Abstract
Fatty liver disease is caused by lipid accumulation in the liver, insulin resistance (IR), reactive oxygen species (ROS), and endoplasmic reticulum (ER) stress. Dehydrocostus lactone (DHE) has anticancer, anti-inflammatory, and anti-ulcer effects. However, its effects on hepatic steatosis and IR remain unclear. In this study, we investigated whether DHE has antisteatotic effect on fatty liver in vitro. Hepatocytes HepG2 and SNU-449 cells were exposed to 0.25 mM palmitate (PA), and then antisteatotic effect was evaluated by treatment with 10 μM DHE. DHE treatment reduced lipid accumulation and lipogenesis factor protein levels, compared with PA-treated hepatocytes. DHE treatment also decreased gluconeogenesis marker expression and recovered IR in PA-treated hepatocytes, and promoted glucose uptake in PA-treated HepG2 cells. Additionally, the levels of ROS and ER stress factors in PA-treated HepG2 cells were reduced by DHE treatment, compared with PA-treated HepG2 cells. Overall, DHE decreased lipid accumulation and lipogenesis factors as well as recovered IR, gluconeogenesis, and glucose uptake by reducing ER stress and ROS levels in PA-treated hepatocytes. Thus, DHE is a potential antisteatotic agent.
Similar content being viewed by others
Introduction
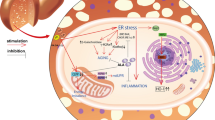
The incidence of non-alcoholic fatty liver disease (NAFLD), a chronic liver disease, is increasing worldwide, and NAFLD occurs in 45–75% of patients with type 2 diabetes (T2D) (Masarone et al. 2014; Lonardo 2015). NAFLD can present as simple steatosis to the more aggressive and serious non-alcoholic steatohepatitis. Excess lipid accumulation in the liver, which is strongly related to hepatic insulin resistance (IR), is essential in the pathogenesis of NAFLD (Marusic et al. 2021). Hepatic lipid accumulation occurs because of the absorption and synthesis of excess free fatty acids (FFAs), which exceeds the oxidation of FFAs. This abnormal FFA metabolism is caused by excess dietary FFAs as well as synthesis of adipocytes (Taylor et al. 2019). Excessive fat accumulation in the liver weakens insulin responsiveness, resulting in IR and increased glucose production (Qiao et al. 2022). In addition, increased ectopic lipid accumulation in the liver leads to hepatic endoplasmic reticulum (ER) and oxidative stress. With the activation of the unfolded protein response by free fatty acids, hepatic ER stress triggers IR and apoptosis (Kallen et al. 2009).
Saussurea lappa is known to have anticancer, anti-inflammatory, anti-ulcer, and anti-obesity effects, and is used as an herbal medicine in Asian countries including Korea, India, and China (Wang et al. 2020; Yu et al. 2007). Dehydrocostus lactone (DHE) is a single compound isolated from S. lappa and is known to have anti-inflammatory, anti-ulcer, and antiviral effects (Kim and Choi 2019; Yuan et al. 2022; Zheng et al. 2016). A recent study has reported that DHE attenuates osteoclastogenesis via the downregulation of the IκB kinase (IKK)/c-Jun N-terminal kinase (JNK) signaling pathways (Lee et al. 2020). Moreover, the extract of S. lappa, which includes DHE, decreases adipogenesis and lipid accumulation in 3T3-L1 cells, resulting in a therapeutic effect against obesity (Karamfilova et al. 2022; Yoon et al. 2010). Although DHE has been shown to have various therapeutic effects, its antisteatotic effects are not known. In this study, we aimed to investigate whether DHE has an antisteatotic effect and recover IR in palmitate (PA)-treated hepatocytes.
Methods
Cell culture and treatment
Hepatocytes, HepG2 and SNU-449 cells, were purchased from the Korean Cell Line Bank (KOREA) and grown in high-glucose Dulbecco’s modified Eagle medium (Hyclone, San Angelo, TX, USA) with 10% fetal bovine serum (Gibco, Thermo Fisher Scientific, MA, USA) and 1% penicillin/streptomycin (Hyclone) at 37 °C in a humidified atmosphere of 5% CO2. The cells were transferred to the other cell culture dish every 3 days and maintained.
