Abstract
Background
The clinical value of alpha-fetoprotein (AFP) in patients with AFP-negative (< 20 ng/ml) hepatocellular carcinoma (HCC) who underwent curative resection remained controversial.
Aims
To investigate clinical relevance and prognostic effect of preoperative serum AFP level in this subgroup.
Methods
A total of 1879 patients with AFP-negative HCC who underwent curative resection were included in the study. Overall survival (OS) and disease-free survival (DFS) rate were displayed by Kaplan–Meier method and compared by log-rank test. Multivariate cox proportional hazard regression analysis was used to identify the independent prognostic factors. The prognostic predictive performance was analyzed by time-dependent areas under receiver operating characteristic curve (AUC).
Results
Even in AFP-negative HCC, patients with high preoperative serum AFP level tended to have multiple tumor (P < 0.001), poorer differentiation of tumor cell (P < 0.001), presence of satellite nodules (P < 0.001), and MVI (P = 0.002). Kaplan–Meier analysis showed the adverse impact of AFP level on prognosis, especially for DFS. Multivariate analysis identified AFP as the independent unfavorable factor for OS and DFS (P < 0.001 for both). Time-dependent AUC analysis showed that the combination with AFP could improve the prognostic predictive performance of 8th AJCC and BCLC staging system.
Conclusions
AFP was still the surrogate of aggressive behavior of HCC and independent prognostic factor for patients with AFP-negative HCC underwent curative resection. Even combining with such a low level of AFP could significantly improve the predictive performance of conventional staging system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC), which accounts for 90% liver cancer, ranks as the fifth most common cancer, and also the third leading cause of cancer-related death worldwide [1]. Despite the improvement of diagnosis and treatment over the years, the long-term prognosis remains unsatisfactory due to high incidence of postoperative recurrence and metastasis [2]. To address the issue, several prognostic markers with abilities to classify and prognosticate HCC were identified to provide guidance for treatment strategies and postoperative follow-up managements.
Although numerous biomarkers have been identified, alpha-fetoprotein (AFP) is still the most widely accepted serum biomarker for HCC in a daily clinical practice [3]. The clinical application of AFP was mainly focused on the following four aspects: screening and diagnosing, predicting prognosis, and monitoring response to treatment [4]. For healthy adults, elevated AFP in serum was the indication of HCC; furthermore, for the patients diagnosed as HCC, higher AFP was associated with more aggressive tumor characteristics, poorer outcomes, and poorer therapy responses [5,6,7].
The subgroup without elevated AFP is account for around 38.1–39.4% in the group of HCC patients [8, 9]. Some studies have classified the subgroup as the AFP-negative HCC and have also researched the clinical characteristic and risk factors of the subgroup. However, the clinical value of AFP in the subgroup remained controversial [10, 11]. Zhang [10], Gan [11], and Wang et al. [12] investigated the prognostic factors of AFP-negative HCC, but none of their studies included AFP as the prognostic factor. Besides, Blank [13] and Lu et al. [14] reported the controversial prognostic effect of AFP. Blank et al. reported that AFP was an independent prognostic factor for HBV-HCC patients underwent hepatectomy, and slight changes of AFP within the range of normality may affect prognosis of HBV-HCC; however, Lu et al. failed to find the prognostic effect of AFP in their study.
It was worth noting that AFP-negative was classified by the cutoff point of AFP for the purpose of HCC screening rather than HCC prognostic. Therefore, this study, which included a large cohort of patients with AFP-negative HCC underwent curative resection, aims to investigate the clinical relevance and prognostic effect of preoperative AFP level in serum in this subgroup.
Methods
Patients
The data of patients who met following criteria underwent hepatectomy between June, 20, 2008, and December, 30, 2014, were extracted from Primary Liver Cancer Big Data (PLCBD). The inclusion criteria of patients in the study were as follows: (1) accepted hepatectomy as the primary treatment for HCC; (2) with a preoperative serum AFP level lower than 20 ng/ml; (3) with tolerable preoperative liver function (Child–Pugh A or B7); (4) without distant metastasis; and (5) receipt of R0 resection, which means the complete removal and histological tumor-free surgical margins of all detectable tumor nodes. The exclusion criteria used in the study were as follows: (1) receipt of palliative tumor resection or preoperative anti-HCC treatment; (2) with medical history of any other malignant diseases; and (3) incomplete clinicopathologic data. All data in this study were verified by three independent researchers (Kongying Lin, Jianxing Zeng, and Qizhen Huang), and the study was conducted to the ethical guideline of the 1975 Declaration of Helsinki and was approved by the institutional ethics committee of Mengchao Hepatobiliary Hospital of Fujian Medial University. Informed consent was obtained from each patient for their data to be used for research purposes.
