective
It is unknown whether ustekinumab (UST) levels can predict clinical outcomes in Crohn’s disease (CD) patients. We assessed the exposure–response relationship of UST trough concentrations with biochemical outcomes at week 24 in a prospective, real-world setting.
Methods
We performed a prospective study in patients with CD starting UST in four academic centres in the Netherlands. All patients received a weight-adjusted intravenous (IV) UST induction dose, followed by one subcutaneous (SC) dose of 90 mg UST at 8 weeks. Maintenance therapy consisted of 90 mg subcutaneous UST every 8 or 12 weeks. Individual UST concentration time course during treatment were estimated using a population pharmacokinetic (PK) model. Quartile analysis and logistic regression were performed to analyse if UST concentrations at week 8 were associated with biochemical remission rates at week 24 (C-reactive protein (CRP) ≤ 5 mg/L and / or faecal calprotectin (FC) ≤ 250 mg/kg).
Results
In total, 124 patients with CD were included. Patients achieving biochemical remission at week 12 and 24 had significantly higher UST levels at week 8 compared to patients without biochemical remission (6.6 µg/mL versus 3.9 µg/mL, P < 0.01 and 6.3 µg/mL versus 3.9 µg/mL, P < 0.01, respectively). In quartile analysis, patients with UST levels in the highest quartile (≥ 6.3 µg/mL at week 8) had higher biochemical remission rates at week 12 and week 24. There was no association between UST levels at and corticosteroid-free clinical remission rates.
Conclusion
In this real-world cohort of patients with CD, UST levels in the highest quartile (≥ 6.3 µg/mL) at week 8 were associated with higher biochemical remission rates at week 24.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Crohn’s disease (CD) is a chronic inflammatory bowel disease (IBD) affecting the gastro-intestinal tract [1]. Obtaining long-term deep remission is essential to prevent irreversible gastrointestinal damage. Ustekinumab (UST) is a monoclonal antibody that binds to the p40 subunit of interleukin(IL)-12 and IL-23 and thereby inhibits its bioactivity [2, 3]. Also, it prevents signalling, differentiation and release of different cytokines which play an important role in the pathophysiology of CD [4].
The efficacy and safety of UST for moderate to severely active CD was investigated in phase 3 placebo-controlled studies [5]. Patients treated with UST 90 mg Q8 or Q12 weeks, following an induction IV dose and one UST 90 mg SC dose at week 8, demonstrated greater clinical response and remission rates compared to placebo at week 44 in a phase 3 study [6].
To individualize dosing for the purpose of maximizing treatment response in IBD, therapeutic drug monitoring (TDM) is a promising tool [7]. TDM is especially useful if there is an observed association between serum drug concentrations and therapeutic outcomes [8]. It may also contribute to reduced drug expenses and longer dose intervals which are convenient for patients. Anti-TNF trough concentrations are associated with important outcomes in IBD and guides physicians in their decision to change therapy within or out-of-class [9, 10]. However, limited data are available to guide clinicians on which drug-level thresholds to aim for when treating patients with CD with UST.
Several studies described UST levels associated with clinical response in CD. The UNITI programme showed that serum levels of UST were proportional to received dose and levels were associated with clinical efficacy [11]. Also, real-world studies suggested an association between UST levels and clinical outcome measures [12,13,14,15]. These findings were not validated by others [14]. It remains subject of debate whether reactive and / or proactive TDM improves therapeutic outcomes in UST treatment in patients with CD.
We previously reported on real-world clinical outcomes of UST for CD in a multi-centre prospective cohort [16, 17]. Here, we designed a multicentre cohort study to assess the exposure–response association of UST in patients with CD.
Methods
Study Design
The Initiative on Crohn and Colitis (ICC) Registry is a prospective, nationwide, observational registry of patients with IBD starting novel IBD therapies in regular care in the Netherlands. The design and rationale as well as the clinical outcomes of UST have been previously described in more detail [16, 17]. Patients were prospectively evaluated with scheduled outpatient clinic visits at week 12 and 24 designed to closely follow regular care.
Participants
Adult (aged ≥ 18) patients with CD who initiated UST treatment were included in four participating centres. The decision to start UST was at the discretion of the treating physician. Patients received a weight-adjusted initial intravenous (IV) infusion with UST (260 mg if body weight < 55 kg, 390 mg if weight was between 55 and 85 kg and 520 mg if weight > 85 kg), followed by one SC dose of 90 mg UST at 8 weeks. Maintenance therapy consisted of 90 mg SC UST every 8 or 12 weeks. Patients without objective disease activity at baseline (either biochemical (C-reactive protein (CRP) < 5 mg/L and faecal calprotectin (FC) < 250 mg/kg) or endoscopic) or without determined UST levels during follow-up were excluded.