Cell viability
For the cell viability assay, HepG2 cells were seeded at 4 × 103 cells in a 96-well plate and incubated at 37 °C in CO2 for 24 h. The cells were treated with 0.25 mM PA as well as with different concentrations of DHE (0, 1, 5, 10, 15, 20, and 40 μΜ) and incubated at 37 °C with CO2. After 24 h, Cell Counting Kit-8 (CCK-8) (Dojindo Molecular Technologies, Inc., Rockville, MD, USA) was added to the cells in a 96-well plate and incubated for 1 h. The treated cells were evaluated for cell viability using SpectroMax M4 (Molecular Devices, San Jose, USA) at an optical density of 450 nm.
Nile red staining
HepG2 cells were seeded at 1 × 104 cells in a 96-well plate and incubated at 37 °C with CO2 for 24 h. To measure the effective concentration of DHE, HepG2 cells were treated with 0.25 mM PA or PA and various concentrations of DHE (0, 1, 5, and 10 μΜ), and then incubated at 37 °C with CO2 for a further 24 h. In addition, to confirm the treatment time of DHE, HepG2 cells were treated with 0.25 mM PA or PA and 10 μΜ DHE, and then incubated at 37 °C with CO2 for 0, 6, 12, 24, and 48 h.
For the assay of lipid accumulation in PA-treated HepG2 and SNU-449 cells, the cells were seeded at 1 × 104 cells in a 96-well plate and incubated at 37 °C with CO2 for 24 h. The seeded cells were treated with 0.25 mM PA or 0.25 mM PA and 10 μΜ DHE and incubated at 37 °C with CO2 for 24 h.
The treated hepatocytes were fixed with 4% paraformaldehyde fixation solution (Thermo Fisher Scientific) for 15 min at 25 °C. The fixed cells were washed twice with phosphate-buffered saline (PBS) and incubated with Nile Red dye (1 μg/mL) (Invitrogen™, MA, USA) at 37 °C for 10 min in the dark. The cells were visualized using the Opera Quadruple Enhanced High Sensitivity (QEHS) microscope (PerkinElmer, MA, USA) with water immersion objective lens at 20×, and the relative intensity was quantified using Columbus software (PerkinElmer).
Glucose uptake
The Glucose Uptake Cell-Based Assay Kit (Cayman, Ann Arbor, MI, USA) was used to evaluate glucose uptake, following the manufacturer’s protocol. The treated HepG2 cells were incubated with 100 μg/mL 2-NBDG (a fluorescent glucose analog) mixed with glucose-free buffer at 37 °C for 30 min in the dark. The cells were washed once with the cell-based assay buffer, and the final cell-based assay buffer was added to each well. The intensity of glucose uptake was measured using SpectroMax M4.
ROS production
For the analysis of ROS production, the treated HepG2 cells were washed twice with PBS and replaced with PBS containing the cell-permeant 2,7-dichlorodihydrofluorescein diacetate (Thermo Fisher Scientific). The cells were visualized using the Opera QEHS microscope with water immersion objective lens at 20×, and the relative intensity was quantified using Columbus software.
Western blotting
Proteins were isolated from the PA-treated hepatocytes (HepG2 and SNU-449) with/without DHE in RIPA lysis and extraction buffer (ThermoFisher Scientific) containing a proteinase inhibitor cocktail (Thermo Fisher Scientific) and phosphatase inhibitor cocktail (GenDEPOT, TX, USA). The protein concentration was measured using a BCA protein assay kit (ThermoFisher Scientific). The proteins were loaded in SDS-PAGE gels and a western blot analysis was performed as described previously (Baldwin 1996). The following antibodies were used: anti-actin (sc7210, Santa Cruz Biotechnology, CA, USA); lipogenesis factors: anti-fatty acid synthase (FAS) (sc20140, Santa Cruz Biotechnology), anti-stearoyl-CoA desaturase-1 (SCD1) (sc14719, Santa Cruz Biotechnology), anti-lipin1 (sc98450, Santa Cruz Biotechnology), and anti-diacylglycerol O-Acyltransferase 2 (DGAT2) (ab102831, Abcam, MA, USA); IR factors: anti-p-insulin receptor substrate 1 (p-IRS1) (sc33956 (S307), Santa Cruz Biotechnology), anti-IRS1 (sc559, Santa Cruz Biotechnology), anti-p-protein kinase B (p-AKT) (4060 (S473), Cell Signaling Technology), and anti-AKT (9272, Cell Signaling Technology); gluconeogenesis markers: anti-glucose-6-phosphatase (G6Pase) (sc25840, Santa Cruz Biotechnology), anti-phosphoenolpyruvate carboxykinase (PEPCK) (sc32879, Santa Cruz Biotechnology), and anti-peroxisome proliferator-activated receptor-gamma coactivator-1α (PGC1α) (2178 s, Cell Signaling Technology, MA, USA); ER stress markers: anti-binding immunoglobulin protein, (Bip) (3177, Cell Signaling Technology), anti-activating transcription factor 6 (ATF6) (65880, Cell Signaling Technology), anti-inositol-requiring transmembrane kinase/endoribonuclease 1α (IRE1α) (3294, Cell Signaling Technology), anti-protein kinase R-like endoplasmic reticulum kinase (PERK) (3191 s, Cell signaling), anti-p-eukaryotic initiation factor 2α (p-eIF2α) (3597 (S51), Cell Signaling Technology), anti-eIF2α (Cell Signaling Technology), anti-p-c-Jun N-terminal kinases (p-JNK) (9251 s (T1183/Y185) Cell Signaling Technology), and anti-JNK (9252, Cell Signaling Technology). The band intensity was evaluated using ImageJ software (Schneider et al. 2012).
