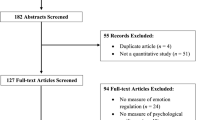
Abstract
Background
Uncertainty-related distress is considered a hallmark of anxiety and obsessive compulsive disorders (OCD). Previous research in community samples has demonstrated that individuals with high Intolerance of Uncertainty (IU), the tendency to find uncertainty aversive, display altered threat extinction learning and retention.
Methods
Here, we conducted an exploratory secondary analysis of an existing dataset (Steinman et al., 2022) to examine the extent to which IU in a clinical sample with anxiety and OCD predicts threat extinction learning and retention. Participants with an anxiety disorder and/or OCD completed a differential threat learning task across two days (n = 27). Skin conductance response (SCR) was used as an index of conditioned responding.
Results
No significant effects of self-reported IU were observed for differential SCR during any of the experimental phases. However, higher self-reported IU, while controlling for trait anxiety, was specifically associated with greater SCR overall during same-day extinction training, next-day extinction training, and next-day reinstatement test.
Conclusions
Such findings provide preliminary evidence that higher IU within clinical samples with anxiety and/or OCD may be associated with heightened arousal under uncertainty, and highlight IU as a promising treatment target for anxiety and OCD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Principles of associative threat and safety learning have supported human models of the development, treatment, and relapse of anxiety and obsessive-compulsive disorders (OCD) (Craske et al., 2008; Jacoby & Abramowitz, 2016). In particular, principles of associative threat and safety learning have been used to inform evidence-based therapies for anxiety and OCD such as exposure therapy (Boschen et al., 2009; Carpenter et al., 2019; McNally, 2007). The aim of exposure-based therapies is to reduce anxiety or OCD symptoms gradually by exposing patients to the situations or objects that make them feel anxious (Barlow, 2002; Foa & Kozak, 1996). Exposure-based therapies are thought to allow the patient to challenge old threat associations (e.g. in the past, I went to the shop and had a panic attack) by forming new safe associations (e.g. recently, I went to the shop and did not have a panic attack). Unfortunately, after exposure therapy completion, many anxiety and OCD patients relapse (Bandelow et al., 2017).
A potential factor that may maintain anxiety and OCD during exposure-based therapy is uncertainty (e.g. how can I be sure that when I go to the shop next, that I won’t have a panic attack?). Indeed, alterations in contingency, such as threat to safety, may not always be easy to identify in the first instance, as ‘it may take a few experiences to recognise that something that once signalled threat may now signal safety’ (Morriss et al., 2020, p. 8). Uncertainty about changes in contingency from threat to safety may delay threat extinction learning and retention (e.g. the reduction of anxious responses to old threat associations) (Bouton, 2002). Notably, uncertainty-related distress plays a fundamental role in anxiety and OCD (Brosschot et al., 2016; Carleton, 2016; Grupe & Nitschke, 2013; Pulcu & Browning, 2019). Initially, individual differences in Intolerance of Uncertainty (IU), the tendency to interpret and react to uncertainty negatively (Birrell et al., 2011; Freeston et al., 1994), was thought to underlie worry in Generalised Anxiety Disorder (Dugas et al., 2004). However, IU is now considered a transdiagnostic dimension, as high levels of IU are observed across many different types of mental health disorders, including anxiety and OCD (McEvoy et al., 2019). Crucially, IU shows promise as a trans-therapy change process, as reductions in IU have been found using evidence-based treatments such as cognitive behavioural therapy for youth and adults with anxiety disorders (Mahoney & McEvoy, 2012; McEvoy & Erceg-Hurn, 2016; Palitz et al., 2019; Sperling, 2022).
In community samples, individual differences in IU have been linked to threat extinction learning and retention (Lonsdorf & Merz, 2017; Morriss, Zuj et al., 2021), core principles underscoring exposure-based therapies (e.g. the reduction of anxious responses to old threat associations) (Craske et al., 2014; McNally, 2007). More specifically, in a recent meta-analysis of eighteen studies, higher IU was associated with poorer threat extinction learning (e.g. difficulty updating an ‘old’ learned threat cue to a learned safety cue). Essentially, individuals with higher IU continue to display greater skin conductance response (SCR) to ‘old’ learned threat cues, relative to learned safety cues (Morriss, Wake et al., 2021). Additionally, higher IU is associated with poorer threat extinction retention, as demonstrated by greater SCR to ‘old’ learned threat cues, relative to learned safety cues: (1) during next-day (24 h later) extinction training (Dunsmoor et al., 2015; Morriss et al., 2020; Wake, Dodd et al., 2021; Morriss, Wake et al., 2021) and (2) during same-day reinstatement test (e.g. unsignalled presentation of an unconditioned stimulus such as electric shock) (Lucas et al., 2018).
The majority of research on IU and threat extinction learning and retention has been conducted in community samples (Morriss, Zuj et al., 2021). As far as we are aware, research is limited on the role of IU in threat extinction learning and retention in clinical samples with anxiety and OCD. Clarifying the generalisability of previous IU and threat extinction learning/retention findings from community samples to clinical samples has important implications for understanding IU-related mechanisms and translation of experimental research to clinical therapies in general (Einstein, 2014; Shihata et al., 2016) and especially in relation to exposure-based therapies (Gee & Odriozola, 2021; Knowles & Olatunji, 2018). Notably, the literature remains mixed as to whether difficulties in threat extinction learning/retention consistently occur across different anxiety disorders (for reviews and meta-analyses see, Cooper & Dunsmoor, 2021; Duits et al., 2015; Wake et al., 2023). Thus, examining whether IU, a transdiagnostic dimension, predicts threat extinction learning/retention in clinical samples with anxiety and OCD may help contextualise previous research based on categorical dimensions (e.g. categorical diagnosis of anxiety and OCD).