Ustekinumab Levels
UST levels were measured during follow-up. Centres were not blinded for UST level results and dose could proactively be intensified based on UST levels on discretion of the treating physician. Since not all levels were measured at exactly week 8 or 16 as a trough level, a population PK model was used to estimate individual UST concentration time course during treatment. The population PK model was based on measured UST levels in patients in a primary cohort in three participating ICC centres (centre 1, 2, 3) and the FDA clinical pharmacology review document (see Sect. 2.4). The population PK model was then validated in patients included in two ICC centres (centre 3 and 4). This was done performing a prediction-corrected visual predictive check (VPC) of the final model on the validation cohort [18]. Patients in the validation cohort met the same inclusion criteria as the patients included in the primary cohort. UST levels at week 8 and 16 were estimated using the population PK model in all patients. For further analysis, clinical and biochemical data of all patients were analysed together to determine the remission rates and exposure–response relationship.
Population Pharmacokinetic Modelling
Nonlinear mixed-effect modelling was used to estimate ustekinumab pharmacokinetic parameters from plasma concentration–time data from both intravenous and subcutaneous administration. NONMEM (v7.4.4, Icon Development Solutions, Ellicott City, MD) was used for modelling ustekinumab pharmacokinetics, using PsN toolkit (v5.0.0)[19,20,21], and Piranã version 2.9.8[22] as modelling environment. R statistics (v. 3.4.4) was used for exploratory graphical analysis and for evaluation of the GOF and VPC [23]. First-order conditional estimation method with interaction (FOCE-I) was used throughout the analysis. The final model was evaluated by means of a prediction-corrected visual predictive check (VPC) based on 500 Monte-Carlo simulations. In addition, the precision of the parameter estimates was further assessed by means of a non-parametric bootstrap with resampling the dataset (n = 1000 times).
The population PK model was based on the UST plasma concentrations of the patients included in the primary cohort and the UST FDA clinical pharmacology review documents [24].
Outcomes and Definitions
A primary analysis determined whether UST drug concentrations at week 8 were associated with biochemical remission (CRP ≤ 5 mg/L and FC ≤ 250 mg/kg) at week 24. Biochemical remission was chosen over clinical remission since it is known that clinical scores are neither sensitive nor specific for inflammation or true disease states [25]. Secondary, we assessed the association between serum UST trough levels at week 8 and biochemical remission at week 12. Furthermore, the association between UST trough levels at week 16 with the biochemical remission to UST treatment at week 24 was evaluated. Corticosteroid-free clinical remission rates were evaluated. Corticosteroid-free clinical remission was defined by a Harvey Bradshaw Index (HBI) score ≤ 4 without concomitant corticosteroid use. Besides clinical remission and biochemical remission rates, we also assessed adverse events and infections at week 12 and 24. If data were missing, patients were considered non-responders.
Bioanalytical Assays
Venous blood was collected during outpatient clinic visits at discretion of the treating physician. Samples were centrifuged for 10 min (2000 g) and serum aliquots were frozen (-80 °C). Samples were sent to Sanquin Biologics, Amsterdam, the Netherlands, for measurement of UST levels and antidrug antibodies (ADA). The UST level assay was an enzyme-linked immunosorbent assay (ELISA). This assay has been shown to have high reproducibility and sensitivity, with low inter- and intra-assay variability [26]. ADA were measured using a radioimmunoassay [27].
Statistical Methods
Differences in UST levels between patients achieving biochemical remission and patients not achieving biochemical remission was determined by Mann–Whitney U tests. Quartile analysis was used to evaluate whether there was an association between UST trough levels and clinical and biochemical outcomes. Logistic regression was performed to identify whether UST levels were predictive for biochemical remission, adverse events and infections. Depending on the normality of the underlying distribution, continuous variables were presented as means with standard deviation (SD) of as median with interquartile range (IQR). Categorical variables were presented as percentages and compared by using the χ2 test. Factors predictive for biochemical remission and high UST levels were assessed using univariate analysis. Multivariate analysis was performed when P < 0.2 in univariate analysis.
To correct for multiple testing, Bonferroni correction was used and P < 0.0125 was considered to be statistically significant. Statistical analyses were performed using IBM® SPSS® Statistics version 26.0.0.1.