Statistical analysis
All data are presented as mean ± standard deviation (SD). Statistical analysis was performed using a one-way ANOVA with the GraphPad Prism 5 analysis package (GraphPad, CA, USA). Statistical significance was set at p < 0.05. All data were obtained from at least three independent experiments.
Results
DHE decreases lipid accumulation and lipogenesis factors in PA-treated HepG2 cells
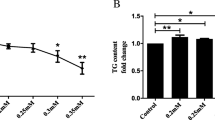
We treated HepG2 cells with PA, one of the FFAs, to generate an in vitro model with steatosis features (Meng et al. 2017; Xiao et al. 2019). First, to evaluate cytotoxicity caused by DHE, the PA-treated HepG2 cells were treated with DHE at different doses, and then cell viability was measured. DHE exerted cytotoxicity of DHE at concentrations in the range of 15–40 μM (Fig. 1a). We measured lipid accumulation in PA-treated HepG2 cells after DHE treatment at each concentration and time. Lipid accumulation significantly reduced when PA-treated HepG2 cells were treated with 10 μM DHE for 24 h (Fig. 1b, c). Based on these results, 10 μM DHE, which did not exert cytotoxicity and reduce lipid accumulation, was used in the subsequent experiments to study the therapeutic efficacy of DHE in fatty liver disease.
DHE is not cytotoxic and decreases lipid accumulation and lipogenesis factors in PA-treated HepG2 cells. a HepG2 cells were treated with 0.25 mM PA and different concentrations of DHE (0, 1, 5, 10, 15, 20, and 40 μΜ) for 24 h, and then cell viability was analyzed using Cell Counting Kit-8. ***p < 0.001, **p < 0.01, *p < 0.05 compared with 0 μM DHE. n = 9. b HepG2 cells were treated with 0.25 mM PA and 0, 1, 5, 10 μΜ DHE for 24 h, and then lipid accumulation was evaluated using Nile Red staining. c HepG2 cells were treated with 0.25 mM PA and 10 μΜ DHE for 0, 1, 6, 12, 24, and 48 h, and lipid accumulation was examined using Nile Red staining. d HepG2 cells were treated with 0.25 mM PA or with 0.25 mM PA and 10 μM DHE for 24 h, and were stained using Nile Red. The stained cells were analyzed using Columbus software. e Lipid accumulation was detected using Nile Red staining (Scalebar = 50 μm). f The protein expression of lipogenesis factors, including FAS, SCD1, Lipin1, and DGAT2, was confirmed in treated HepG2 cells using western blotting. g Relative protein levels of lipogenesis factors were assessed using the Image J program. All data are indicated as mean ± SD of at least three independent experiments. ***p < 0.001, **p < 0.01, *p < 0.05. n = 3–4. CTL; control
We investigated whether DHE affects lipid accumulation. Lipid accumulation increased in PA-treated HepG2 cells, but was significantly decreased by DHE treatment (Fig. 1d, e). To examine the lipogenesis factors related to lipid accumulation, these factors were evaluated in PA-treated HepG2 cells with/without DHE. Lipogenesis factors including FAS, SCD1, Lipin1 and DGAT2 were downregulated in PA-treated HepG2 cells with DHE compared with PA-treated HepG2 cells without DHE (Fig. 1f, g). These results indicate that the low concentration of DHE had no cytotoxicity, and that DHE’s effect on PA-induced lipid accumulation was accompanied with the reduction in lipogenesis factors in PA-treated HepG2 cells.