Here, we conducted an exploratory secondary analysis of an existing dataset (Steinman et al., 2022) to examine the extent to which IU in a clinical sample with anxiety and OCD predicts extinction learning and retention. The differential threat learning task was conducted across two days and included four experimental phases: acquisition training, same-day extinction training (extinction learning), next-day extinction training (extinction retention), and next-day reinstatement test. SCR was used as an index of conditioned responding. Similar to previous research (Morriss, Wake et al., 2021; Sjouwerman et al., 2020), the specificity of IU was assessed against trait anxiety (Spielberger et al., 1983), a measure of characterological anxiety (Barlow et al., 2014; Clark & Beck, 2011).
Based on prior literature, we hypothesised that higher IU would be associated with greater SCR to the learned threat (CS+) versus safe (CS-) cue during: (1) same-day extinction training (Morriss, Wake et al., 2021), (2) next-day extinction training (Dunsmoor et al., 2015; Morriss et al., 2020; Wake, Dodd et al., 2021; Morriss, Wake et al., 2021), and (3) next-day reinstatement test (Dunsmoor et al., 2015).
Methods
For a detailed account of the procedure and design please refer to the original study (Steinman et al., 2022).
Participants
The sample consisted of 27 participants (for demographic information, see Table 1) who met criteria for one or more anxiety disorders or OCD (for diagnosis information, see Table 1), were free of psychotropic medication, and did not have other mental health disorders such as depression, substance abuse etc. Participants were assessed and screened for mental health disorders by trained clinicians using a structured clinical interview (First et al., 1995).
Procedure
The current study was conducted at the Anxiety Disorders Clinic, an outpatient research clinic, at the New York State Psychiatric Institute (NYSPI) and Columbia University Medical Center, and was approved by the NYSPI Institutional Review Board. Following informed consent, participants completed measures of intolerance of uncertainty, trait anxiety, interpretation bias (not reported here), and a first session of the differential threat learning task, consisting of acquisition training and extinction training. Participants were randomized to either standard extinction (discussed in this paper) or novelty-facilitated extinction training (not reported here). Participants returned to the lab 24 h later, where they completed a second session of the differential threat learning, consisting of extinction training and extinction training after reinstatement.
Electric Shock Work-Up
On Day 1 and two Ag/AgCl electrodes were attached to the participants’ right wrist, to deliver electric shocks. The electric shock was generated by the SHK1 Pain Stimulation Shocker (Contact Precision Instruments, Massachusetts, USA) and lasted 200 ms. An electric shock work-up was conducted to determine the level of electric shock that each participant found to be highly annoying, but not painful (for more detailed information on the electric shock work up procedure used in this study, see Steinman et al., 2022).
Differential Threat Learning Task
Participants completed the differential threat learning task over the course of two days (see Fig. 1). PsyLab (Contact Precision Instruments, Massachusetts, USA) was used to run the differential threat learning task. Conditioned stimuli (CS) consisted of two angry white male faces (Dunsmoor et al., 2015). In each trial, the CS was presented for 6 s, followed by a 12 s intertrial interval. In CS + paired trials, the electric shock co-terminated with the presentation of the CS. The trial order was pseudorandomized so that no more than 3 trials of the same type occurred in a row.
On Day 1, following the electric shock work-up, participants were instructed to sit comfortably and pay attention to the images displayed on the screen. They were told they may or may not receive electric shocks, and that there would be an association between pictures and electric shocks, but they would need to learn it themselves. Participants wore headphones (Sennheiser PRO) to block out noise. On Day 1, the task began with habituation (5 trials each of CS + and CS-). Then, there were two runs of acquisition training (first run: 4 CS + trials that co-terminated with a shock to the wrist, 7 CS + trials unpaired with shock, and 7 CS- trials; second run: 4 CS + trials paired with shock, 8 CS + trials unpaired with shock, and 8 CS- trials). Next, there were two runs of extinction training (the first and second run were identical and included 10 CS + trials and 10 CS- trials).
On Day 2, no new instructions were given that would indicate any departure from the procedures from the previous day. Electrodes were reattached and the electric shock intensity was set at the level determined on Day 1. On Day 2, the task began with an extinction training session (10 CS + trials and 10 CS- trials). After this, participants received three unsignaled electric shocks to the wrist to reinstate conditioned responses. Then, there was a run of reinstatement test (10 CS + and 10 CS- trials).
Questionnaires
The Intolerance of Uncertainty Scale (IUS: Freeston et al., 1994) consists of 27 items and the Trait subscale of the State-Trait Anxiety Inventory (STAI-T: Spielberger et al., 1983) consists of 20 items.
Data Collection and Reduction of SCR
SCRs were collected using PsyLab (Contact Precision Instruments, Massachusetts, USA) at 500 Hz. SCRs were collected on Day 1 and 2, by attaching two Ag/AgCl electrodes to the hypothenar eminence of the participants’ left hand. SignaGel Electrode Gel (Parker Labs, New Jersey, USA), a highly conductive saline gel, was used.
SCRs were calculated using an automated MATLAB (The MathWorks, Inc., Natick, Massachusetts, USA) script (Green et al., 2014). SCRs to the CS were considered valid if: (1) the trough-to-peak deflection occurred within a 0.5 to 6.0 s time window starting at CS onset, (2) the response lasted between 0.5 and 5.0 s, and (3) the response was greater than 0.02 microsiemens. If a given response on a trial did not meet these criteria, it was scored as zero. Raw SCR values were square-root transformed to reduce skewness. No SCR exclusion criteria (e.g. non-responding or non-learning) were applied.