Statistical Methods PK Model
Population PK Model selection was based on statistical significance, goodness of fit and stability. Throughout the model building process, an altered model was chosen over a precursor model if a difference in the objective functions (-2 log likelihood) was > 6.63 (P < 0.01, with 1 degree of freedom, assuming X2 distribution). In addition, basic goodness-of-fit (GOF) plots, in which the observed concentration is plotted against the individual- and population-predicted concentrations, and the conditional weighted residual errors are plotted against time and against population-predicted concentrations, were assessed. Also, parameter precision, shrinkage and interindividual variability (IIV) were taken into account during the modelling process.
Ethical Consideration
This study was reviewed and approved by the Committee on Research Involving Human Subjects at the Radboudumc (institutional review board: 2018-4076).
Results
Baseline Characteristics
In the primary cohort, a total of 90 patients were included. In the validation cohort, 34 patients were included. In total, 124 patients with CD treated with UST were enrolled in this study and used in the analysis for remission rates and exposure–response relationship. Baseline characteristics are presented in Table 1.
Most of the patients were female (62.1%). The mean age at UST induction was 41 years (IQR 32–54) and the median disease duration was 16 years (IQR 6–26). Only five patients (4%) were anti-TNF naive. Twenty-four patients (19.4%) were on concomitant immunosuppressive treatment and 30 patients (24.2%) were concomitantly treated with corticosteroids at baseline. All patients had biochemical disease activity at initiation of therapy. None of the patients initiated ustekinumab as post-surgical prophylaxis.
Clinical and Biochemical Outcomes
Of the included patients, 46/124 (37.1%) and 60/124 (48.4%) were in corticosteroid-free clinical remission at week 12 and 24, respectively. Eighty-seven patients (70.2%) had clinical disease activity at baseline. Biochemical remission was achieved in 32/124 (25.8%) and 29/124 (23.4%) patients at week 12 and 24, respectively. No predictors of biochemical remission at week 24 were found (in supplementary table 1).
Exposure–Response Relationship (ER)
Ustekinumab Serum Concentrations
In the primary cohort, 265 UST levels were measured in 90 patients. UST levels were measured as part of standard clinical care. However, the timing of UST level measurement was different between the participating centres. The UST levels were measured at week 8 (n = 19), week 16 (n = 36), week 24 (n = 25), week 32 (n = 18), week 40 (n = 20) and week 48 (n = 13) or in-between these time points. In the validation cohort of 34 patients, 108 UST levels were measured during follow-up (mostly at week 8 (n = 17), week 16 (n = 18), week 24 (n = 6), week 32 (n = 12) and week 48 (n = 12)). All measured UST levels in the primary cohort were used to design our Population PK model and all measured UST levels in our validation cohort were used to validate our Population PK model. The prediction-corrected visual predictive checks of both cohorts are shown in Fig. 1 and show adequate predictive performance of the model in both cohorts. Population Pharmacokinetic parameters of the final model and 1000 Bootstrap runs are presented in Table 2. The median values of the parameters estimated from the bootstrap analysis were in good agreement with the point estimates and the 95% CIs were reasonably narrow, demonstrating acceptable precision.
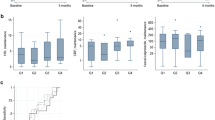
Prediction-Corrected Visual Predictive Checks for the primary (A) and validation (B) cohort. Comparisons were performed between the 10th, 90th (blue solid) and 50th (red solid line) percentiles of the observed ustekinumab serum levels (closed circles) vs. time after first dose (days) and the 80% confidence interval (shaded area) obtained from 500 simulations
Median concentrations of UST were 4.2 µg/mL (IQR 2.8–5.8) at week 8 and 1.8 µg/mL (IQR 1.0–2.9) at week 16 in the primary cohort. In the validation cohort, median concentrations of UST were similar (4.5 (IQR 3.0–6.7) at week 8 and 1.8 (IQR 1.2–4.5) at week 16). Predictors for high UST levels (> 6.3 µg/mL) are displayed in Table 3. There was no association between serum albumin levels or CRP and UST serum levels (P = 0.43 and P = 0.07, respectively).
ER Ustekinumab Trough Levels at Week 8
Patients achieving biochemical remission at week 12 had significantly higher UST levels at week 8 compared to patients without biochemical remission (6.6 µg/mL versus 3.9 µg/mL, P < 0.01) (Fig. 2). Also, patients achieving biochemical remission at week 24 had significant higher UST levels at week 8 compared to patients without biochemical remission (6.3 µg/mL versus 3.9 µg/mL, P < 0.01, respectively) (Fig. 2).
