Abstract
Introduction
The aim of this study was to evaluate the association between serum ustekinumab (UST) trough levels and response to induction and maintenance UST treatment in refractory Crohn’s Disease (CD) patients.
Methods
We performed a prospective study including CD patients who received UST from September 2015 to January 2017. Patients received 90 mg of UST subcutaneously at weeks 0, 4, and 12, then every 8 weeks. Two cohorts of patients were analyzed: an induction cohort and a maintenance cohort. We evaluated clinical, biological, and imaging/endoscopic response to UST treatment. UST trough levels and anti-UST antibodies were dosed at weeks 12 and 28 in the induction cohort, and at a single time point in the maintenance cohort.
Results
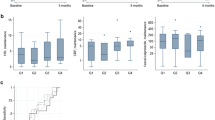
Forty-nine patients were enrolled in the maintenance cohort. Mean concentrations of UST were 1.88 ± 1.40 µg/mL. UST trough levels were not significantly different in patients with or without clinical, biological, or imaging/endoscopic responses to UST treatment (p > 0.11). Twenty-three consecutive patients were included in the induction cohort. At week 12, mean UST concentrations were 1.45 ± 1.15 µg/mL. Patients with a biological response to UST treatment had significant higher serum UST trough concentration (median 1.72 µg/mL) than non-responders (median 0.56 µg/mL, p = 0.02). A UST trough level ≥ 1.10 µg/mL at week 12 was associated with a biological response to UST treatment at 6 months.
Conclusion
UST trough levels were associated with a biological response at the end of the induction phase. In patients with low levels of UST, optimization treatment may be necessary to obtain a sustained response.




Similar content being viewed by others
Abbreviations
- UST:
-
Ustekinumab
- CD:
-
Crohn’s disease
- IBD:
-
Inflammatory bowel disease
- TNF:
-
Tumor necrosis factor
- IFX:
-
Infliximab
- ADA:
-
Adalimumab
- HBI:
-
Harvey–Bradshaw index
- PGA:
-
Physician global assessment
- CRP:
-
C-reactive protein
- MRI:
-
Magnetic resonance imaging
- ELISA:
-
Enzyme-linked immunosorbent assay
- ROC:
-
Receiver operating characteristic
- IQR:
-
Interquartile range
References
Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet Lond Engl. 2012;380:1590–1605.
Peyrin-Biroulet L, Loftus EV, Colombel J-F, Sandborn WJ. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol. 2010;105:289–297.
Colombel J-F, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52–65.
Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet Lond Engl. 2002;359:1541–1549.
Yanai H, Hanauer SB. Assessing response and loss of response to biological therapies in IBD. Am J Gastroenterol. 2011;106:685–698.
Peluso I, Pallone F, Monteleone G. Interleukin-12 and Th1 immune response in Crohn’s disease: pathogenetic relevance and therapeutic implication. World J Gastroenterol. 2006;12:5606–5610.
Neurath MF. IL-23: a master regulator in Crohn disease. Nat Med. 2007;13:26–28.
Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375:1946–1960.
Wils P, Bouhnik Y, Michetti P, et al. Long-term efficacy and safety of ustekinumab in 122 refractory Crohn’s disease patients: a multicentre experience. Aliment Pharmacol Ther. 2018;47:588–595.
Wils P, Bouhnik Y, Michetti P, et al. Subcutaneous ustekinumab provides clinical benefit for two-thirds of patients with Crohn’s disease refractory to anti-tumor necrosis factor agents. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2016;14:242–250.e1-2.
Ma C, Fedorak RN, Kaplan GG, et al. Clinical, endoscopic and radiographic outcomes with ustekinumab in medically-refractory Crohn’s disease: real world experience from a multicentre cohort. Aliment Pharmacol Ther. 2017;45:1232–1243.
Kopylov U, Afif W, Cohen A, et al. Subcutaneous ustekinumab for the treatment of anti-TNF resistant Crohn’s disease—the McGill experience. J Crohns Colitis. 2014;8:1516–1522.
Vande Casteele N, Herfarth H, Katz J, Falck-Ytter Y, Singh S. American Gastroenterological Association Institute technical review on the role of therapeutic drug monitoring in the management of inflammatory bowel diseases. Gastroenterology. 2017;153:835–857.e6.
Verstockt B, Moors G, Bian S, et al. Influence of early adalimumab serum levels on immunogenicity and long-term outcome of anti-TNF naive Crohn’s disease patients: the usefulness of rapid testing. Aliment Pharmacol Ther. 2018;48:731–739.
Roblin X, Rinaudo M, Sparrow MP, et al. Pharmacokinetics in IBD: ready for prime time? Curr Drug Targets. 2014;15:1049–1055.
Mitrev N, Vande Casteele N, Seow CH, et al. Review article: consensus statements on therapeutic drug monitoring of anti-tumour necrosis factor therapy in inflammatory bowel diseases. Aliment Pharmacol Ther. 2017;46:1037–1053.
Peyrin-Biroulet L, Bouhnik Y, Roblin X, et al. French national consensus clinical guidelines for the management of Crohn’s disease. Dig Liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver. 2017;49:368–377.
Roblin X, Rinaudo M, Del Tedesco E, et al. Development of an algorithm incorporating pharmacokinetics of adalimumab in inflammatory bowel diseases. Am J Gastroenterol. 2014;109:1250–1256.
Sandborn WJ, Feagan BG, Fedorak RN, et al. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology. 2008;135:1130–1141.
Sandborn WJ, Gasink C, Gao L-L, et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. 2012;367:1519–1528.
Battat R, Kopylov U, Bessissow T, et al. Association between ustekinumab trough concentrations and clinical, biomarker, and endoscopic outcomes in patients with Crohn’s disease. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2017;15:1427–1434.e2.
Adedokun OJ, Xu Z, Gasink C, et al. Pharmacokinetics and exposure response relationships of ustekinumab in patients with Crohn’s disease. Gastroenterology. 2018;154:1660–1671.
Menting SP, van den Reek JMPA, Baerveldt EM, et al. The correlation of clinical efficacy, serum trough levels and antidrug antibodies in ustekinumab-treated patients with psoriasis in a clinical-practice setting. Br J Dermatol. 2015;173:855–857.
Harris KA, Horst S, Gadani A, et al. Patients with refractory Crohn’s disease successfully treated with ustekinumab. Inflamm Bowel Dis. 2016;22:397–401.
Menter A, Papp KA, Gooderham M, et al. Drug survival of biologic therapy in a large, disease-based registry of patients with psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Eur Acad Dermatol Venereol JEADV. 2016;30:1148–1158.
Funding
None.
Author information
Authors and Affiliations
Contributions
CP and BP conceived and designed the study, interpreted the data, drafted the manuscript, and supervised the study. CP, BP and ND gave administrative, technical, or material support. All authors took part in data acquisition, critical revision of the manuscript for important intellectual content, and approval of the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig.
1: Serum UST trough levels at the end of the induction phase (week 12) in the induction cohort according to the clinical response to UST treatment. Boxplot of serum UST trough levels in patients with (left box) or without (right box) clinical response to UST therapy at week 12. The box represents the 25th–75th percentiles, and the whiskers correspond to the 5th–95th percentiles. W. Abbreviations: UST, ustekinumab (TIFF 48 kb)
Rights and permissions
About this article
Cite this article
Painchart, C., Brabant, S., Duveau, N. et al. Ustekinumab Serum Trough Levels May Identify Suboptimal Responders to Ustekinumab in Crohn’s Disease. Dig Dis Sci 65, 1445–1452 (2020). https://doi.org/10.1007/s10620-019-05865-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-05865-3