SCRs from the CS + trials paired with the shock during the acquisition training phase were not included in the analysis due to potential contamination of the SCR signal from the shock (Dunsmoor et al., 2015). SCR-transformed values for the CS + and CS- were averaged across all trials separately for the acquisition training, next-day extinction training, and next-day reinstatement test phases. SCR-transformed values for the CS + and CS- were averaged across early (first 10) and late (last 10) trials separately for the same-day extinction training phase, in line with prior research on IU and threat extinction learning (Morriss, Wake et al., 2021).
Analysis Plan
We conducted multilevel models (MLMs) in R version 4.3.1 using the lmer function from the lme4 package (Bates et al., 2015). The data and analysis scripts are available at https://osf.io/p258h/. To first establish whether successful threat acquisition and subsequent extinction and reinstatement effects were observed, separate MLMs were conducted for skin conductance response for (i) acquisition, (ii) extinction learning, (iii) extinction retention, and (iv) reinstatement test. For the acquisition, extinction retention, and reinstatement test phases, Stimulus (CS+, CS-) was entered at level 1 and individual subjects at level 2. For the extinction learning phase, Stimulus (CS+, CS-) and Time (Early Extinction: first 10 CS+/CS- trials, Late Extinction: last 10 CS+/CS- trials) were entered at level 1 and individual subjects at level 2. Furthermore, as the sample consisted of patients with either one or multiple diagnoses, we entered Diagnosis Status (One, Multiple) as a group factor at level 1 in each of the MLMs to ascertain whether this would influence our findings. Footnote 1 Fixed effects included Stimulus, Time and Diagnosis Status, and random effects included a random intercept for each individual subject. A maximum likelihood estimator was utilised in all models. Level 1 variables were categorical and therefore effect coded (Stimulus: CS + = 1, CS- = -1; Time: Early Extinction = 1, Late Extinction = -1; Diagnosis Status: Multiple = 1, One = -1).
We then carried out separate MLMs to investigate the effect of individual difference predictors IUS and STAI-T, where grand-mean centred IUS and STAI-T scores were included as continuous predictor variables in the MLMs, with all parameters as described above. Separate MLMs were initially carried out to investigate the effect of each individual difference predictor on SCRs (i.e., separate models for IUS and STAI-T). In the case of significant effects or interactions observed with IUS or STAI-T scores, follow-up MLMs were conducted with both IUS and STAI-T scores included to assess specificity. A significant interaction with one of these predictors (IUS or STAI-T) but not the other would indicate specificity of that predictor. Footnote 2 In line with previous work (Klingelhöfer-Jens et al., 2022; Mertens & Morriss, 2021; Morriss et al., 2022), to further understand any significant IUS/STAI-T effects or interactions from the MLMs, we conducted follow-up two-tailed partial correlations between the relevant self-report measure (IUS or STAI-T) and SCRs during the relevant phase(s).
A sensitivity analysis based on a point biserial correlation model was conducted (one tailed/two tailed, α = 0.05, 1 - β err prob = 0.8, n = 27). The effect size that we were able to detect in the current study was between 0.44 and 0.48. This effect size is larger than that reported in a recent meta-analysis of correlational data between IU and SCR during threat extinction learning (e.g. 0.28–0.31) (Morriss, Wake et al., 2021). Even though the current study is underpowered to detect individual differences in IU, this exploratory study provides an opportunity to examine the relationships between IU and conditioned responding in a clinical sample with diagnosed anxiety disorders and/or OCD.
Results
Questionnaires
Scores on the IUS (M = 75.22; SD = 19.82) and STAI-T (M = 51.37; SD = 9.32) were similar to that previously reported for clinical samples with anxiety and OCD (Abramowitz & Deacon, 2006; Khawaja & Yu, 2010; Yook et al., 2010) (see Fig. 2 for frequency distributions). Higher IUS was associated with higher STAI-T [r(27) = 0.76, p < .001, 95% CI [0.53, 0.88]] in all participants (see Fig. 3), as well as when grouped by Multiple [r(14) = 0.82, p < .001 [95% CI 0.51, 0.94]] or One diagnosis [r(13) = 0.61, p = .026, 95% CI [0.09, 0.98]].
Scatterplot With Histograms Depicting Correlation Between IUS and STAI-T. Note The distribution of IUS scores is displayed on top of the figures in yellow, and the distribution of STAI-T is displayed on the right side of the figures in orange. Shaded areas represent 95% confidence intervals. Higher IUS was associated with higher STAI-T [r(27) = 0.76, p < .001, 95% CI [0.53, 0.88]] in all participants, as well as when grouped by Multiple [r(14) = 0.82, p < .001 [95% CI 0.51, 0.94]] or One diagnosis [r(13) = 0.61, p = .026, 95% CI [0.09, 0.98]].
Skin Conductance Response Data
The results are presented by conditioning phase (acquisition, extinction learning, extinction retention, reinstatement test). In each section, the first paragraph reports the results of the initial MLM analyses which included Stimulus, Time, and Stimulus x Time interactions (where applicable). These results are also visualised in Fig. 4 (see figure note for a summary of these effects). The second paragraph of each section reports the results for the MLM analyses including the main effects of IUS, as well as Stimulus/Time x IUS interactions. The third paragraph of each section reports the MLM analysis results including the main effects of STAI-T, as well as Stimulus/Time x STAI-T interactions. These results are also presented in Table 2. Follow-up tests (i.e. MLMs with both IUS and STAI-T assessing their specificity) are presented in the text only. For overall model effect sizes, please refer to Table 3, and for standardised betas from the MLM analyses indicating the effects of IUS and STAI-T, please refer to Table 4.