Higher UST levels at week 8 were associated with higher biochemical remission rates at week 12 (OR 1.35, 95%CI 1.16–1.56, P < 0.01) and 24 (OR 1.31, 95%CI 1.13–1.52, P < 0.01) in logistic regression analysis (Fig. 3). In quartile analysis, patients with UST levels in the highest quartile (≥ 6.3 µg/mL) at week 8 had higher biochemical remission rates at week 12 and week 24 (Fig. 4).
Quartile analysis depicting the exposure–response relationship between ustekinumab serum levels at week 8 (A) and week 16 (B) and biochemical remission rates at week 12 and 24. Biological remission was defined as a C-reactive protein (CRP) ≤ 5 mg/L and/or faecal calprotectin (FC) ≤ 250 mg/kg. A Q1 ≤ 2.8 µg/mL, Q2: 2.9–4.3 µg/mL, Q3: 4.4–6.3 µg/mL, Q4 ≥ 6.4 µg/mL. B Q1 ≤ 1.1 µg/mL, Q2: 1.2–1.8 µg/mL, Q3: 1.9–3.0 µg/mL, Q4 ≥ 3.1 µg/mL
Patients achieving corticosteroid-free clinical remission at week 12 and 24 had no significant higher UST levels at week 8 compared to patients without corticosteroid-free clinical remission (4.52 µg/mL versus 4.16 µg/mL, P = 0.99 and 4.69 µg/mL versus 4.13 µg/mL, P = 0.59). There was no association between UST levels at week 8 and corticosteroid-free clinical remission rates at week 12 and 24 in logistic regression (OR 0.97, 95%CI 0.86–1.09, P = 0.35 and OR 1.10, 95%CI 0.90–1.13, P = 0.86) and quartile analysis (P = 0.32 and P = 0.74).
ER Ustekinumab Trough Levels at Week 16 as Predictor for Outcomes at Week 24
Patients achieving biochemical remission at week 24 had significantly higher UST levels at week 16 compared to patients without biochemical remission (2.84 µg/mL versus 1.61 µg/mL, P < 0.01) (Fig. 2). Higher UST levels at week 16 were associated with higher biochemical remission rates at week 24 in logistic regression analysis (OR 1.19, 95%CI 1.02–1.40, P < 0.01) (Fig. 3). In quartile analysis, patients with UST levels in the highest quartile (≥ 3.00 µg/mL) at week 16 had higher biochemical remission rates at week 24 (Fig. 4).
Patients achieving corticosteroid-free clinical remission at week 24 had no significant higher UST levels at week 16 compared to patients without corticosteroid-free clinical remission (1.78 µg/mL versus 1.91 µg/mL, P = 0.91). There was no association between UST levels at week 16 and corticosteroid-free clinical remission rates at week 24 in quartile analysis (P = 0.65) and logistic regression (OR 0.94, 95%CI 0.81–1.08, P = 0.45).
Immunogenicity
In 113 patients, anti-drug antibodies (ADA) were determined. In none of these patients, ADA antibodies were detectable.
Safety Outcomes
In total, 124 patients were treated with ustekinumab for 113.5 patient-years. Fifty-eight adverse events and 29 infections were noted (supplementary table 2). Most common adverse events were headache and skin reactions. Ten patients required intestinal resection during follow-up. There was no association between UST levels and adverse events at week 8 (P = 0.26) and week 16 (P = 0.06) in logistic regression. In addition, there was no association between UST level and infections at week 8 (P = 0.52) and week 16 (P = 0.36).
Ustekinumab Interval
After the first SQ injection, 16 (13.1%) patients started SQ UST therapy at a 12-week interval (q12w), 103 (84.4%) patients at an eight-week (q8w) interval, two patients at a six-week interval and one patient at a four-week interval. The remaining two patients discontinued treatment before 12 weeks after initiation. Q8w or q12w week interval was centre dependent. At week 24, seven (9%) patients were on a q12w dosing scheme, 67 (85.9%) on a q8w dosing scheme, one on a q6w dosing scheme and one on a q4w dosing scheme. Trough levels at week 16 were not statistically significant different in patients on a q8w or a q12w dosing scheme (1.7 µg/mL vs 1.0 µg/mL, P = 0.70). Also, biochemical remission rates were not statistically significant different between patients on a q8w or a q12w dosing scheme at week 24 (P = 0.45).
