Abstract
Purpose
The study aimed to investigate the prognostic role of the preoperative carbohydrate antigen 19-9 (CA19-9) level in alpha-fetoprotein (AFP)-negative (AFP < 25 ng/ml) hepatocellular carcinoma (HCC) patients.
Methods
From December 2004 to December 2013, 750 patients diagnosed with AFP-negative HCC following curative resection were enrolled. Both univariate and multivariate analyses were performed to clarify the prognostic factors for overall survival (OS) and disease-free survival (DFS).
Results
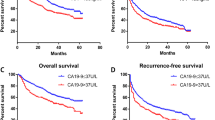
The optimal cutoff point for preoperative CA19-9 was 32.6 U/ml, and the value of the area under the curve was 0.640. The 1-, 3-, and 5-year OS rates were 88.4, 72.2, and 57.0%, respectively, for the CA19-9 > 32.6 U/ml group and 97.0, 83.3, and 79.9%, respectively, for the CA19-9 ≤ 32.6 U/ml group (P < 0.001). The 1-, 3-, and 5-year DFS rates were 71.8, 47.7, and 34.8%, respectively, for the CA19-9 > 32.6 U/ml group and 80.8, 63.6, and 55.5%, respectively, for the CA19-9 ≤ 32.6 U/ml group (P < 0.001). The multivariate analysis showed that CA19-9 > 32.6 U/ml was one of the most significant unfavorable predictors of OS and DFS (P < 0.001 for both).
Conclusions
Preoperative CA19-9 > 32.6 U/ml is a predictor of dismal prognosis and can be employed as a prognostic marker for patient selection in AFP-negative HCC management.


Similar content being viewed by others
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: a cancer journal for clinicians. 2015;65(2):87–108. doi:10.3322/caac.21262.
Bruix J, Sherman M, Practice Guidelines Committee AAftSoLD. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–36. doi:10.1002/hep.20933. Bruix J and Sherman M shared co-first authorship.
European Association For The Study Of The L, European Organisation For R, Treatment Of C. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Journal of hepatology. 2012;56(4):908–43. doi:10.1016/j.jhep.2011.12.001.
Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatology international. 2010;4(2):439–74. doi:10.1007/s12072-010-9165-7.
Shah SA, Cleary SP, Wei AC, Yang I, Taylor BR, Hemming AW et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery. 2007;141(3):330–9. doi:10.1016/j.surg.2006.06.028.
Tangkijvanich P, Anukulkarnkusol N, Suwangool P, Lertmaharit S, Hanvivatvong O, Kullavanijaya P et al. Clinical characteristics and prognosis of hepatocellular carcinoma: analysis based on serum alpha-fetoprotein levels. Journal of clinical gastroenterology. 2000;31(4):302–8.
Tyson GL, Duan Z, Kramer JR, Davila JA, Richardson PA, El-Serag HB. Level of alpha-fetoprotein predicts mortality among patients with hepatitis C-related hepatocellular carcinoma. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2011;9(11):989–94. doi:10.1016/j.cgh.2011.07.026.
Ding T, Xu J, Wang F, Shi M, Zhang Y, Li SP et al. High tumor-infiltrating macrophage density predicts poor prognosis in patients with primary hepatocellular carcinoma after resection. Human pathology. 2009; 40(3):381–9. doi:10.1016/j.humpath.2008.08.011.
Hainsworth JD, Williams SD, Einhorn LH, Birch R, Greco FA. Successful treatment of resistant germinal neoplasms with VP-16 and cisplatin: results of a Southeastern Cancer Study Group trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1985; 3(5):666–71. doi:10.1200/jco.1985.3.5.666.
Hoskin P, Dilly S, Easton D, Horwich A, Hendry W, Peckham MJ. Prognostic factors in stage I non-seminomatous germ-cell testicular tumors managed by orchiectomy and surveillance: implications for adjuvant chemotherapy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1986; 4(7):1031–6. doi:10.1200/jco.1986.4.7.1031.