Preoperative Assessment, Hepatectomy, and Follow-Up
Preoperative assessments of patients contained routine examination of liver, renal, cardiopulmonary function, AFP, and hepatitis B/C immunology. Imaging examinations included abdominal ultrasonography, contrast-enhanced magnetic resonance (MRI) or computed tomography (CT) of abdomen, and X-ray check or CT scan of chest. The diagnosis of HCC complied with practice guidelines which recommended by American Association for the study of Liver Diseases [15]. The adoption of anatomical or partial hepatectomy depends on the tumor variables, such as diameter, number and location, and patient’s liver function status. Intraoperative ultrasound test was routinely performed to ensure all detectable tumor nodes were removed.
The followed-up procedure was described as follows. In simple terms, patients routinely accepted serum AFP test, abdominal ultrasonography every 2–3 months in the first 2 years after surgery and later every 3–6 months. The contrast-enhanced CT or MRI was routinely performed every 6 months, or earlier if patients with the result suggestive of recurrence. The diagnosis of recurrence of tumor was similar to the initial diagnostic criteria. The end-points of study were overall survival (OS) and disease-free survival (DFS). Overall survival (OS) was defined as the interval between the date of resection and the date of either death or the last follow-up taken. Disease-free survival (DFS) was defined as the interval between date of resection and the date of recurrence, metastasis, or last follow-up.
Clinicopathologic Variables
The preoperative serologic AFP level and other serologic variables of patients selected in the study were the most recent test within 15 days prior to surgery. The formula of albumin–bilirubin score (ALBI) was reported in previous studies, and we further divided patients into three grades according to the previous cutoff point (grade 1 ≤ −2.60, grade 2 > −2.60 ~ − 1.39; grade 3 > −1.39) [6, 16]. The pathological examination of surgical specimens was performed by three independent pathologists. Tumor diameter means the largest diameter of the largest tumor nodule. The differentiation of tumor cells was based on the Edmondson–Steiner classification, and if there were multiple tumor nodes, the differentiation of tumor cells was depended on which was worst. Vascular invasion was divided into macrovascular invasion and microvascular invasion (MVI).
Statistical Analysis
Continuous variables were expressed as the median (25th to 75th percentiles), and the comparison of continuous variables was tested by the Student’s t test or the Mann–Whitney U test. Categorical variables were expressed by number of patients (percentage) and compared by the χ2 test or Fisher exact test. The comparisons of OS and DFS rate were displayed by Kaplan–Meier method and tested by log-rank test. The independent risk factors for OS and DFS were acquired using the univariate and multivariate cox proportional hazard regression analysis. The predictive performance was analyzed by time-dependent areas under the receiver operating characteristic cure (AUC). Time-dependent AUC of AFP, 8th AJCC, and BCLC staging system for OS and DFS at each time point was calculated by the function of “timeROC” using “timeROC” R package, and the comparison between two time-dependent AUCs for each time point was tested by the function of “compare” using “timeROC” R package [17]. All statistical tests were two-sided tests, and the P value < 0.05 was regarded statistically significant. The SPSS software 20.0 and R software 3.0 (“rms,” “survival,” and “timeROC”) were used in this study.
Results
Patients’ Characteristics
According to the inclusion and exclusion criteria accepted in the study, 1879 patients with AFP-negative HCC were included for further analysis. The basic clinicopathologic characteristics of patients are shown in Table 1. The median AFP level of the patients was 4.6 (25th to 75th percentiles: 2.8, 8.2) ng/ml. Most patients were male (91.0%) and HBsAg positive (82.2%). Liver cirrhosis was present in 65.7% of patients. In ALBI grading, the majority of patients showed well liver function, 77.2% of patients were ALBI grade1 and 22.8% of patients were ALBI grade 2/3. (We grouped together ALBI grades 2 and 3 for further statistical analysis, because only a few patients (2 patients) were classified as ALBI 3 grade.) Regarding to tumor characteristics, most of patients were BCLC A stage (78.6%) or AJCC I stage (66.5%). The median diameter of tumor was 4.6 (25th to 75th percentiles: 3.2, 7.1) cm, 85.7% of patients presented solitary tumor, 24.5% of patients presented MVI, and 5.7% of patients presented macrovascular invasion.
