Abstract
Background
Elevated serum saturated fatty acid levels and hepatocyte lipoapoptosis are features of nonalcoholic fatty liver disease (NAFLD).
Aim
The purpose of this study was to investigate saturated fatty acid induction of lipoapoptosis in human liver cells and the underlying mechanisms.
Methods
Human liver L02 and HepG2 cells were treated with sodium palmitate, a saturated fatty acid, for up to 48 h with or without lithium chloride, a glycogen synthase kinase-3β (GSK-3β) inhibitor, or GSK-3β shRNA transfection. Transmission electron microscopy was used to detect morphological changes, flow cytometry was used to detect apoptosis, a colorimetric assay was used to detect caspase-3 activity, and western blot analysis was used to detect protein expression.
Results
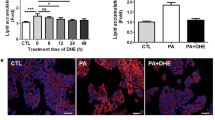
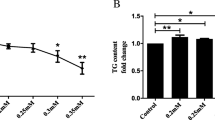
The data showed that sodium palmitate was able to induce lipoapoptosis in L02 and HepG2 cells. Western blot analysis showed that sodium palmitate activated GSK-3β protein, which was indicated by dephosphorylation of GSK-3β at Ser-9. However, inhibition of GSK-3β activity with lithium chloride treatment or knockdown of GSK-3β expression with shRNA suppressed sodium palmitate-induced lipoapoptosis in L02 and HepG2 cells. On a molecular level, inhibition of GSK-3β expression or activity suppressed sodium palmitate-induced c-Jun-N-terminal kinase (JNK) phosphorylation and Bax upregulation, whereas GSK-3β inhibition did not affect endoplasmic reticulum stress-induced activation of unfolded protein response.
Conclusions
The present data demonstrated that saturated fatty acid sodium palmitate-induced lipoapoptosis in human liver L02 and HepG2 cells was regulated by GSK-3β activation, which led to JNK activation and Bax upregulation. This finding indicates that GSK-3β inhibition may be a potential therapeutic target to control NAFLD.






Similar content being viewed by others
Abbreviations
- NAFLD:
-
Nonalcoholic fatty liver disease
- NASH:
-
Nonalcoholic steatohepatitis
- FFA:
-
Free fatty acid
- ER:
-
Endoplasmic reticulum
- UPR:
-
Unfolded protein response
- PERK:
-
Protein kinase RNA-like ER kinase
- IRE1:
-
Inositol-requiring protein 1
- GRP78:
-
Glucose-regulated protein 78
- GSK-3:
-
Glycogen synthase kinase-3
- JNK:
-
c-Jun-N-terminal kinase
- Bax:
-
Bcl-2-associated X protein
- BSA:
-
Bovine serum albumin
- 7-AAD:
-
7-amino actinomycin D
- ATF6:
-
Activating transcription factor 6
- ATF4:
-
Activating transcription factor 4
- CHOP:
-
C/EBP-homologous protein
- XBP-1:
-
X-box-binding protein 1
- shRNA:
-
Short hairpin RNA
- PI3K:
-
Phosphatidylinositide-3-OH kinase
References
Parekh S, Anania FA. Abnormal lipid and glucose metabolism in obesity: implications for nonalcoholic fatty liver disease. Gastroenterology. 2007;132:2191–2207.
Targher G, Marra F, Marchesini G. Increased risk of cardiovascular disease in nonalcoholic fatty liver disease: causal effect or epiphenomenon? Diabetologia. 2008;51:1947–1953.
Adams LA, Lymp JF. St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121.
Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231.
Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. Clin Invest. 2005;115:1343–1351.
Diraison F, Moulin P, Beylot M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab. 2003;29:478–485.
Kusminski CM, Shetty S, Orcil L, Unger RH, Scherer PE. Diabetes and apoptosis: lipotoxicity. Apoptosis. 2009;14:1484–1495.
Unger RH, Orci L. Lipoapoptosis: its mechanism and its diseases. Biochim Biophys Acta. 2002;1585:202–212.
Wei Y, Wang D, Pagliassotti MJ. Saturated fatty acid mediated endoplasmic reticulum stress and apoptosis are augmented by trans-10, cis-12-conjugated linoleic acid in liver cells. Mol Cell Biochem. 2007;3031:105–113.