Discussion
Ustekinumab is an effective and relatively safe therapy for patients with CD, while it remains debated whether UST levels are predictive for therapy response [28]. In this prospective real-world cohort study of 124 patients with CD, UST levels at week 8 and 16 were associated with biochemical remission outcomes. More specific, UST levels at week 8 in the highest quartile (≥ 6.3 µg/mL) were associated with higher biochemical remission rates at week 12 and 24. UST levels were not associated with corticosteroid-free clinical remission rates.
Median UST levels were 4.2 µg/mL (IQR 2.8–5.8) at week 8 and 1.8 µg/mL (IQR 1.0–2.9) at week 16. UST trough levels at week 8 in our cohort were lower compared to the post hoc UNITI analyses in patients receiving the 6 mg/kg UST induction dose (6.4 µg/mL at week 8).[11] Patients in our cohort had higher CRP and FC levels at initiation of UST therapy. More severe disease activity may be the explanation of the lower UST trough levels. In the same study, trough levels between 24 and 40 weeks of UST treatment in patients with q8w interval (2.0 µg/mL—2.2 µg/mL) were comparable to our UST levels at week 16. In the UNITI study, the trough levels in patients treated with the q12w dosing scheme were three-fold lower (ranging from 0.6 to 0.8 µg/mL at week 12, 24 and 36).
Only few studies have evaluated UST exposure–response relation in induction phase at week 8 and during maintenance at week 16. The results are heterogeneous. In 86 anti-TNF-experienced patients with CD, UST levels of at least 4.2 µg/mL at week 8 were needed to achieve a biochemical response at week 8 [13]. This is lower comparable to our UST level quartile of ≥ 6.3 µg/mL at week 8 that was associated with higher biochemical remission rates. This might be explained by the difference in outcomes. We described biochemical remission which may require a higher UST level threshold compared to biochemical response. In the same cohort, UST levels of 2.3 µg/mL at week 16 were identified as the minimal exposure needed to maximize the likelihood of endoscopic response after six months. A prospective study of 23 patients with CD described that trough UST concentrations of ≥ 1.10 µg/mL at week 12, more than five times lower compared to our threshold, were associated with biological response to UST treatment at 6 months [14]. Differences might be explained by the induction dosing scheme in which patients received UST SQ induction at week 0, 4 and 12 in contrast to our IV induction scheme. Also, the number of patients included was limited.
Other studies showed the exposure–response relation of UST levels during maintenance phase after 16 weeks of treatment. UST pharmacokinetics based on the phase 3 induction (UNITI-1 and UNITI-2) and maintenance trials (IM-UNITI) reported median UST concentrations ranging from 2.0 to 2.2 µg/mL at week 44 in patients receiving 90 mg every 8 weeks [11]. However, only patients on clinical response were included in the maintenance phase. In a combined prospective, longitudinal and cross-sectional cohort of 62 anti-TNF-experienced patients showed that maintenance trough levels of UST > 4.5 µg/mL were associated with biomarker reduction and endoscopic response [12]. Of these patients, 75% received UST 90 mg every 4 weeks. These results emphasize that patients can benefit from early UST optimization at the end of induction therapy. Several real-world studies confirmed that dose escalation was successful in more than 50% of the cases [29, 30]. In our cohort, the number of patients receiving dose escalation was too limited to draw meaningful conclusions.
Only two cohorts evaluated the association between UST trough levels and clinical outcomes. In the first study, there was no association found [12]. Post hoc UNITI analysis showed that serum UST concentrations ≥ 0.8 µg/mL were positively associated with clinical remission in both induction trials and maintenance phase serum [11]. Though it was a prospective cohort with large number of patients, it was a phase 3 study with highly selected patients and only less than half of the patients were anti-TNF experienced. In our cohort, there was only an association between UST levels and biochemical outcomes, not clinical outcomes. It is known that clinical scores are neither sensitive nor specific for inflammation or true disease states [25]. This may explain the discordance of clinical and biochemical outcomes seen in our study. In IBD, mucosal healing is the most reliable factor associated with improved outcomes [31]. In our study, endoscopy was not mandatory and insufficient endoscopic data were available for analysis. Instead, we assessed disease activity by using biochemical outcomes (FC and CRP). Biochemical outcomes as faecal biomarker concentrations are associated with endoscopic but not clinical disease activity in patients with CD [32].
In our cohort, none of the patients developed UST antibodies. This is in line with other prospective real-life studies [14, 33]. In the UNITI trials, only in 0.2% of patients antibodies were detected after induction therapy and in 2.3% of patients at 44 weeks of treatment [11]. These results confirm the low immunogenicity of UST.