Association Between AFP and Clinicopathologic Characteristics
As shown in Figure (S1-2), AFP level was significantly different within different contexts of liver or tumor characteristics. The patients with higher AFP level were associated with undesirable liver characteristic, such as positive HBsAg (P < 0.001), liver cirrhosis (P < 0.001), decreased albumin (P = 0.015), decreased platelets (P < 0.001), high ALT level (P < 0.001), and undesirable ALBI grade (P < 0.001) (Figure S1). In addition, higher AFP was also associated with aggressive pathologic characteristics of HCC even within such low level. Higher AFP level was significantly associated with multiple tumor nodules (P < 0.001), poorer differentiation of tumor cell (P < 0.001), presence of satellite nodules (P < 0.001), and MVI (P = 0.002) (Figure S2).
Prognostic Effect of AFP
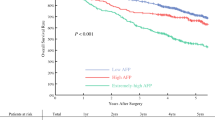
The median of follow-up was 49.1 months (range 1.1–119.9 months). We classified patients into two cohorts based on the median AFP level (4.6 ng/ml) of the whole cohort. Kaplan–Meier analysis showed that the OS and DFS were significantly poorer for the higher AFP level cohort than the lower AFP level cohort (P < 0.001 for both) (Fig. 1a, b). The 1-, 3-, and 5-year OS rates of higher AFP level cohort were 91.6%, 75.0%, 53.7%, respectively, and the corresponding DFS rates were 66.3%, 44.9%, 30.5%, respectively, whereas the postoperative 1-, 3, 5-year OS rates of lower AFP level cohort were 93.4%, 81.4%, 64.0%, respectively, and the corresponding DFS rates were 76.5%, 56.5%, 42.3%, respectively. Although the OS and DFS rates between two cohorts were both significantly different, the difference was relatively small in OS rates, while the difference in the DFS rates was more distinctive. The DFS curve between two cohorts was distinctly different within 1 year after resection, and then almost parallel thereafter.
After stratification according to cirrhosis, higher AFP level was associated with poorer outcome regardless of cirrhosis (Fig. 1c–f). When the prognostic analysis was conducted in the subgroup stratified by etiology, the OS and DFS rates in higher AFP level cohort were both poorer than in lower AFP level cohort, excepting the OS in HCV-HCC (Fig. 2a–f). The distinctive DFS curve trend mentioned above was both found in HBV-HCC and NBNC-HCC cohorts, especially clear in NBNC-HCC cohort. In further prognostic analysis that performed in the subgroup stratified by the 8th AJCC staging system, the higher AFP level cohort had a significantly poorer OS and DFS than lower AFP level cohort in the stage I and II patients (Fig. 3a–f).
Prognostic Factors for OS and DFS
Univariate and multivariate cox proportional hazard regression analysis was used to acquire independent risk factor of OS and DFS (Tables 2, 3). Multivariate analysis for OS showed that higher AFP level remained as independent risk factor of OS (HR with 95% CI, 1.036 (1.022–1.051), P < 0.001), and other variables were albumin [0.961 (0.943–0.979), P < 0.001)], tumor diameter [1.067 (1.047–1.087), P < 0.001)], multiple tumor [1.472 (1.228–1.764), P < 0.001], poorer tumor differentiation [1.299 (1.080–1.563), P = 0.005], tumor capsule [1.248 (1.051–1.482), P = 0.011, 1.618(1.297–2.018), P < 0.001], MVI [1.545 (1.300–1.836), P < 0.001], and macrovascular invasion [2.057 (1.576–2.685), P < 0.001] (Table 3).
Multivariate analysis for DFS also identified AFP level as the independent risk factor for DFS [1.031 (1.019–1.043), P < 0.001], in addition to albumin [0.977 (0.961–0.993), P = 0.005], cirrhosis [1.335 (1.175–1.517), P < 0.001], tumor diameter [1.075 (1.059–1.092), P < 0.001], multiple tumor [1.600 (1.376–1.860), P < 0.001], tumor capsule [1.289 (1.126–1.476), P < 0.001, 1.511 (1.259–1.814), P < 0.001], MVI [1.290 (1.118–1.489), P < 0.001], and macrovascular invasion [2.433 (1.913–3.093), P < 0.001] (Table 3).