Pfaffenbach KT, Gentile CL, Nivala AM, Wang D, Wei Y, Pagliassotti MJ. Linking endoplasmic reticulum stress to cell death in hepatocytes: roles of C/EBP homologous protein and chemical chaperones in palmitate-mediated cell death. Am J Physiol Endocrinol Metab. 2010;298:E1027–E1035.
Wang D, Wei Y, Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology. 2006;147:943–951.
Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529.
Cheng J, Kaplowitz N. ER stress: can the liver cope? Hepatol. 2006;45:321–333.
Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–190.
Kaplowitz N, Than TA, Shinohara M, Ji C. Endoplasmic reticulum stress and liver injury. Semin Liver Dis. 2007;27:367–377.
Puri P, Mirshahi P, Cheung P, et al. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology. 2008;134:568–576.
Cao J, Dai DL, Yao L, et al. Saturated fatty acid induction of endoplasmic reticulum stress and apoptosis in human liver cells via the PERK/ATF4/CHOP signaling pathway. Mol Cell Biochem. 2012;364:115–129.
Eldar-Finkelman H. Glycogen synthase kinase 3: an emerging therapeutic target. Trends Mol Med. 2002;8:126–132.
Jope RS, Yuskaitis CJ, Beurel E. Glycogen synthase kinase-3 (GSK-3): inflammation, diseases, and therapeutics. Neurochem Res. 2007;32:577–595.
Wojtaszewski JF, Nielsen P, Kiens B, Richter EA. Regulation of glycogen synthase kinase-3 in human skeletal muscle: effects of food intake and bicycle exercise. Diabetes. 2001;50:265–269.
Markuns JF, Wojtaszewski JF, Goodyear LJ. Insulin and exercise decrease glycogen synthase kinase-3 activity by different mechanisms in rat skeletal muscle. J Biol Chem. 1999;274:24896–24900.
Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositon 3-kinase/Akt cell survival pathway. J Biol Chem. 1998;273:19929–19932.
Takadera T, Fujibayashi M, Kaniyu H, Sakota N, Ohyashiki T. Caspase-dependent apoptosis induced by thapsigargin was prevented by glycogen synthase kinase-3 inhibitors in cultured rat cortical neurons. Neurochem Res. 2007;32:1336–1342.
Linseman DA, Butts BD, Precht TA, et al. Glycogen synthase kinase-3beta phosphorylates Bax and promotes its mitochondrial localization during neuronal apoptosis. J Neurosci. 2004;24:9993–10002.
Shinohara M, Ybanez MD, Win S, et al. Silencing glycogen synthase kinase-3beta inhibits acetaminophen hepatotoxicity and attenuates JNK activation and loss of glutamate cysteine ligase and myeloid cell leukemia sequence 1. J Biol Chem. 2010;285:8244–8255.
Nehra V, Angulo P, Buchman AL, Lindor KD. Nutritional and metabolic considerations in the etiology of nonalcoholic steatohepatitis. Dig Dis Sci. 2001;46:2347–2352.
Mu YM, Yanase TH, Nishi YH, et al. Saturated FFAs, palmitic acid and steaeic acid, induce apoptosis in human granulosa cells. Endocrinology. 2001;142:3590–3597.
Lu AH, Mu YM, Li BA, et al. Saturated free fatty acids, palmitic acid and stearic acid, induce apoptosis by stimulation of ceramide generation in rat testicular Leydig cell. Biochem Biophys Res Commun. 2003;303:1002–1007.
Ji J, Zhang L, Wang P, et al. Saturated free fatty acid, palmitic acid, induces apoptosis in fetal hepatocytes in culture. Exp Toxicol Pathol. 2005;56:369–376.
Li Q, Liu Z, Guo J, et al. Cholesterol overloading leads to hepatic L02 cell damage through activation of the unfolded protein response. Int J Mol Med. 2009;24:459–464.
Yu HH, Wu JF, Yang M, et al. Involvement of liver X receptor alpha in histone modifications across the target fatty acid synthase gene. Lipids. 2012;47:249–257.