He C, Xu J, Zhang J, Xie D, Ye H, Xiao Z et al. High expression of trimethylated histone H3 lysine 4 is associated with poor prognosis in hepatocellular carcinoma. Human pathology. 2012; 43(9):1425–35. doi:10.1016/j.humpath.2011.11.003. He C, Xu J, Zhang J, shared co-first authorship.
Sun YF, Xu Y, Yang XR, Guo W, Zhang X, Qiu SJ et al. Circulating stem cell-like epithelial cell adhesion molecule-positive tumor cells indicate poor prognosis of hepatocellular carcinoma after curative resection. Hepatology. 2013; 57(4):1458–68. doi:10.1002/hep.26151. Sun YF, Xu Y, Yang XR, shared co-first authorship.
Wang X, Ren H, Zhao T, Chen J, Sun W, Sun Y et al. Stem cell factor is a novel independent prognostic biomarker for hepatocellular carcinoma after curative resection. Carcinogenesis. 2014; 35 (10):2283–90. doi:10.1093/carcin/bgu162. Wang X and Ren H, shared co-first authorship.
Xu YF, Yi Y, Qiu SJ, Gao Q, Li YW, Dai CX et al. PEBP1 downregulation is associated to poor prognosis in HCC related to hepatitis B infection. Journal of hepatology. 2010; 53(5):872–9. doi:10.1016/j.jhep.2010.05.019. Xu YF, Yi Y, Qiu SJ shared co-first authorship.
Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. Journal of gastrointestinal oncology. 2012; 3(2):105–19. doi:10.3978/j.issn.2078-6891.2011.021.
Ballehaninna UK, Chamberlain RS. Biomarkers for pancreatic cancer: promising new markers and options beyond CA 19-9. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2013; 34(6):3279–92. doi:10.1007/s13277-013-1033-3.
Tao LY, Cai L, He XD, Liu W, Qu Q. Comparison of serum tumor markers for intrahepatic cholangiocarcinoma and hepatocellular carcinoma. The American surgeon. 2010; 76 (11):1210–3.
Kim KH, Lee SG, Park EH, Hwang S, Ahn CS, Moon DB et al. Surgical treatments and prognoses of patients with combined hepatocellular carcinoma and cholangiocarcinoma. Annals of surgical oncology. 2009; 16(3):623–9. doi:10.1245/s10434-008-0278-3.
Tsuji M, Kashihara T, Terada N, Mori H. An immunohistochemical study of hepatic atypical adenomatous hyperplasia, hepatocellular carcinoma, and cholangiocarcinoma with alpha-fetoprotein, carcinoembryonic antigen, CA19-9, epithelial membrane antigen, and cytokeratins 18 and 19. Pathology international. 1999; 49(4):310–7.
Chen YL, Chen CH, Hu RH, Ho MC, Jeng YM. Elevated preoperative serum CA19-9 levels in patients with hepatocellular carcinoma is associated with poor prognosis after resection. TheScientificWorldJournal. 2013; 2013:380797. doi:10.1155/2013/380797.
Hsu CC, Goyal A, Iuga A, Krishnamoorthy S, Lee V, Verna EC et al. Elevated CA19-9 is associated with increased mortality in a prospective cohort of hepatocellular carcinoma patients. Clinical and translational gastroenterology. 2015; 6:e74. doi:10.1038/ctg.2014.22.
Wan P, Zhang J, Long X, Li Q, Xu N, Zhang M et al. Serum levels of preoperative alpha-fetoprotein and CA19-9 predict survival of hepatic carcinoma patients after liver transplantation. European journal of gastroenterology & hepatology. 2014; 26(5):553–61. doi:10.1097/MEG.0000000000000070.
Zhang J, Huang T, Zhang F, Xu J, Chen G, Wang X et al. Prognostic role of serum carbohydrate antigen 19-9 levels in patients with resectable hepatocellular carcinoma. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2015; 36(4):2257–61. doi:10.1007/s13277-014-2435-6.