Violin plots depicting main effects of stimulus and time on skin conductance responses (scrs) per conditioning phase.Note Individual datapoints presented here are SCRs averaged for each participant per stimulus during acquisition (A), per stimulus and time during Extinction Learning (B), per stimulus during Extinction Retention (C), and per stimulus during Reinstatement Test (D). Filled circles denote mean of SCRs per stimulus, (time for Extinction Learning only) and conditioning phase. SCR is square root transformed (√) and in microsiemens (µS). Error bars denote standard error of the mean. Early = first 10 CS+/CS- Extinction Learning trials; Late = last 10 CS+/CS- Extinction Learning trials. MLM analyses indicated that significantly decreased SCRs for the CS + vs. CS- were observed during all four phases, and there were no significant differences in SCRs during early vs. late extinction learning
Descriptive statistics of SCR data and scatterplots depicting the relationships between IUS and SCRs across all four phases, as well as IUS and SCRs in response to the CS + and CS-, and IUS and SCR CS discrimination (CS+ - CS-) are presented in the Supplementary Materials.
Acquisition
During acquisition, SCRs were significantly larger to the CS+ (M = 0.45; SD = 0.32), compared to the CS- (M = 0.39; SD = 0.32) [Stimulus: F(1, 27) = 7.15, p = .013].
Individual differences in IUS were not significantly related to SCRs across acquisition [IUS: F(1, 27) = 3.80, p = .062] or to differential SCRs in response to the CS + vs. CS- [Stimulus x IUS: F(1, 27) = 0.01, p = .908].
No significant effects of STAI-T were observed across acquisition [STAI-T: F(1, 27) = 0.02, p = .878] or to differential SCRs in response to the CS + vs. CS- throughout acquisition [Stimulus x STAI-T: F(1, 27) = 0.01, p = .933].
Extinction Learning
During extinction learning, SCRs were significantly larger to the CS+ (M = 0.50; SD = 0.36), compared to the CS- (M = 0.31; SD = 0.23) [Stimulus: F(1, 81) = 21.30, p < .001]. SCRs to the CS + versus CS- did not fall across time, suggesting little evidence of extinction learning [Stimulus x Time: F(1, 81) = 0.05, p = .830; Time: F(1, 81) = 0.05, p = .817]
A significant main effect of IUS for SCRs throughout extinction learning was observed both when IUS was entered into the model alone (p = .013, see Table 2) and when entered together with STAI-T [IUS: F(1, 27) = 8.44, p = .007; STAI-T: F(1, 27) = 2.51, p = .125]. A follow up partial correlation confirmed that higher IUS, while controlling for STAI-T, was associated with greater SCRs overall during extinction learning [r(27) = 0.48, p = .013; see Fig. 5]. IUS was not significantly associated with differential SCRs to the CS + vs. the CS- during extinction learning [Stimulus x Time x IUS: F(1, 81) = 0.04, p = .851; Stimulus x IUS: F(1, 81) = 2.22, p = .140] or with SCRs across time during extinction learning [Time x IUS: F(1, 81) = 0.76, p = .387].
Scatterplot With Histograms Depicting the Partial Correlation Between IUS (Controlling for STAI-T) and Skin Conductance Responses (SCRs) across the Extinction Learning Phase. Note The distribution of IUS scores is displayed on top of the figures in yellow, and the distribution of SCRs across Extinction Learning is displayed on the right side of the figures in blue. SCR is square root transformed (?) and in microsiemens (?S). Shaded areas represent 95% confidence intervals. Higher IUS was associated with increased SCRs across Extinction Learning
STAI-T was not significantly associated with SCRs during extinction learning [STAI-T: F(1, 27) = 0.22, p = .645], and individual differences in STAI-T were not significantly related to differential SCRs to the CS + vs. CS- [Stimulus x Time x STAI-T: F(1, 81) = 0.00, p = .954; Stimulus x STAI-T: F(1, 81) = 0.70, p = .405], or with SCRs across time during extinction learning [Time x STAI-T: F(1, 81) = 0.07, p = .795; ].
Extinction Retention
During extinction retention, SCRs were significantly larger to the CS+ (M = 0.54; SD = 0.34), compared to the CS- (M = 0.39; SD = 0.32) [Stimulus: F(1, 27) = 19.16, p < .001]. IUS was significantly associated with SCRs across extinction retention both when IUS was entered into the model alone (p = .015, see Table 2), and when entered into the model together with STAI-T [IUS: F(1, 27) = 8.20, p = .008; STAI-T: F(1, 27) = 2.30, p = .141]. A follow up partial correlation confirmed that higher IUS, while controlling for STAI-T, was associated with greater SCRs overall during extinction retention [r(27) = 0.48, p = .012; see Fig. 6]. IUS was not significantly related to differential SCRs to the CS + vs. CS- throughout extinction retention [Stimulus x IUS: F(1, 27) = 1.09, p = .306].