The prospective, real-world study design is one of the strengths of this study. Also, the use of a validation cohort to confirm our PK model is a major strength. A limitation of our study is the use of biochemical outcomes instead of endoscopic outcomes, although both are aligned to endoscopic outcomes in patients with CD [32]. However, FC does not always accurately reflect small-bowel CD [34]. Also, when either CRP or FC was missing, only one marker was used to determine whether the patient achieved biochemical remission. Since patients with missing data were classified as non-responders, remission rates might be underestimated. All patients were included in the Netherlands, which is a relatively small European country. Though patients included in this study had comparable baseline characteristics (e.g. disease duration, medical history and prior treatment) compared to other real-world cohorts [35,36,37,38], other factors such as lifestyle, diet, socioeconomic status and ethnical background were not assessed. Therefore, our population might be not representative of a more global population. Another limitation is the lack of systematic assessment of UST levels. UST levels were determined at different time points during follow-up. To correct for this, we developed a PK model which we validated in another 34 patients with CD. At last, we did not correct for corticosteroid use in the determination of biochemical remission rates.
In conclusion, we demonstrated that UST levels at week 8 and 16 are associated with biochemical remission in patients with CD. These outcomes can be used clinically to help decide whether UST treatment should be continued eight weeks after induction therapy. Prospective randomized trials are needed to determine whether treatment outcomes can be improved by dose escalation based on trough levels.
References
Baumgart DC, Sandborn WJ. “Crohn’s disease,” (in eng). Lancet 2012;380:1590–1605. https://doi.org/10.1016/s0140-6736(12)60026-9.
Deepak P, Sandborn WJ. “Ustekinumab and Anti-Interleukin-23 Agents in Crohn’s Disease,” (in eng). Gastroenterol Clin North Am 2017;46:603–626. https://doi.org/10.1016/j.gtc.2017.05.013.
Peluso I, Pallone F, Monteleone G. “Interleukin-12 and Th1 immune response in Crohn’s disease: pathogenetic relevance and therapeutic implication,” (in eng). World J Gastroenterol 2006;12:5606–5610. https://doi.org/10.3748/wjg.v12.i35.5606.
Neurath MF. “IL-23: a master regulator in Crohn disease,” (in eng). Nat Med 2007;13:26–28. https://doi.org/10.1038/nm0107-26.
Feagan BG, Sandborn WJ, Gasink C et al. “Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease,” (in eng). N Engl J Med 2016;375:1946–1960. https://doi.org/10.1056/NEJMoa1602773.
Sandborn WJ, Rutgeers P, Gasink C et al. “Long-term efficacy and safety of ustekinumab for Crohn’s disease through the second year of therapy,” (in eng). Alimentary Pharmacology & Therapeutics 2018;48:65–77. https://doi.org/10.1111/apt.14794.
Vande Casteele N, Herfarth H, Katz J, Falck-Ytter Y, Singh S. “American Gastroenterological Association Institute Technical Review on the Role of Therapeutic Drug Monitoring in the Management of Inflammatory Bowel Diseases,” (in eng). Gastroenterology 2017;153:835–857. https://doi.org/10.1053/j.gastro.2017.07.031.
Papamichael K, Vogelzang EH, Lambert J, Wolbink G, Cheifetz AS. “Therapeutic drug monitoring with biologic agents in immune mediated inflammatory diseases,” (in eng). Expert Rev Clin Immunol 2019;15:837–848. https://doi.org/10.1080/1744666x.2019.1630273.
Khanna R, Levesque BG, Sandborn WJ, Feagan BG. “Therapeutic Drug Monitoring of TNF Antagonists in Inflammatory Bowel Disease,” (in eng). Gastroenterol Hepatol (N Y) 2014;10:478–489.
Mitrev N, vande Casteele N, Seow CH et al. “Review article: consensus statements on therapeutic drug monitoring of anti-tumour necrosis factor therapy in inflammatory bowel diseases,” (in eng). Aliment Pharmacol Ther 2017;46:1037–1053. https://doi.org/10.1111/apt.14368.
Adedokun OJ, Xu Z, Gasink C et al. “Pharmacokinetics and Exposure Response Relationships of Ustekinumab in Patients With Crohn’s Disease,” (in eng). Gastroenterology 2018;154:1660–1671. https://doi.org/10.1053/j.gastro.2018.01.043.