Predictive Effect of AFP for Prognosis
The time-dependent AUC analysis was performed to assess the predictive effect of the AFP on OS and DFS (Fig. 4a, b). The median time-dependent AUC of AFP was 0.572 (range 0.558–0.576) for OS and 0.587 (range 0.575–0.594) for DFS, respectively. In addition to conventional BCLC and 8th AJCC staging systems, we further added the factor of AFP to analyze the time-dependent AUCs and compared it with the conventional ones. As shown in Fig. 4c, d, the combination with AFP could significantly improve the predictive capability of 8th AJCC and BCLC staging systems. As shown in Table S1, by comparing the original BCLC and 8th AJCC staging systems and the staging systems combined with APF factor, the time-dependent AUCs of DFS showed significantly different for each time points (P < 0.001 for both). Concerning the time-dependent AUCs of OS, the comparison showed significantly different at 3-, 4-, and 5-year time point (Table 1).
Discussion
The present study included a large cohort of patients with AFP-negative HCC who underwent curative resection into the investigation, in order to find out the clinicopathologic and prognostic relevance of AFP in the subgroup. Our results showed that preoperative AFP level was still associated with the aggressive behavior of tumor cells and poor prognostic outcome in AFP-negative HCC.
High serologic AFP level was not only clinically indication of HCC, but also the surrogate for tumor aggressive biology [5, 18]. Some studies have also reported the association among AFP level and different molecular subclasses of HCC [19, 20]. In our study, we showed that preoperative serologic AFP level was also significantly associated with more frequency of multiple tumor numbers, satellite nodules, poorly differentiation, and MVI. The findings may demonstrate that AFP is still the surrogate of aggressive characteristic of tumor cells in AFP-negative HCC.
The Kaplan–Meier analysis showed the adverse impact of AFP level on prognosis, especially for DFS. The DFS curves between higher and lower AFP level cohorts were distinctly different within 1 year after resection and then almost parallel thereafter. There were two different recurrence types of HCC: One is the “early recurrence” which was mainly caused by the initial tumor, and the other is the “late recurrence” which was mainly due to clonal origin [21]. The distinctive trend of DFS curve between different AFP levels suggested that the frequency of early recurrence was higher in higher AFP cohort. Some studies have reported that early recurrence of HCC is mainly associated with aggressive tumor behavior, such as multiple tumors nodules, poorly differentiation, and MVI [6, 22]. The findings may suggest that the adverse impact of AFP on prognosis of AFP-negative HCC was caused by its association of tumor aggressive behavior.
Interestingly, the prognostic analysis according to etiology showed that the changed trend of DFS curve was more distinctive in NBNC-HCC rather than the one in HBV-HCC. It may be caused by the difference pathogenic mechanisms of hepatocarcinogenesis between HBV-HCC and NBNC-HCC. Chronic hepatitis B virus infection was unique pathogenic mechanisms of hepatocarcinogenesis in HBV-HCC. The role of HBV in elevation of AFP has been previously reported in some studies [23, 24]. Our results also showed higher AFP level in HBsAg-positive patients. Zhang et al. [24] reported that HBX could directly upregulate the expression of AFP via binding to and activate the promoter of AFP gene. In view of this, the prognostic effect of serum AFP level in HBV-HCC may be affected by HBV factors, such as HBV DNA load. However, more detailed mechanism of such difference between HBV-HCC and NBNC-HCC remains unknown and needs further study.
The study also analyzed the prognostic effect of AFP in the subgroup stratified by 8th AJCC staging system. The AFP level showed a significantly adverse effect on prognosis in the stage I and II. The AJCC staging system was worldwide accepted and conventionally used in a daily clinical practice [25]. However, given the heterogeneity of tumor cells, the patients, even within same stage, may manifest different prognoses [26]. The result showed that AFP may be an additional biomarker, which could be joint with conventional AJCC staging system for clinical usage, in order to identify the high-risk patients. This may be valuable to guide the postoperative adjuvant therapy and monitoring.