Scatterplot With Histograms Depicting the Partial Correlation Between IUS (Controlling for STAI-T) and Skin Conductance Responses (SCRs) across the Extinction Retention Phase Note The distribution of IUS scores is displayed on top of the figures in yellow, and the distribution of SCRs across Extinction Retention is displayed on the right side of the figures in blue. SCR is square root transformed (?) and in microsiemens (?S). Shaded areas represent 95% confidence intervals. Higher IUS was associated with increased SCRs across Extinction Retention
There were no significant effects of STAI-T on SCRs throughout extinction retention [STAI-T: F(1, 27) = 0.34, p = .563], and SCRs in response to the CS + vs. CS- were not significantly related to individual differences in STAI-T across extinction retention [Stimulus x STAI-T: F(1, 27) = 0.03, p = .864].
Reinstatement Test
For the reinstatement test, SCRs were larger to the CS+ (M = 0.46; SD = 0.40), compared to the CS- (M = 0.37; SD = 0.32) [Stimulus: F(1, 27) = 9.01, p = .006].
A significant main effect of IUS for SCRs was observed during reinstatement test both when IUS was entered into the model alone (p = .024, see Table 2) and when entered with STAI-T [IUS: F(1, 27) = 9.19, p = .005; STAI-T: F(1, 27) = 3.65, p = .067]. The follow-up partial correlation showed that higher IUS, while controlling for STAI-T, was significantly associated with greater SCRs across the reinstatement test [r(27) = 0.50, p = .009] (see Fig. 7). IUS was not significantly associated with differential SCRs to the CS + vs. CS- across reinstatement test [Stimulus x IUS: F(1, 27) = 0.08, p = .781].
Scatterplot With Histograms Depicting the Partial Correlation Between IUS (Controlling for STAI-T) and Skin Conductance Responses (SCRs) across the Reinstatement Test Phase. Note The distribution of IUS scores is displayed on top of the figures in yellow, and the distribution of SCRs across Reinstatement Test is displayed on the right side of the figures in blue. SCR is square root transformed (?) and in microsiemens (?S). Shaded areas represent 95% confidence intervals. Higher IUS was not significantly associated with increased SCRs across Reinstatement Test
Scatterplot With Histograms Depicting the Partial Correlation Between IUS (Controlling for STAI-T) and Skin Conductance Responses (SCRs) across the Reinstatement Test Phase. Note The distribution of IUS scores is displayed on top of the figures in yellow, and the distribution of SCRs across Reinstatement Test is displayed on the right side of the figures in blue. SCR is square root transformed (?) and in microsiemens (?S). Shaded areas represent 95% confidence intervals. Higher IUS was not significantly associated with increased SCRs across Reinstatement Test
Individual differences in STAI-T were not significantly associated with SCRs across reinstatement test [STAI-T: F(1, 27) = 0.05, p = .818], or with differential responses to the CS + vs. CS- during reinstatement test [Stimulus x STAI-T: F(1, 27) = 0.05, p = .831].
Discussion
The current study examined whether self-reported IU in a clinical sample with diagnosed anxiety disorders and/or OCD was associated with differential threat extinction learning and retention. No significant effects of IU were observed for differential SCR (threat vs. safe cues) during same-day extinction training, next-day extinction training and next-day reinstatement test. Interestingly, higher IU was specifically associated with greater SCR overall during same-day extinction, next-day extinction training, and next-day reinstatement test. These findings provide preliminary evidence that individuals with higher levels of IU within clinical samples with anxiety and/or OCD may be more susceptible to heightened arousal under uncertainty. Such findings further highlight the relevance of IU as a potential treatment target in therapies for anxiety and OCD that rely on principles of associative threat and safety learning, such as exposure-based therapies.
Typical effects of threat conditioning were observed e.g. greater SCR to learned threat versus safety cues (for review see, Lonsdorf et al., 2017). However, there was little evidence of threat extinction learning in this clinical sample with anxiety and OCD e.g. SCR’s did not decrease to the learned threat versus safety cues across the threat extinction learning phase. This finding is in line with prior research suggesting poorer threat extinction learning in clinical samples with anxiety and OCD (for reviews and meta analyses see, Cooper & Dunsmoor, 2021; Duits et al., 2015). This explanation is more likely, given that previous research from different lab groups have found successful threat extinction learning in community samples using this exact experimental design (Dunsmoor et al., 2015; Morriss, Wake et al., 2021).
Prior research in community samples has shown that higher IU is associated with greater SCR to learned threat versus safety cues during same-day and next-day extinction training (Dunsmoor et al., 2015; Morriss et al., 2020; Wake, Dodd et al., 2021; Morriss, Wake et al., 2021), as well as during same-day reinstatement test (Lucas et al., 2018). However, in this clinical sample with anxiety and OCD, no significant effects of IU (although means were in the expected direction) were observed for SCR to learned threat versus safe cues during any of the experimental phases. Notably, the majority of the sample in this study had higher than average IU (Abramowitz & Deacon, 2006; Khawaja & Yu, 2010; Yook et al., 2010), and maintained a differential SCR throughout the same-day extinction training phase (Steinman et al., 2022), similar to individuals with high IU in community samples (Morriss, Wake et al., 2021). Thus, it is possible that IU-related effects for differential SCR are difficult to observe without the full range of IU scores (e.g. both the low and high end). Furthermore, perhaps in a clinical sample, more experimental trials would be required to observe an IU-related effect on differential SCR.
In this clinical sample, higher IU, controlling for trait anxiety and diagnosis status, was associated with greater SCR overall during same-day extinction training, next-day extinction training, and next-day reinstatement test. Individuals with high IU may have displayed greater SCR overall because they found the unexpected uncertainty during these phases more anxiety-provoking (e.g. the uninstructed change in contingency from threat to safety). While not expected, these findings are in line with recent research in patients with anxiety disorders, who demonstrate greater SCR overall during same-day extinction training (Abend et al., 2020). Further replication of IU-related effects in larger samples is required to clarify the extent to which higher IU leads to greater physiological arousal in anxiety and OCD populations, and whether this results in longer-term disruption of new safety learning and retention.