Mann DV, Edwards R, Ho S, Lau WY, Glazer G. Elevated tumour marker CA19-9: clinical interpretation and influence of obstructive jaundice. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2000; 26(5):474–9. doi:10.1053/ejso.1999.0925.
Schlieman MG, Ho HS, Bold RJ. Utility of tumor markers in determining resectability of pancreatic cancer. Archives of surgery. 2003; 138(9):951–5; discussion 5-6. doi:10.1001/archsurg.138.9.951.
Zhang YF, Zhou J, Wei W, Zou RH, Chen MS, Lau WY et al. Intermediate-stage hepatocellular carcinoma treated with hepatic resection: the NSP score as an aid to decision-making. British journal of cancer. 2016;115(9):1039–47. doi:10.1038/bjc. 2016.301.Zhang YF and Zhou J, shared co-first authorship.
Shi M, Guo RP, Lin XJ, Zhang YQ, Chen MS, Zhang CQ et al. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Annals of surgery. 2007; 245(1):36–43. doi:10.1097/01.sla.0000231758.07868.71.
Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clinical cancer research: an official journal of the American Association for Cancer Research. 2004; 10 (21):7252–9. doi:10.1158/1078-0432.CCR-04-0713.
Tandon P, Garcia-Tsao G. Prognostic indicators in hepatocellular carcinoma: a systematic review of 72 studies. Liver international: official journal of the International Association for the Study of the Liver. 2009; 29(4):502–10. doi:10.1111/j.1478-3231.2008.01957.x.
Fabris C, Falleti E, Pirisi M, Soardo G, Toniutto P, Vitulli D et al. Non-specific increase of serum carbohydrate antigen 19-9 in patients with liver disease associated with increased circulating levels of adhesion molecules. Clinica chimica acta; international journal of clinical chemistry. 1995; 243(1):25–33.
Ong SL, Sachdeva A, Garcea G, Gravante G, Metcalfe MS, Lloyd DM et al. Elevation of carbohydrate antigen 19.9 in benign hepatobiliary conditions and its correlation with serum bilirubin concentration. Digestive diseases and sciences. 2008; 53 (12):3213–7. doi:10.1007/s10620-008-0289-8.
Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016; 388 (10039):73–85. doi:10.1016/S0140-6736(16)00141-0.
Lee HS, Lee SH, Roh YH, Chung MJ, Park JY, Park SW et al. Efficacy of adjuvant chemotherapy and prognostic factors for patients with extrahepatic bile duct cancer. Chemotherapy. 2016; 61(3):152–8. doi:10.1159/000441377.
Kassahun WT, Hauss J. Management of combined hepatocellular and cholangiocarcinoma. International journal of clinical practice. 2008; 62(8):1271–8. doi:10.1111/j.1742-1241.2007.01694.x.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (No. 81172037/H1606) and the Guangdong Province Science and Technology Project of China (No. 2013B021800159). We are also grateful to and thank all the patients who participated in this study.
Author information
Authors and Affiliations
Contributions
Guo RP and Lu LH designed the study; Lu LH and Zhan YF collected and analyzed the data, performed the statistical analysis, and wrote the manuscript (Lu LH and Zhan YF made equal contributions); and Guo RP, Wei W, and Shi M analyzed the data and contributed to the discussion and revision.
Corresponding author
Ethics declarations
Financial Support
This study was supported by grants from the Guangdong Province Science and Technology Project of China (No. 2013B021800159).
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Lu, LH., Zhang, YF., Wei, W. et al. Preoperative Carbohydrate Antigen 19-9: Its Neglected Role in Alpha-Fetoprotein-Negative Hepatocellular Carcinoma Patients. J Gastrointest Surg 21, 2025–2032 (2017). https://doi.org/10.1007/s11605-017-3528-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-017-3528-5