DHE recovers IR and glucose metabolism in PA-treated HepG2 cells
Insulin promotes glucose uptake from the blood to the liver through the insulin signaling pathway. However, insulin does not function properly owing to IR, resulting in hyperglycemia due to the impaired suppression of glucose production and the reduction in hepatic glucose uptake (Jiang et al. 2020). We examined whether DHE regulates IR and glucose metabolism in PA-treated HepG2 cells. DHE treatment increased the phosphorylated AKT (Ser473) protein level in PA-treated HepG2 cells, whereas it decreased the phosphorylated IRS1 (Ser307) level compared with those in just PA-treated HepG2 cells, resulting in improved IR (Fig. 2a, b). Gluconeogenesis markers such as G6Pase, PEPCK, and PGC1α, were downregulated by DHE treatment in PA-treated HepG2 cells compared with those in PA-treated HepG2 cells without DHE (Fig. 2c, d). Moreover, glucose uptake was promoted by DHE treatment compared with that in PA-treated HepG2 cells without DHE (Fig. 2e). These findings show that DHE recovers the protein levels of IR factors and gluconeogenesis markers as well as glucose uptake in PA-treated HepG2 cells.
DHE influences insulin resistance and glucose metabolism in PA-treated HepG2 cells. HepG2 cells were treated with 0.25 mM PA or with 0.25 mM PA and 10 μΜ DHE for 24 h, and then the cells were harvested and protein was isolated. a The insulin resistance factors, including p-AKT (S473) and p-IRS1 (S307), were evaluated using western blotting. b Relative protein levels of insulin resistance factors were assessed using the Image J program. Each value was normalized to AKT or IRS1. c The gluconeogenesis markers, such as G6Pase, PEPCK, and PGC1α, were examined using western blotting. d Relative protein levels of gluconeogenesis markers were assessed using the Image J program. Values were normalized to actin. e. Glucose uptake in the treated cells was assessed using a glucose uptake cell-based assay kit. All data were indicated as mean ± SD of at least three independent experiments. ***p < 0.001, **p < 0.01, *p < 0.05. n = 3–4. CTL; control
DHE suppresses ROS levels and ER stress in PA-treated HepG2 cells
Studies have reported that PA increases ER stress and ROS levels in HepG2 cells (Gao et al. 2010; Tang et al. 2015). We examined whether DHE regulates ROS levels in PA-treated HepG2 cells with or without DHE using an ROS assay kit. DHE treatment significantly suppressed ROS levels in PA-treated HepG2 cells compared with those in PA-treated HepG2 cells without DHE treatment (Fig. 3a, b). Moreover, we evaluated ER stress markers such as PERK, IRE1α, ATF6, Bip, phosphorylated eIF2α (Ser51), and phosphorylated JNK (Tyr1183/Y185) in PA-treated HepG2 hepatocytes with/without DHE. DHE treatment suppressed ER stress marker levels in PA-treated HepG2 cells compared with that in PA-treated HepG2 cells without DHE (Fig. 3c, d). These results indicate that DHE decreased ER stress and ROS levels in PA-treated HepG2 cells.
DHE decreases ROS levels and protein levels of ER stress markers in PA-treated HepG2 cells. a HepG2 cells were treated with 0.25 mM PA or with 0.25 mM PA and 10 μM DHE for 24 h, and ROS levels were assessed (Scale bar = 50 μm). b ROS levels were evaluated in the treated cells using SpectroMax M4. c The treated HepG2 cells were assessed for the protein expression of ER stress markers (PERK, IRE1α, ATF6, Bip, p-JNK(T1183/Y185), and p-eIF2α (S51)) using western blotting. d Relative protein expression levels of ER stress markers were assessed using the Image J program. Values were normalized to β-actin, JNK, or eIF2α. All data are indicated as mean ± SD of at least three independent experiments. ***p < 0.001, **p < 0.01, *p < 0.05. n = 3–5. CTL; control
DHE decreases lipid accumulation, and recuperates IR and glucose metabolism in PA-treated SNU-449 cells
We investigated whether DHE also has antisteatotic effects on SNU-449 cells, one of human hepatocyte. Previous studies have reported that PA induces steatosis in SNU-449 cells (Sullivan et al. 2022). We measured lipid accumulation in PA- or PA and DHE-treated SNU-449 cells using Nile Red staining. DHE treatment significantly decreased lipid accumulation in PA-treated SNU-449 cells (Fig. 4a, b). The increased lipogenesis factors in PA-treated SNU-449 cells were decreased by DHE treatment, compared with those in PA-treated SNU-449 cells (Fig. 4c, d). Besides, DHE treatment restored IR factors (Fig. 4e, f), and consequently decreased gluconeogenesis markers in PA-treated SNU-449 cells (Fig. 4g, h). These findings indicate that DHE decreases lipid accumulation and protein levels of lipogenesis factors as well as recovers IR and glucose metabolism in PA-treated SNU-449 cells.