The study did have several limitations. Firstly, the sample size was small, limiting statistical power. Secondly, there was considerable variation in anxiety and OCD diagnoses, making it difficult to fully contextualise the results in relation to past research on threat conditioning in different clinical samples (for reviews and meta analyses see, Cooper & Dunsmoor, 2021; Duits et al., 2015; Wake et al., 2023). Thirdly, the lack of control group (e.g. healthy sample without anxiety and OCD diagnoses) does not allow us to ascertain whether the IU-related heightened arousal in the anxiety and OCD sample in this study is related to threat generalisation, which is commonly observed across clinical samples with anxiety and OCD (for meta-analysis see, Cooper et al., 2022).
In sum, no significant effects of IU were observed for differential SCR (threat vs. safe cues) during same-day and next-day extinction training or next-day reinstatement test. However, higher IU was specifically associated with greater SCR overall during same-day extinction training, next-day extinction training, and next-day reinstatement test. These findings provide preliminary evidence that higher IU within clinical samples with anxiety and OCD may be associated with heightened arousal under uncertainty. Further research is warranted on the role of IU in clinical samples with anxiety and OCD to understand the utility of targeting IU in evidence-based therapies (Hui & Zhihui, 2017; Oglesby et al., 2017; Robichaud & Dugas, 2006; Wahlund et al., 2020) that rely on principles of associative threat and safety learning, such as exposure-based therapies.
Notes
There were not any significant effects or interactions observed with Diagnosis Status for any of the MLMs. See Supplementary Materials for outputs of results.
As there were not any significant effects or interactions observed with IUS or STAI-T throughout acquisition, we did not run additional MLM analyses to examine specificity of IUS or STAI-T during the acquisition phase.
References
Abend, R., Gold, A. L., Britton, J. C., Michalska, K. J., Shechner, T., Sachs, J. F., & Pine, D. S. (2020). Anticipatory threat responding: Associations with anxiety, development, and brain structure. Biological Psychiatry, 87(10), 916–925.
Abramowitz, J. S., & Deacon, B. J. (2006). Psychometric properties and construct validity of the obsessive–compulsive Inventory—Revised: Replication and extension with a clinical sample. Journal of Anxiety Disorders, 20(8), 1016–1035.
Bandelow, B., Michaelis, S., & Wedekind, D. (2017). Treatment of anxiety disorders. Dialogues in Clinical Neuroscience, 19(2), 93.
Barlow, D. H. (2002). Anxiety and its disorders: The nature and treatment of anxiety and panic (2nd ed.). Guilford Press.
Barlow, D. H., Sauer-Zavala, S., Carl, J. R., Bullis, J. R., & Ellard, K. K. (2014). The nature, diagnosis, and treatment of neuroticism: Back to the future. Clinical Psychological Science, 2(3), 344–365.
Bates, D., Mächler, M., Bolker, B., & Walker, S. (2015). Fitting Linear mixed-effects models using lme4. Journal of Statistical Software, 67(1).
Birrell, J., Meares, K., Wilkinson, A., & Freeston, M. (2011). Toward a definition of intolerance of uncertainty: A review of factor analytical studies of the intolerance of uncertainty scale. Clinical Psychology Review, 31(7), 1198–1208.
Boschen, M. J., Neumann, D. L., & Waters, A. M. (2009). Relapse of successfully treated anxiety and fear: Theoretical issues and recommendations for clinical practice. Australian & New Zealand Journal of Psychiatry, 43(2), 89–100.
Bouton, M. E. (2002). Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biological Psychiatry, 52(10), 976–986.
Brosschot, J. F., Verkuil, B., & Thayer, J. F. (2016). The default response to uncertainty and the importance of perceived safety in anxiety and stress: An evolution-theoretical perspective. Journal of Anxiety Disorders, 41, 22–34.
Carleton, R. N. (2016). Fear of the unknown: One fear to rule them all? Journal of Anxiety Disorders, 41, 5–21.
Carpenter, J. K., Pinaire, M., & Hofmann, S. G. (2019). From extinction learning to anxiety treatment: Mind the gap. Brain Sciences, 9(7), 164.
Clark, D. A., & Beck, A. T. (2011). Cognitive therapy of anxiety disorders: Science and practice. Guilford Press.
Cooper, S. E., & Dunsmoor, J. E. (2021). Fear conditioning and extinction in obsessive-compulsive disorder: A systematic review. Neuroscience & Biobehavioral Reviews, 129, 75–94.
Cooper, S. E., van Dis, E. A., Hagenaars, M. A., Krypotos, A. M., Nemeroff, C. B., Lissek, S., & Dunsmoor, J. E. (2022). A meta-analysis of conditioned fear generalization in anxiety-related disorders. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 47(9), 1652–1661.
Craske, M. G., Kircanski, K., Zelikowsky, M., Mystkowski, J., Chowdhury, N., & Baker, A. (2008). Optimizing inhibitory learning during exposure therapy. Behaviour Research and Therapy, 46(1), 5–27.
Craske, M. G., Treanor, M., Conway, C. C., Zbozinek, T., & Vervliet, B. (2014). Maximizing exposure therapy: An inhibitory learning approach. Behaviour Research and Therapy, 58, 10–23.