Meijer L, Flajolet M, Greengard P. Pharmacological inhibitors of glycogen synthase kinase 3. Trends Pharmacol Sci. 2004;25:471–480.
Yun SI, Yoon HY, Chung YS. Glycogen synthase kinase-3β regulates etoposide-induced apoptosis via Bcl-2 mediated caspase-3 activation in C3H10T1/2 cells. Apoptosis. 2009;14:771–777.
Kim AJ, Shi Y, Austin RC, Werstuck GH. Valproate protects cells from ER stress-induced lipid accumulation and apoptosis by inhibiting glycogen synthase kinase-3. J Cell Sci. 2005;118:89–99.
Huang WC, Lin YS, Chen CL, Wang CY, Chiu WH, Lin CF. Glycogen synthase kinase-3β mediates endoplasmic reticulum stress-induced lysosomal apoptosis in leukemia. J Pharmacolm Exp Ther. 2009;329:524–531.
Johnson GL, Nakamura K. The c-jun kinase/stress-activated pathway: regulation, function and role in human disease. Biochim Biophys Acta. 2007;1773:1341–1348.
Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336.
Schattenberg JM, Singh R, Wang Y, et al. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology. 2006;43:163–172.
Singh R, Wang Y, Xiang Y, Tanaka KE, Gaarde WA, Czaja MJ. Differential effects of JNK1 and JNK2 inhibition on murine steatohepatitis and insulin resistance. Hepatology. 2009;49:87–96.
Cazanave SC, Mott JL, Elmi NA, et al. JNK1-dependent PUMA expression contributes to hepatocyte lipoapoptosis. J Biol Chem. 2009;284:26591–26602.
Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem. 2006;281:12093–12101.
Mishra R, Barthwal MK, Sondarva G, et al. Glycogen synthase kinase-3beta induces neuronal cell death via direct phosphorylation of mixed lineage kinase 3. J Biol Chem. 2007;282:30393–30405.
Kim JW, Lee JE, Kim MJ, Cho EG, Cho SG, Choi EJ. Glycogen synthase kinase 3 beta is a natural activator of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase kinase 1 (MEKK1). J Biol Chem. 2003;278:13995–14001.
Kim BJ, Ryu SW, Song BJ. JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J Biol Chem. 2006;281:21256–21265.
Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27:5621–6245.
Malhi H, Gores GJ. Cellular and molecular mechanisms of liver injury. Gastroenterology. 2008;134:1641–1654.
Song L, De Sarno P, Jope RS. Central role of glycogen synthase kinase-3β in endoplasmic reticulum stress-induced caspase-3 activation. Biol Chem. 2002;277:44701–44708.
Hiroi T, Wei H, Hough C, Leeds P, Chuang DM. Protracted lithium treatment protects against the ER stress elicited by thapsigargin in rat PC12 cells: roles of intracellular calcium, GRP78 and Bcl-2. Phamacogenomics. 2005;5:102–111.
Meares GP, Mines MA, Beulel E, et al. Glycogen synthase kinase-3 regulates endoplasmic reticulum (ER) stress-induced CHOP expression in neuronal cells. Exp Cell Res. 2011;317:1621–1628.
Hosoia T, Hyoda K, Okuma Y, Nomura Y, Ozawa K. Akt up- and down-regulation in response to endoplasmic reticulum stress. Brain Res. 2007;1152:27–31.
Acknowledgments
We sincerely thank the staff of the Department of Gastroenterology and Hepatology at the Second Affiliated Hospital of Chongqing Medical University for their constant and unselfish help. We thank Ms. Yingxia Xiang and Mr. Jing Wang for their consistent and illuminating technical assistance. We also thank Medjaden Bioscience, Hong Kong, China for assistance in preparation of this manuscript. This study was supported in part by grants from the Natural Science Foundation of China (#81270494 and #30871160) and the Project of Medical Science and Technology of Chongqing (#2012-1-033).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cao, J., Feng, XX., Yao, L. et al. Saturated Free Fatty Acid Sodium Palmitate-Induced Lipoapoptosis by Targeting Glycogen Synthase Kinase-3β Activation in Human Liver Cells. Dig Dis Sci 59, 346–357 (2014). https://doi.org/10.1007/s10620-013-2896-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-013-2896-2