DHE decreases lipid accumulation and restores IR and glucose metabolism in PA-treated SNU-449 cells. SNU-449 cells were treated with 0.25 mM PA or 0.25 mM PA and 10 μΜ DHE for 24 h. a Lipid accumulation was confirmed in the treated SNU-449 using Nile Red staining (Scale bar = 50 μm). b The stained cells were analyzed using Columbus software. c The treated cells were harvested and protein was isolated, and then the lipogenesis factors, including FAS, SCD1, Lipin1, and DGAT2, were measured using western blotting. d Relative protein levels of lipogenesis factors were assessed using the Image J program. Values were normalized to actin. e The insulin resistance factors, including p-AKT (S473) and p-IRS1 (S307), were evaluated using western blotting. f Relative protein levels of insulin resistance factors were assessed using the Image J program. Each value was normalized to AKT or IRS1. g The gluconeogenesis markers, such as G6Pase, PEPCK, and PGC1α, were examined using western blotting. h Relative protein levels of gluconeogenesis markers were assessed using the Image J program. Values were normalized to actin. All data are indicated as mean ± SD of at least three independent experiments. ***p < 0.001, **p < 0.01, *p < 0.05. n = 3–5. CTL; control
Discussion
Natural products, as well as their structural analogs and natural product-based drugs, have historically made a great contribution to the treatment of various diseases (Atanasov et al. 2021). DHE is a natural product obtained from the roots of S. lappa, that has therapeutic effects against inflammatory and cancer diseases (Zhou et al. 2020; Sheng et al. 2018). High concentrations of DHE, such as 15 and 30 μM, are toxic to HepG2 cells owing to the inhibition of cell proliferation and promotion of apoptosis (Jeong et al. 2007). Consistent with these findings, our results also showed cytotoxicity of DHE at over 15 μM. Furthermore, our results indicated that 10 μM DHE was not cytotoxic but had an antisteatotic effect. Another study reported that a low concentration of DHE upregulated the viability of oxidative stress-induced HepG2 cells and increased cellular resistance as an inducer of cytoprotective HO-1 expression (Yadollah et al. 2017). Therefore, we suggest that a high concentration of DHE is cytotoxic owing to its anticancer effect on HepG2 cells, but that treatment with a low concentration of DHE is not toxic to PA-treated HepG2 cells and has antisteatotic effects.
NAFLD is currently one of the most common liver diseases, affecting up to 25% of the adult population (Tanase et al. 2020). Hepatic steatosis refers to a form in which more than 5% of the liver’s is accounted by lipids (Nassir et al. 2015), and it has been reported that DHE inhibits lipogenesis in adipocytes (Yoon et al. 2010). We expected that DHE would affect lipid accumulation and lipogenesis in hepatic steatosis. To induce fatty liver conditions, we treated HepG2 and SNU-449 cells with PA, a free fatty acid. PA-treated hepatocytes have been used in previous studies as a fatty liver model to simulate lipid accumulation and IR (Xiao et al. 2019; Gao et al. 2010; Liu et al. 2019; Zhang et al. 2019). As shown in Figs. 1d–g and 4a–d, DHE treatment decreased lipid accumulation and protein levels of lipogenesis factors in PA-induced steatotic hepatocytes.
Insulin is an important factor in regulating blood glucose and mediates hepatic glucose metabolism by suppressing the production of hepatic glucose and stimulating glucose uptake (Hatting et al. 2018). Insulin binds to the insulin receptor on the cell surface activates IRS1, intracellularly, which in turn activates PI3K and AKT to increase insulin sensitivity (Dong et al. 2006). In addition, the IRS1-AKT pathway is a major signaling pathway mediating the effects of insulin on anabolic metabolism and insulin-stimulated glucose uptake (Huang et al. 2018). These signaling pathways regulate gluconeogenesis genes such as G6Pase and PEPCK (Huang et al. 2018; Guo et al. 2009). However, under IR condition, insulin action becomes dysfunctional, gluconeogenesis increases, and glucose uptake decreases due to the weakening of insulin signaling, including IRS1 and AKT (Sangwung et al. 2020). Furthermore, FFAs inhibit insulin-stimulated glucose uptake (Boden et al. 1994; Homko et al. 2003). Our observations indicate that DHE recovered IR by activating IRS1/AKT signaling in PA-treated HepG2 and SNU-449 cells (Figs. 2a, b and 4e, f). Moreover, our results indicate that DHE downregulated gluconeogenesis markers in PA-treated HepG2 and SNU-449 cells (Figs. 2c, d and 4g, h) and promoted glucose uptake in PA-treated HepG2 cells (Fig. 2e). The effect of DHE on glucose uptake might result from decreased FA uptake and utilization.