Furthermore, the present study analyzed the prognostic predictive effect of AFP. The prognostic effect of AFP has been acknowledged by serval scoring systems, such as BALAD score, [27] Cancer of the Liver Italian Program (CLIP) score [28], Chinese University Prognostic Index (CUPI) [29], and The Taipei Integrated score (TIS) [30]. However, the cutoff point of AFP accepted in these scoring systems was in the range from 400 to 500 ng/ml, which may be unable to differentiate between patients with low AFP level. In the study, we also showed that the time-dependent AUC of AFP in AFP-negative HCC was 0.572 (range 0.558–0.576) for OS and 0.587 (range 0.575–0.594) for DFS, respectively, which were approximated to those reported in previous studies that included the HCC with various ranges of AFP level [31, 32]. In consideration that HCC is a complicated disease with diverse pathogenic mechanisms caused by various risk factors, a single biomarker may be difficult to stably predict prognosis. Hence, the study further analyzed the predictive performance of conventionally used BCLC and 8th AJCC staging system combined with AFP factor. The result showed that even combining with such a low level of AFP could significantly improve the predictive performance of conventional 8th AJCC and BCLC staging systems.
The study has several limitations. The first one is single-center retrospective study only, and thus, the selection bias was unavoidable. The second one is that the patients included in the study were from a hepatitis B virus endemic area, the majority of patients were suffering from HBV infection, and only 26 patients were HCV-associated HCC, and thus, the clinicopathologic relevance and prognostic effect of AFP in HCV-associated HCC need further study. The third one is that although present study demonstrated that AFP was still the surrogate of aggressive behavior of tumor cell and independent risk prognostic factor for patients with AFP-negative HCC, the time-dependent AUC suggested that AFP alone was not a powerful prognostic predictor. Combination with other serologic biomarkers, such as AFP-L3 and PIVKA-II, may improve predictive performance of AFP. In addition, the study included only the patients who underwent resection and the results need to be further verified in patients who received other treatments.
Conclusion
AFP was still the surrogate of aggressive behavior of HCC and independent risk factor of prognosis in patients with AFP-negative HCC who underwent curative resection. Even combining with such a low level of AFP could significantly improve the predictive performance of conventional staging system.
Abbreviations
- AFP:
-
Alpha fetoprotein
- HBsAg:
-
Hepatitis B virus surface antigen
- HCVAb:
-
Anti-hepatitis c virus antibody
- HBV-HCC:
-
HBV associated hepatocellular carcinoma
- HCV-HCC:
-
HCV associated hepatocellular carcinoma
- HB/CV-HCC:
-
HBV, HCV associated hepatocellular carcinoma
- NBNC-HCC:
-
Non-B, non-C hepatocellular carcinoma
- WBCs:
-
White blood cells
- RBCs:
-
Red blood cells
- Hb:
-
Hemoglobin
- ALT:
-
Alanine transaminase
- GGT:
-
Gamma-glutamyl transpeptidase
- ALBI:
-
Albumin–bilirubin
- BCLC:
-
Barcelona Clinic Liver Cancer staging system
- AJCC:
-
American Joint Committee on Cancer
- MVI:
-
Microvascular invasion
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
Dhir M, Melin AA, Douaiher J, et al. A review and update of treatment options and controversies in the management of hepatocellular carcinoma. Ann Surg. 2016;263:1112–1125.
European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L: eASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236.
Galle PR, Foerster F. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019;39:2214–2229.
Cucchetti A, Piscaglia F, Grigioni AD, et al. Preoperative prediction of hepatocellular carcinoma tumour grade and micro-vascular invasion by means of artificial neural network: a pilot study. J Hepatol. 2010;52:880–888.
Chan AWH, Zhong J, Berhane S, et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol. 2018;69:1284–1293.
Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–296.
Zhang B, Zhang B, Zhang Z, et al. 42,573 cases of hepatectomy in China: a multicenter retrospective investigation. Sci China Life Sci. 2018;61:660–670.
Chan MY, She WH, Dai WC, et al. Prognostic value of preoperative alpha-fetoprotein (AFP) level in patients receiving curative hepatectomy- an analysis of 1,182 patients in Hong Kong. Transl Gastroenterol Hepatol. 2019;4:52.
Zhang XF, Qi X, Meng B, et al. Prognosis evaluation in alpha-fetoprotein negative hepatocellular carcinoma after hepatectomy: comparison of five staging systems. Eur J Surg Oncol. 2010;36:718–724.
