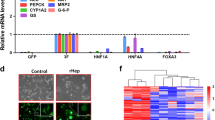
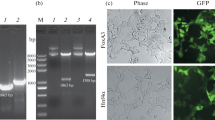
Abstract
Hepatoma cells are a promising cell source for the construction of bioartificial liver (BAL) systems owing to their high proliferative capability. However, their low liver function compared with primary hepatocytes is a major problem. In a previous study, we established a genetically modified hepatoma cell line, Hepa/8F5, in which eight liver-enriched transcription factor (LETF) genes were transduced into mouse hepatoma Hepa1-6 cells using a drug-inducible transactivator system. These cells proliferate actively under normal culture conditions, meaning that large quantities can be prepared easily. When the overexpression of the LETFs is induced by the addition of an inducer drug, cell growth stops and cell morphology changes with concomitant high expression of liver functions. However, the liver functions largely depend on the presence of the inducer drug, which must be continuously added to maintain these enhanced functions. In the present study, we attempted to modify the method of induction of LETF overexpression in Hepa/8F5 cells to remove the requirement for continual drug addition. To this end, we constructed a system in which the artificial transactivator was transcribed and amplified under the control of a heat-shock protein promoter, and introduced the system into the genome of Hepa/8F5 cells. In our modified cell line, heat-triggered LETF expression was confirmed to induce high liver function. After drug-screening of transfected cells, we established a hepatoma cell line (Hepa/HS), which exhibited high, heat-inducible liver functions. The Hepa/HS cells may represent a new cell source for hepatic studies such as the construction of BAL systems.





Similar content being viewed by others
References
Bou-Nader M, Caruso S, Donne R, Celton-Morizur S, Calderaro J, Gentric G, Cadoux M, L’Hermitte A, Klein C, Guilbert T, Albuquerque M, Couchy G, Paradis V, Couty JP, Zucman-Rossi J, Desdouets C (2020) Polyploidy spectrum: a new marker in HCC classification. Gut 69:355–364
Chamuleau RA, Deurholt T, Hoekstra R (2005) Which are the right cells to be used in a bioartificial liver? Metab Brain Dis 20:327–335
Cotto JJ, Kline M, Morimoto RI (1996) Activation of heat shock factor 1 DNA binding precedes stress-induced serine phosphorylation. Evidence for a multistep pathway of regulation. J Biol Chem 271:3355–3358
Fujii Y, Higashi K, Mizumoto H, Kamihira M, Kajiwara T (2020) A bioartificial liver device based on three-dimensional culture of genetically engineered hepatoma cells using hollow fibers. Cytotechnology 72:227–237
García Martínez JJ, Bendjelid K (2018) Artificial liver support systems: what is new over the last decade? Ann Intensive Care 8:109
Gómez-Lechón MJ, Tolosa L, Donato MT (2017) Upgrading HepG2 cells with adenoviral vectors that encode drug-metabolizing enzymes: application for drug hepatotoxicity testing. Expert Opin Drug Metab Toxicol 13:137–148
Gong Z, Yu J, Yang S, Lai PBS, Chen GG (2020) FOX transcription factor family in hepatocellular carcinoma. Biochim Biophys Acta Rev Cancer 1874:188376
Gossen M, Bujard H (1992) Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA 89:5547–5551
Hoekstra R, Nibourg GA, van der Hoeven TV, Ackermans MT, Hakvoort TB, van Gulik TM, Lamers WH, Elferink RP, Chamuleau RA (2011) The HepaRG cell line is suitable for bioartificial liver application. Int J Biochem Cell Biol 43:1483–1489
Ito A, Okamoto N, Yamaguchi M, Kawabe Y, Kamihira M (2012) Heat-inducible transgene expression with transcriptional amplification mediated by a transactivator. Int J Hyperth 28:788–798
Katsuda T, Kawamata M, Hagiwara K, Takahashi RU, Yamamoto Y, Camargo FD, Ochiya T (2017) Conversion of terminally committed hepatocytes to culturable bipotent progenitor cells with regenerative capacity. Cell Stem Cell 20:41–55
Lee KC, Stadlbauer V, Jalan R (2016) Extracorporeal liver support devices for listed patients. Liver Transpl 22:839–848
Messina A, Luce E, Hussein M, Dubart-Kupperschmitt A (2020) Pluripotent-stem-cell-derived hepatic cells: hepatocytes and organoids for liver therapy and regeneration. Cells 9:420
Miyashita T, Enosawa S, Suzuki S, Tamura A, Tanaka H, Amemiya H, Matsumura T, Omasa T, Suga K, Aoki T, Koyanagi Y (2000) Development of a bioartificial liver with glutamine synthetase-transduced recombinant human hepatoblastoma cell line, HepG2. Transpl Proc 32:2355–2358
Park JK, Lee DH (2005) Bioartificial liver systems: current status and future perspective. J Biosci Bioeng 99:311–319
Sarkar J, Kumari J, Tonello JM, Kamihira M, Kumar A (2017) Enhanced hepatic functions of genetically modified mouse hepatoma cells by spheroid culture for drug toxicity screening. Biotechnol J 12:1700274
Takebe T, Sekine K, Kimura M, Yoshizawa E, Ayano S, Koido M, Funayama S, Nakanishi N, Hisai T, Kobayashi T, Kasai T, Kitada R, Mori A, Ayabe H, Ejiri Y, Amimoto N, Yamazaki Y, Ogawa S, Ishikawa M, Kiyota Y, Sato Y, Nozawa K, Okamoto S, Ueno Y, Taniguchi H (2017) Massive and reproducible production of liver buds entirely from human pluripotent stem cells. Cell Rep 21:2661–2670
Tonello JM, Kawashima S, Sato K, Kawabe Y, Ito A, Kamihira M (2017) Three-dimensional culture of a genetically modified hepatoma cell line using macroporous gelatin beads. Cytotechnology 69:925–931
Yamaguchi M, Ito A, Okamoto N, Kawabe Y, Kamihira M (2012) Heat-inducible transgene expression system incorporating a positive feedback loop of transcriptional amplification for hyperthermia-induced gene therapy. J Biosci Bioeng 114:460–465
Yamaguchi M, Ito A, Ono A, Kawabe Y, Kamihira M (2014) Heat-inducible gene expression system by applying alternating magnetic field to magnetic nanoparticles. ACS Synth Biol 3:273–279
Yamamoto H, Kawabe Y, Ito A, Kamihira M (2012) Enhanced liver functions in mouse hepatoma cells by induced overexpression of liver-enriched transcription factors. Biochem Eng J 60:67–73
Yamamoto H, Tonello JM, Sambuichi T, Kawabe Y, Ito A, Kamihira M (2018) Characterization of genetically engineered mouse hepatoma cells with inducible liver functions by overexpression of liver-enriched transcription factors. J Biosci Bioeng 125:131–139
Acknowledgements
We thank Amy Phillips, PhD, from Edanz Group (https://en-author-services.edanzgroup.com/) for editing a draft of this manuscript.
Funding
This work was financially supported in part by Grants-in-Aid for Scientific Research (no. 26560216 and 20H00322) from the Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kitano, H., Nagae, Y., Kawabe, Y. et al. Development of a genetically modified hepatoma cell line with heat-inducible high liver function. Cytotechnology 73, 353–362 (2021). https://doi.org/10.1007/s10616-021-00457-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-021-00457-4