ER stress has been reported to play an important role in both pathogenesis of steatosis and progression to NAFLD (Zhang et al. 2014). The various stress pathways by which ER stress activates IR and inflammation, or pathologically induces physiological mechanisms include fat accumulation, enhanced ROS production, inflammation, and apoptosis that play an important role in NAFLD (Sreejayan et al. 2008; Rahman et al. 2007). ROS production has been linked to ER stress and unfolded protein reaction, and redox homeostasis induces ER stress and can induce ROS production in the ER and mitochondria (Sangwung et al. 2020; Bhattarai et al. 2021). Moreover, the increased FFAs induce protein unfolding, and increased levels of unfolded proteins bind to PERK, IRE1α, and ATF6 in the ER lumen, consequently inducing ER stress and activating the JNK pathway (Kaneto et al. 2010). Therefore, we hypothesized that DHE affects the ROS and ER stress signaling pathways. Consistent with this hypothesis, our results indicated that DHE inhibited ROS levels and ER stress, including the JNK pathway (Fig. 3). We propose that DHE reduces lipid accumulation with a decrease in ER stress and ROS level, and influences the regulation of IR and glucose metabolism. Future studies are needed to determine whether DHE has the same therapeutic effect in animal models with fatty liver.
Conclusions
In conclusion, we used an in vitro model of fatty hepatocytes by treating two types of hepatocytes, HepG2 and SNU-449, with PA to measure the antisteatotic efficacy of DHE. Our results showed that DHE decreases lipid accumulation and recovers IR and glucose metabolism by reducing ER stress and ROS levels in PA-induced fatty hepatocytes. Furthermore, treatment with a low concentration of DHE had no cytotoxicity and exerted antisteatotic effects on PA-induced fatty hepatocytes. Therefore, we propose that DHE could be used as a treatment for hepatic steatosis.
Availability of data and materials
All data and materials are available on request.
Abbreviations
- DHE:
-
Dehydrocostus lactone
- ER:
-
Endoplasmic reticulum
- FFA:
-
Free fatty acid
- IR:
-
Insulin resistance
- NAFLD:
-
Non-alcoholic fatty liver disease
- PA:
-
Palmitate
- ROS:
-
Reactive oxygen species
- T2D:
-
Type 2 diabetes
- QEHS:
-
Quadruple enhanced high sensitivity
References
Atanasov AG, Zotchev SB, Dirsch VM, Supuran CT, International Natural Product Sciences. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov. 2021;20(3):200–16.
Baldwin AS Jr. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–83.
Bhattarai KR, Riaz TA, Kim HR, Chae HJ. The aftermath of the interplay between the endoplasmic reticulum stress response and redox signaling. Exp Mol Med. 2021;53(2):151–67.
Boden G, Chen X, Ruiz J, White JV, Rossetti L. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Investig. 1994;93(6):2438–46.
Dong X, Park S, Lin X, Copps K, Yi X, White MF. Irs1 and Irs2 signaling is essential for hepatic glucose homeostasis and systemic growth. J Clin Invest. 2006;116(1):101–14.
Gao D, Nong S, Huang X, Lu Y, Zhao H, Lin Y, et al. The effects of palmitate on hepatic insulin resistance are mediated by NADPH Oxidase 3-derived reactive oxygen species through JNK and p38MAPK pathways. J Biol Chem. 2010;285(39):29965–73.
Guo S, Copps KD, Dong X, Park S, Cheng Z, Pocai A, et al. The Irs1 branch of the insulin signaling cascade plays a dominant role in hepatic nutrient homeostasis. Mol Cell Biol. 2009;29(18):5070–83.
Hatting M, Tavares CDJ, Sharabi K, Rines AK, Puigserver P. Insulin regulation of gluconeogenesis. Ann N Y Acad Sci. 2018;1411(1):21–35.
Homko CJ, Cheung P, Boden G. Effects of free fatty acids on glucose uptake and utilization in healthy women. Diabetes. 2003;52(2):487–91.
Huang X, Liu G, Guo J, Su Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci. 2018;14(11):1483–96.