Dugas, M. J., Buhr, K., & Ladouceur, R. (2004). The role of intolerance of uncertainty in etiology and maintenance of generalized anxiety disorder. In R. G. Heimberg, C. L. Turk, & D. S. Mennin (Eds.), Generalized anxiety disorder: Advances in research and practice (pp. 143–163). Guilford Press.
Duits, P., Cath, D. C., Lissek, S., Hox, J. J., Hamm, A. O., Engelhard, I. M., & Baas, J. M. (2015). Updated meta-analysis of classical fear conditioning in the anxiety disorders. Depression and Anxiety, 32(4), 239–253.
Dunsmoor, J. E., Campese, V. D., Ceceli, A. O., LeDoux, J. E., & Phelps, E. A. (2015). Novelty-facilitated extinction: Providing a novel outcome in place of an expected threat diminishes recovery of defensive responses. Biological Psychiatry, 78(3), 203–209.
Einstein, D. A. (2014). Extension of the transdiagnostic model to focus on intolerance of uncertainty: A review of the literature and implications for treatment. Clinical Psychology: Science and Practice, 21(3), 280–300.
First, M. B., Spitzer, R. L., Gibbon, M., & Williams, J. B. (1995). Structured clinical interview for DSM-IV axis I disorders-patient edition (SCID-I/P, Version 2.0). New York: Biometrics Research Department, New York State Psychiatric Institute, 722.
Foa, E. B., & Kozak, M. J. (1996). Psychological treatment for obsessive-compulsive disorder. In P. R. Mavissakalian M (Ed.), Long-term treatments of anxiety disorders (pp. 285–309). American Psychiatric Press, Inc.
Freeston, M. H., Rhéaume, J., Letarte, H., Dugas, M. J., & Ladouceur, R. (1994). Why do people worry? Personality and Individual Differences, 17(6), 791–802.
Gee, D. G., & Odriozola, P. (2021). When uncertainty is a certainty: Optimizing exposure-based therapies. Biological Psychiatry: Global Open Science, 1(3), 166–167.
Green, S. R., Kragel, P. A., Fecteau, M. E., & LaBar, K. S. (2014). Development and validation of an unsupervised scoring system (autonomate) for skin conductance response analysis. International Journal of Psychophysiology, 91(3), 186–193.
Grupe, D. W., & Nitschke, J. B. (2013). Uncertainty and anticipation in anxiety: An integrated neurobiological and psychological perspective. Nature Reviews Neuroscience, 14(7), 488–501.
Hui, C., & Zhihui, Y. (2017). Group cognitive behavioral therapy targeting intolerance of uncertainty: A randomized trial for older Chinese adults with generalized anxiety disorder. Aging & Mental Health, 21(12), 1294–1302.
Jacoby, R. J., & Abramowitz, J. S. (2016). Inhibitory learning approaches to exposure therapy: A critical review and translation to obsessive-compulsive disorder. Clinical Psychology Review, 49, 28–40.
Khawaja, N. G., & Yu, L. N. H. (2010). A comparison of the 27-item and 12-item intolerance of uncertainty scales. Clinical Psychologist, 14(3), 97–106.
Klingelhöfer-Jens, M., Morriss, J., & Lonsdorf, T. B. (2022). Effects of intolerance of uncertainty on subjective and psychophysiological measures during fear acquisition and delayed extinction. International Journal of Psychophysiology.
Knowles, K. A., & Olatunji, B. O. (2018). Enhancing Inhibitory Learning: The utility of variability in exposure. Cognitive and Behavioral Practice, 26(1), 186–200.
Lonsdorf, T. B., Menz, M. M., Andreatta, M., Fullana, M. A., Golkar, A., Haaker, J., & Merz, C. J. (2017). Don’t fear ‘fear conditioning’: Methodological considerations for the design and analysis of studies on human fear acquisition, extinction, and return of fear. Neuroscience & Biobehavioral Reviews, 77, 247–285.
Lonsdorf, T. B., & Merz, C. J. (2017). More than just noise: Inter-individual differences in fear acquisition, extinction and return of fear in humans-Biological, experiential, temperamental factors, and methodological pitfalls. Neuroscience & Biobehavioral Reviews, 80, 703–728.
Lucas, K., Luck, C. C., & Lipp, O. V. (2018). Novelty-facilitated extinction and the reinstatement of conditional human fear. Behaviour Research and Therapy, 109, 68–74.
Mahoney, A. E., & McEvoy, P. M. (2012). Changes in intolerance of uncertainty during cognitive behavior group therapy for social phobia. Journal of Behavior Therapy and Experimental Psychiatry, 43(2), 849–854.
McEvoy, P. M., & Erceg-Hurn, D. M. (2016). The search for universal transdiagnostic and trans-therapy change processes: Evidence for intolerance of uncertainty. Journal of Anxiety Disorders, 41, 96–107.
McEvoy, P. M., Hyett, M. P., Shihata, S., Price, J. E., & Strachan, L. (2019). The impact of methodological and measurement factors on transdiagnostic associations with intolerance of uncertainty: A meta-analysis. Clinical Psychology Review, 73, 101778.
McNally, R. J. (2007). Mechanisms of exposure therapy: How neuroscience can improve psychological treatments for anxiety disorders. Clinical Psychology Review, 27(6), 750–759.
Mertens, G., & Morriss, J. (2021). Intolerance of uncertainty and threat reversal: A conceptual replication of. Behaviour Research and Therapy, 103799.