Battat R, Kopylov U, Bessissow T et al. “Association Between Ustekinumab Trough Concentrations and Clinical, Biomarker, and Endoscopic Outcomes in Patients With Crohn’s Disease,” (in eng). Clin Gastroenterol Hepatol 2017;15:1427–1434. https://doi.org/10.1016/j.cgh.2017.03.032.
Verstockt B, Dreesen E, Noman M et al. “Ustekinumab Exposure-outcome Analysis in Crohn’s Disease Only in Part Explains Limited Endoscopic Remission Rates,” (in eng). J Crohns Colitis 2019;13:864–872. https://doi.org/10.1093/ecco-jcc/jjz008.
Painchart C, Brabantm S, Duveau N et al. “Ustekinumab Serum Trough Levels May Identify Suboptimal Responders to Ustekinumab in Crohn’s Disease,” (in eng). Dig Dis Sci 2020;65:1445–1452. https://doi.org/10.1007/s10620-019-05865-3.
Soufflet N, Bosschetti G, Robin X et al. “Concentrations of Ustekinumab During Induction Therapy Associate With Remission in Patients With Crohn’s Disease,” (in eng). Clin Gastroenterol Hepatol 2019;17:2610–2612. https://doi.org/10.1016/j.cgh.2019.02.042.
Biemans VBC, van der Meulen AE, van der Woude CJ et al. “Ustekinumab for Crohn’s Disease: Results of the ICC Registry, a Nationwide Prospective Observational Cohort Study,” (in eng). J Crohns Colitis 2020;14:33–45. https://doi.org/10.1093/ecco-jcc/jjz119.
Straatmijer T et al. “Ustekinuma b for Crohn’s Disease: Two-Year Results of the Initiative on Crohn and Colitis (ICC) Registry, a Nationwide Prospective Observational Cohort Study,” (in eng). J Crohns Colitis 2021;15:1920–1930. https://doi.org/10.1093/ecco-jcc/jjab081.
N. Holford. “The visual predictive check - superiority to standard diagnostic (Rorschach) plots. https://www.page-meeting.org/?abstract=972 (accessed.
Jonsson EN, Karlsson MO. “Xpose–an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM,” (in eng). Comput Methods Programs Biomed 1999;58:51–64. https://doi.org/10.1016/s0169-2607(98)00067-4.
Lindbom L, Pihlgren P, Jonsson EN. “PsN-Toolkit–a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM,” (in eng). Comput Methods Programs Biomed 2005;79:241–257. https://doi.org/10.1016/j.cmpb.2005.04.005.
S. L. Beal S, Boeckmann A. NONMEM user's guides. Ellicott City: ICON Development Solutions. (accessed.
Keizer RJ, van Benten M, Beijnen JH, Schellens JH, Huitema AD. “Piraña and PCluster: a modeling environment and cluster infrastructure for NONMEM,” (in eng). Comput Methods Programs Biomed 2011;101:72–79. https://doi.org/10.1016/j.cmpb.2010.04.018.
R. D. C. Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. (accessed.
FDA. Clinical Pharmacology Biopharmaceutics Review(s). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/761044Orig1s000TOC.cfm (accessed.
Peyrin-Biroulet L, Reinisch W, Colombel JF et al. “Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn’s disease in the SONIC trial,” (in eng). Gut 2014;63:88–95. https://doi.org/10.1136/gutjnl-2013-304984.
Salbato J, Parnell J, Reddy R et al. “Validation of a Homogenous Mobility Shift Assay (HSMA) for the Measurement of Golimumab (GLM) and Antibodies to Golimumab (ATG) in Inflammatory Bowel Disease (IBD) Patient Serum: 1794,". Official Journal of the American College of Gastroenterology | ACG 2013;108:S540–S541.
Gorovits B, Baltrukonis DJ, Bhattacharya I et al. “Immunoassay methods used in clinical studies for the detection of anti-drug antibodies to adalimumab and infliximab,” (in eng). Clin Exp Immunol 2018;192:348–365. https://doi.org/10.1111/cei.13112.
Alsoud D, Vermeire S, Verstockt B. “Monitoring vedolizumab and ustekinumab drug levels in patients with inflammatory bowel disease: hype or hope?,” (in eng). Curr Opin Pharmacol 2020;55:17–30. https://doi.org/10.1016/j.coph.2020.09.002.