Jeong GS, Pae HO, Jeong SO, Kim YC, Kwon TO, Lee HS, et al. The alpha-methylene-gamma-butyrolactone moiety in dehydrocostus lactone is responsible for cytoprotective heme oxygenase-1 expression through activation of the nuclear factor E2-related factor 2 in HepG2 cells. Eur J Pharmacol. 2007;565(1–3):37–44.
Jiang S, Young JL, Wang K, Qian Y, Cai L. Diabeticinduced alterations in hepatic glucose and lipid metabolism: the role of type 1 and type 2 diabetes mellitus (Review). Mol Med Rep. 2020;22(2):603–11.
Kaneto H, Katakami N, Matsuhisa M, Matsuoka T. Role of reactive oxygen species in the progression of Type 2 diabetes and atherosclerosis. Mediat Inflamm. 2010;2010:1.
Karamfilova V, Assyov Y, Nedeva I, Gateva A, Ivanova I, Cherkezov N, et al. Increased serum pentraxin 3 is associated with prediabetes and type 2 diabetes in obese patients with nonalcoholic fatty liver disease. Metab Syndr Relat Disord. 2022;20(2):132–6.
Kim DY, Choi BY. Costunolide-A bioactive sesquiterpene lactone with diverse therapeutic potential. Int J Mol Sci. 2019;20(12):2926.
Lee HI, Lee GR, Lee J, Kim N, Kwon M, Kim HJ, et al. Dehydrocostus lactone inhibits NFATc1 via regulation of IKK, JNK, and Nrf2, thereby attenuating osteoclastogenesis. BMB Rep. 2020;53(4):218–22.
Liu J-Y, Zhang Y-C, Song L-N, et al. Nifuroxazide ameliorates lipid and glucose metabolism in palmitate-induced HepG2 cells. RSC Adv. 2019;9(67):39394–404.
Lonardo A, Bellentani S, Argo CK, Ballestri S, Byrne CD, Non-alcoholic Fatty Liver Disease Study G, et al. Epidemiological modifiers of non-alcoholic fatty liver disease: focus on high-risk groups. Dig Liver Dis. 2015;47(12):997–1006.
Marusic M, Paic M, Knobloch M, Liberati Prso AM. NAFLD, insulin resistance, and diabetes mellitus Type 2. Can J Gastroenterol Hepatol. 2021;2021:6613827.
Masarone M, Federico A, Abenavoli L, Loguercio C, Persico M. Non alcoholic fatty liver: epidemiology and natural history. Rev Recent Clin Trials. 2014;9(3):126–33.
Meng XH, Chen B, Zhang JP. Intracellular insulin and impaired autophagy in a zebrafish model and a cell model of type 2 diabetes. Int J Biol Sci. 2017;13(8):985–95.
Nassir F, Rector RS, Hammoud GM, Ibdah JA. Pathogenesis and prevention of hepatic steatosis. Gastroenterol Hepatol. 2015;11(3):167–75.
Qiao L, Men L, Yu S, Yao J, Li Y, Wang M, et al. Hepatic deficiency of selenoprotein S exacerbates hepatic steatosis and insulin resistance. Cell Death Dis. 2022;13(3):275.
Rahman SM, Schroeder-Gloeckler JM, Janssen RC, Jiang H, Qadri I, Maclean KN, et al. CCAAT/enhancing binding protein beta deletion in mice attenuates inflammation, endoplasmic reticulum stress, and lipid accumulation in diet-induced nonalcoholic steatohepatitis. Hepatology. 2007;45(5):1108–17.
Sangwung P, Petersen KF, Shulman GI, Knowles JW. Mitochondrial dysfunction, insulin resistance, and potential genetic implications. Endocrinology. 2020. https://doi.org/10.1210/endocr/bqaa017.
Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–5.
Sheng W, Mao H, Wang C, Yang N, Zhang Z, Han J. Dehydrocostus lactone enhances chemotherapeutic potential of doxorubicin in lung cancer by inducing cell death and limiting metastasis. Med Sci Monit. 2018;24:7850–61.
Sreejayan N, Dong F, Kandadi MR, Yang X, Ren J. Chromium alleviates glucose intolerance, insulin resistance, and hepatic ER stress in obese mice. Obesity. 2008;16(6):1331–7.
Sullivan KE, Kumar S, Liu X, Zhang Y, de Koning E, Li Y, et al. Uncovering the roles of dihydropyrimidine dehydrogenase in fatty-acid induced steatosis using human cellular models. Sci Rep. 2022;12(1):14109.