Morriss, J., Wake, S., Lindner, M., McSorley, E., & Dodd, H. (2020). How many times do I need to see to believe? The impact of intolerance of uncertainty and exposure experience on safety-learning and retention in young adults. International Journal of Psychophysiology, 153, 8–17.
Morriss, J., Wake, S., Elizabeth, C., & van Reekum, C. M. (2021). I doubt it is safe: A meta-analysis of self-reported intolerance of uncertainty and threat extinction training. Biological Psychiatry Global Open Science, 1(3), 171–179.
Morriss, J., Zuj, D. V., & Mertens, G. (2021). The role of intolerance of uncertainty in classical threat conditioning: Recent developments and directions for future research. International Journal of Psychophysiology, 166, 116–126.
Morriss, J., Bradford, D. E., Wake, S., Biagi, N., Tanovic, E., Kaye, J. T., & Joormann, J. (2022). Intolerance of uncertainty and physiological responses during instructed uncertain threat: A multi-lab investigation. Biological Psychology, 167, 108223.
Morriss, J., Abend, R., Zika, O., Bradford, D. E., & Mertens, G. (2023). Neural and psychophysiological markers of intolerance of uncertainty. International Journal of Psychophysiology, 184, 94–99.
Oglesby, M. E., Allan, N. P., & Schmidt, N. B. (2017). Randomized control trial investigating the efficacy of a computer-based intolerance of uncertainty intervention. Behaviour Research and Therapy, 95, 50–57.
Palitz, S. A., Rifkin, L. S., Norris, L. A., Knepley, M., Fleischer, N. J., Steinberg, L., & Kendall, P. C. (2019). But what will the results be? Learning to tolerate uncertainty is associated with treatment-produced gains. Journal of Anxiety Disorders, 68, 102146.
Pulcu, E., & Browning, M. (2019). The misestimation of uncertainty in affective disorders. Trends in Cognitive Sciences, 23(10), 865–875.
Robichaud, M., & Dugas, M. J. (2006). A cognitive-behavioral treatment targeting intolerance of uncertainty. Worry and its psychological disorders: Theory, assessment and treatment, 289–304.
Shihata, S., McEvoy, P. M., Mullan, B. A., & Carleton, R. N. (2016). Intolerance of uncertainty in emotional disorders: What uncertainties remain? Journal of Anxiety Disorders, 41, 115–124.
Sjouwerman, R., Scharfenort, R., & Lonsdorf, T. B. (2020). Individual differences in fear acquisition: Multivariate analyses of different emotional negativity scales, physiological responding, subjective measures, and neural activation. Scientific Reports, 10, 15283.
Sperling, J. (2022). The role of intolerance of uncertainty in treatment for Pediatric anxiety disorders and obsessive-compulsive disorder. Evidence-Based Practice in Child and Adolescent Mental Health, 1–10.
Spielberger, C. D., Gorsuch, R. L., Lushene, R., Vagg, P., & Jacobs, G. (1983). Consulting Psychologists Press, Inc. 2. Palo Alto (CA).
Steinman, S. A., Dunsmoor, J. E., Gazman, Z., Stovezky, Y., Pascucci, O., Pomerenke, J., & Simpson, H. B. (2022). A Preliminary Test of Novelty-Facilitated Extinction in Individuals With Pathological Anxiety. Frontiers in Behavioral Neuroscience, 16.
Wahlund, T., Andersson, E., Jolstedt, M., Perrin, S., Vigerland, S., & Serlachius, E. (2020). Intolerance of uncertainty–focused treatment for adolescents with excessive worry: A pilot feasibility study. Cognitive and Behavioral Practice, 27(2), 215–230.
Wake, S., Dodd, H., & Morriss, J. (2021). Intolerance of uncertainty and novelty facilitated extinction: The impact of reinforcement schedule. British Journal of Psychology, 113(2), 353–369.
Wake, S., Morriss, J., Johnstone, T., Van Reekum, C. M., & Dodd, H. (2021). Intolerance of uncertainty, and not social anxiety, is associated with compromised extinction of social threat. Behaviour Research and Therapy, 139, 103818.
Wake, S., van Reekum, C. M., & Dodd, H. (2023). The Effect of Social Anxiety on Threat Acquisition and Extinction: A Systematic Review. PsyArXiv. https://doi.org/10.31234/osf.io/amn4h.
Yook, K., Kim, K. H., Suh, S. Y., & Lee, K. S. (2010). Intolerance of uncertainty, worry, and rumination in major depressive disorder and generalized anxiety disorder. Journal of Anxiety Disorders, 24(6), 623–628.
Acknowledgements
The write up of this report was supported by an ESRC New Investigator Grant (ES/R01145/1) awarded to Jayne Morriss and a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation awarded to Shari A. Steinman. Original data collection was also supported by the Molberger Scholar Award (awarded to Yael Stovezky), NIMH K24MH091555 (awarded to Helen Blair Simpson) and NIH R00MH106719 (awarded to Joseph E. Dunsmoor).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Research Involving Human Participants and/or Animals
The research involved human participants. The procedure of this study was approved by the NYSPI Institutional Review Board.
Informed Consent
The human participants in this study gave informed consent.
Disclosure of Potential Conflicts of Interest
The authors have no conflicts of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Morriss, J., Rodriguez-Sobstel, C. & Steinman, S.A. Intolerance of Uncertainty is Associated with Heightened Arousal During Extinction Learning and Retention: Preliminary Evidence from a Clinical Sample with Anxiety and Obsessive-Compulsive Disorders. Cogn Ther Res (2024). https://doi.org/10.1007/s10608-024-10491-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s10608-024-10491-z