Wils P, Bouhnik Y, Michetti P et al. “Subcutaneous Ustekinumab Provides Clinical Benefit for Two-Thirds of Patients With Crohn’s Disease Refractory to Anti-Tumor Necrosis Factor Agents,” (in eng). Clin Gastroenterol Hepatol 2016;14:242-50.e1–2. https://doi.org/10.1016/j.cgh.2015.09.018.
Kopylov U, Afif W, Cohen A et al. “Subcutaneous ustekinumab for the treatment of anti-TNF resistant Crohn’s disease–the McGill experience,” (in eng). J Crohns Colitis 2014;8:1516–1522. https://doi.org/10.1016/j.crohns.2014.06.005.
Shah SC, Colombel JF, Sands BE, Narula N. “Systematic review with meta-analysis: mucosal healing is associated with improved long-term outcomes in Crohn’s disease,” (in eng). Aliment Pharmacol Ther 2016;43:317–333. https://doi.org/10.1111/apt.13475.
Jones J, Loftus EV, Panaccione R et al. “Relationships between disease activity and serum and fecal biomarkers in patients with Crohn’s disease,” (in eng). Clin Gastroenterol Hepatol 2008;6:1218–1224. https://doi.org/10.1016/j.cgh.2008.06.010.
Menting SP, van den Reek JMPA, Baerveldt EM et al. “The correlation of clinical efficacy, serum trough levels and antidrug antibodies in ustekinumab-treated patients with psoriasis in a clinical-practice setting,” (in eng). Br J Dermatol 2015;173(3):855–857. https://doi.org/10.1111/bjd.13834.
Kopylov U, Yung DE, Engel T et al. “Fecal calprotectin for the prediction of small-bowel Crohn’s disease by capsule endoscopy: a systematic review and meta-analysis,” (in eng). Eur J Gastroenterol Hepatol 2016;28:1137–1144. https://doi.org/10.1097/meg.0000000000000692.
Iborra M et al. “Real-world long-term effectiveness of ustekinumab in Crohn’s disease: results from the ENEIDA registry,” (in eng). Aliment Pharmacol Ther. 2020. https://doi.org/10.1111/apt.15958.
Khorrami S et al. “Ustekinumab for the Treatment of Refractory Crohn’s Disease: The Spanish Experience in a Large Multicentre Open-label Cohort,” (in eng). Inflamm Bowel Dis 2016;22:1662–1669. https://doi.org/10.1097/mib.0000000000000842.
Kubesch A et al. “Short and Long-Term Effectiveness of Ustekinumab in Patients with Crohn’s Disease: Real-World Data from a German IBD Cohort,” (in eng). J Clin Med. 2019. https://doi.org/10.3390/jcm8122140.
Liefferinckx C et al. “Long-term Clinical Effectiveness of Ustekinumab in Patients with Crohn’s Disease Who Failed Biologic Therapies: A National Cohort Study,” (in eng). J Crohns Colitis 2019;13:1401–1409. https://doi.org/10.1093/ecco-jcc/jjz080.
Acknowledgments
Nothing to declare.
Funding
The ICC fellow is sponsored by AbbVie, Pfizer, Takeda, Celgene, Janssen Pharmaceutica, Teva Pharmaceutical Industries, Cablon Medical, Ferring pharmaceuticals, Mundipharma, Dr. Falk Pharma, Sandoz, MLDS and Tramedico.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
TS, VBC, RT: none. MD: reports advisory fees from Echo Pharma and Robarts Clinical Trials, Inc., speaker fees from Janssen, Merck & Co., Inc., Pfizer, Takeda and Tillotts Pharma, and nonfinancial support from Dr. Falk Pharm. FH has served on advisory boards or as speaker for Abbvie, Janssen-Cilag, MSD, Takeda, Celltrion, Teva, Sandoz and Dr Falk Funding (Grants/Honoraria): Takeda, Janssen-Cilag, Abbvie Consulting Fees: Celgene. MP has served on advisory boards, or as speaker or consultant for Abbvie, Janssen‐Cilag, MSD, Takeda, Ferring, Dr Falk, and Sandoz and has received unrestricted grants from Janssen‐Cilag, Abbvie and Takeda outside the submitted work. AE van der Meulen has received a presentation fee from Janssen and served on the advisory board of Takeda and Galapagos, outside of the submitted work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Straatmijer, T., Biemans, V.B.C., Moes, D.J.A.R. et al. Ustekinumab Trough Concentrations Are Associated with Biochemical Outcomes in Patients with Crohn’s Disease. Dig Dis Sci 68, 2647–2657 (2023). https://doi.org/10.1007/s10620-023-07822-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-023-07822-7