Tanase DM, Gosav EM, Costea CF, Ciocoiu M, Lacatusu CM, Maranduca MA, et al. The intricate relationship between Type 2 diabetes mellitus (T2DM), insulin resistance (IR), and nonalcoholic fatty liver disease (NAFLD). J Diabetes Res. 2020;2020:3920196.
Tang Z, Zhang W, Wan C, Xu G, Nie X, Zhu X, et al. TRAM1 protect HepG2 cells from palmitate induced insulin resistance through ER stress-JNK pathway. Biochem Biophys Res Commun. 2015;457(4):578–84.
Taylor R, Al-Mrabeh A, Sattar N. Understanding the mechanisms of reversal of type 2 diabetes. Lancet Diabetes Endocrinol. 2019;7(9):726–36.
van der Kallen CJ, van Greevenbroek MM, Stehouwer CD, Schalkwijk CG. Endoplasmic reticulum stress-induced apoptosis in the development of diabetes: is there a role for adipose tissue and liver? Apoptosis. 2009;14(12):1424–34.
Wang Y, Fan XX, Yang J, Wang ZQ, Wang N, Chen JQ, et al. Research progress on terpenes and pharmacological effects of Saussurea lappa. Zhongguo Zhong Yao Za Zhi. 2020;45(24):5917–28.
Xiao Q, Zhang S, Yang C, Du R, Zhao J, Li J, et al. Ginsenoside Rg1 ameliorates palmitic acid-induced hepatic steatosis and inflammation in HepG2 Cells via the AMPK/NF-kappaB pathway. Int J Endocrinol. 2019;2019:7514802.
Yadollah S, Kazemipour N, Bakhtiyari S, Nazifi S. Palmitate-induced insulin resistance is attenuated by Pioglitazone and EGCG through reducing the gluconeogenic key enzymes expression in HepG2 cells. J Med Life. 2017;10(4):244–9.
Yoon TS, Sung YY, Jang JY, et al. Anti-obesity activity of extract from Saussurea lappa. Korean J Med Crop Sci. 2010;18(3):151–6.
Yu HH, Lee JS, Lee KH, Kim KY, You YO. Saussurea lappa inhibits the growth, acid production, adhesion, and water-insoluble glucan synthesis of Streptococcus mutans. J Ethnopharmacol. 2007;111(2):413–7.
Yuan Y, Hu Q, Liu L, Xie F, Yang L, Li Y, et al. Dehydrocostus lactone suppresses dextran sulfate sodium-induced colitis by targeting the IKKalpha/beta-NF-kappaB and Keap1-Nrf2 signalling pathways. Front Pharmacol. 2022;13:817596.
Zhang XQ, Xu CF, Yu CH, Chen WX, Li YM. Role of endoplasmic reticulum stress in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20(7):1768–76.
Zhang Q, Kong X, Yuan H, Guan H, Li Y, Niu Y. Mangiferin improved palmitate-induced-insulin resistance by promoting free fatty acid metabolism in HepG2 and C2C12 cells via PPARalpha: mangiferin improved insulin resistance. J Diabetes Res. 2019;2019:2052675.
Zheng H, Chen Y, Zhang J, Wang L, Jin Z, Huang H, et al. Evaluation of protective effects of costunolide and dehydrocostuslactone on ethanol-induced gastric ulcer in mice based on multi-pathway regulation. Chem Biol Interact. 2016;250:68–77.
Zhou Q, Zhang WX, He ZQ, Wu BS, Shen ZF, Shang HT, et al. The possible anti-inflammatory effect of dehydrocostus lactone on DSS-induced colitis in mice. Evid Based Complement Alternat Med. 2020;2020:5659738.
Acknowledgements
We thank you laboratory members for their help in the study. We would like to thank Editage (www.editage.co.kr) for English language editing.
Funding
This work was supported by grants from the Korea Basic Science Institute (Grant number C270300) and the National Research Foundation of Korea (Grant Number NRF 2021R1A2c1008663).
Author information
Authors and Affiliations
Contributions
YH, ML, and GK conceptualized and designed the study. YH, ML, and CK carried out the experiments. YH and ML drafted the manuscript. GK guided the research and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hong, Y., Lee, M., Kim, C. et al. Dehydrocostus lactone ameliorates lipid accumulation, insulin resistance, and endoplasmic reticulum stress in palmitate-treated hepatocytes. J Anal Sci Technol 13, 38 (2022). https://doi.org/10.1186/s40543-022-00349-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40543-022-00349-y