Gan W, Huang JL. New nomogram predicts the recurrence of hepatocellular carcinoma in patients with negative preoperative serum AFP subjected to curative resection. J Surg Oncol. 2018;117:1540–1547.
Wang X, Mao M, He Z, et al. Development and Validation of a Prognostic Nomogram in AFP-negative hepatocellular carcinoma. Int J Biolo Sci. 2019;15:221–228.
Blank S, Wang Q, Fiel MI, et al. Assessing prognostic significance of preoperative alpha-fetoprotein in hepatitis B-associated hepatocellular carcinoma: normal is not the new normal. Ann Surg Oncol. 2014;21:986–994.
Lu LH, Zhang YF, Wei W, Shi M, Guo RP. Preoperative carbohydrate antigen 19-9: its neglected role in alpha-fetoprotein-negative hepatocellular carcinoma patients. J Gastrointest Surg. 2017;21:2025–2032.
Bruix J, Sherman M. Practice Guidelines Committee AAftSoLD: Management of hepatocellular carcinoma. Hepatology (Baltimore, MD). 2005;42:1208–1236.
Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–558.
Blanche P, Dartigues JF, Jacqmin-Gadda H. Estimating and comparing time-dependent areas under receiver operating characteristic curves for censored event times with competing risks. Stat Med. 2013;32:5381–5397.
Halazun KJ, Najjar M, Abdelmessih RM, et al. Recurrence after liver transplantation for hepatocellular carcinoma: a new MORAL to the story. Ann Surg. 2017;265:557–564.
Calderaro J, Couchy G, Imbeaud S, et al. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J Hepatol. 2017;67:727–738.
Hoshida Y, Nijman SM, Kobayashi M, et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Can Res. 2009;69:7385–7392.
Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89:500–507.
Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–207.
Li M, Zhu M, Li W, et al. Alpha-fetoprotein receptor as an early indicator of HBx-driven hepatocarcinogenesis and its applications in tracing cancer cell metastasis. Cancer Lett. 2013;330:170–180.
Zhang C, Chen X, Liu H, et al. Alpha fetoprotein mediates HBx induced carcinogenesis in the hepatocyte cytoplasm. Int J Cancer. 2015;137:1818–1829.
Zhang G, Li R, Zhao X, Meng S, Ye J, Zhao L. Validation of the American Joint Committee on Cancer eighth edition staging system in patients undergoing hepatectomy for hepatocellular carcinoma: a US population-based study. J Surg Res. 2018;222:55–68.
Mao YP, Xie FY, Liu LZ, et al. Re-evaluation of 6th edition of AJCC staging system for nasopharyngeal carcinoma and proposed improvement based on magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2009;73:1326–1334.
Toyoda H, Kumada T, Osaki Y, et al. Staging hepatocellular carcinoma by a novel scoring system (BALAD score) based on serum markers. Clin Gastroenterol Hepatol. 2006;4:1528–1536.
Cancer of the Liver Italian Program (CLIP) Investigators. A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. (Baltimore, Md). 1998;28:751–755.
Leung TW, Tang AM, Zee B, et al. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94:1760–1769.
Hsu CY, Huang YH, Hsia CY, et al. A new prognostic model for hepatocellular carcinoma based on total tumor volume: the Taipei Integrated Scoring System. J Hepatol. 2010;53:108–117.
Yang SL, Liu LP, Yang S, et al. Preoperative serum alpha-fetoprotein and prognosis after hepatectomy for hepatocellular carcinoma. Br J Surg. 2016;103:716–724.
Giannini EG, Marenco S, Borgonovo G, et al. Alpha-fetoprotein has no prognostic role in small hepatocellular carcinoma identified during surveillance in compensated cirrhosis. Hepatology (Baltimore, MD). 2012;56:1371–1379.
Acknowledgments
This study was supported by Science and Technology project of Fuzhou (Grant Number: 2019-SZ-49), Key Clinical Specialty Discipline Construction Program of Fuzhou, Fujian, P.R.C (Grant Number: 201912002), and Fujian provincial medical center of hepatobiliary.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lin, K., Huang, Q., Zeng, J. et al. Clinical Significance of Alpha-Fetoprotein in Alpha-Fetoprotein Negative Hepatocellular Carcinoma Underwent Curative Resection. Dig Dis Sci 66, 4545–4556 (2021). https://doi.org/10.1007/s10620-020-06797-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06797-z