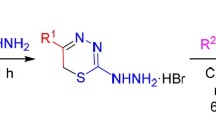
Novel imidazothiazolotriazines were obtained as a result of the reaction of imidazo[4,5-e][1,2,4]triazine-3-thiones with various phenacyl bromides. It was shown that the cyclocondensation proceeds with high regioselectivity leading to the formation of linear 7-arylimidazo-[4,5-e]thiazolo[3,2-b][1,2,4]triazines.



Similar content being viewed by others
References
(a) de Souza, M. V. N. J. Sulfur Chem. 2005, 26, 429. (b) Tomašić, T.; Mašič, L. P. Curr. Med. Chem. 2009, 16, 1596. (c) Ali, S. H.; Sayed, A. R. Synth. Commun. 2021, 51, 670. (d) Chhabria, M. T.; Patel, S.; Modi, P.; Brahmkshatriya, P. S. Curr. Top. Med. Chem. 2016, 16, 2841.
(a) Fleming, A. Lancet 1943, 242, 434. (b) Eagle, H. J. Bacteriol. 1946, 52, 81.
Igual-Adell, R.; Oltra-Alcaraz, C. Expert Opin. Pharmacother. 2004, 5, 2615.
Hotta, N. Biomed. Pharmacother. 1995, 49, 232.
Ye, X.; Zhou, W.; Li, Y.; Sun, Y.; Zhang, Y.; Ji, H.; Lai, Y. Cancer Chemother. Pharmacol. 2010, 66, 277.
Cai, D.; Li, T.; Xie, Q.; Yu, X.; Xu, W.; Chen, Y.; Jin, Z.; Hu, C. Molecules 2020, 25, 1307.
(a) Abdel-Rahman, R. M.; Seada, M.; Fawzy, M.; El-Baz, I. Pharmazie 1994, 49, 729. (b) Liu, K. C.; Shih, B. J.; Lee, C. H. J. Heterocycl. Chem. 1992, 29, 97.
(a) Izmest'ev, A. N.; Anikina, L. V.; Zanin, I. E.; Kolotyrkina, N. G.; Izmalkova, E. S.; Kravchenko, A. N.; Gazieva, G. A. New J. Chem. 2022, 24, 11632. (b) Izmest'ev, A. N.; Gazieva, G. A.; Anikina, L. V.; Pukhov, S. A.; Karnoukhova, V. A.; Kolotyrkina, N. G.; Kravchenko, A. N. New J. Chem. 2021, 45, 12271. (c) Gazieva, G. A.; Izmest'ev, A. N.; Anikina, L. V.; Pukhov, S. A.; Meshchaneva, M. E.; Khakimov, D. V.; Kolotyrkina, N. G.; Kravchenko, A. N. Mol. Diversity 2018, 22, 585. (d) Izmest'ev, A. N.; Gazieva, G. A.; Kulikov, A. S.; Anikina, L. V.; Kolotyrkina, N. G.; Kravchenko, A. N. Rus. J. Org. Chem. 2017, 53, 753.
(a) Khalilpour, A.; Asghari, S.; Pourshab, M. Chem. Biodiversity 2019, 16, e1800563. (b) Izmest'ev, A. N.; Vinogradov, D. B.; Kolotyrkina, N. G.; Kravchenko, A. N.; Gazieva, G. A. Beilstein J. Org. Chem. 2021, 17, 1141.
Kushakova, P. M.; Yulisova, A. I.; Ramsh, S. M.; Garabadgiu, A. V. Chem. Heterocycl. Compd. 2006, 42, 520.
Hantzsch, A.; Traumann, V. Ber. Dtsch. Chem. Ges. 1888, 21, 938.
(a) Ulrich, H.; Sayigh, A. A. R. J. Org. Chem. 1965, 30, 2781. (b) Faidallah, H. M.; Khan, K. A.; Asiri, A. M. J. Fluorine Chem. 2011, 132, 870.
(a) Boga, C.; Forlani, L.; Silvestroni, C.; Bonamartini Corradi, A.; Sgarabotto, P. J. Chem. Soc., Perkin Trans. 1 1999, 10, 1363. (b) Bramley, S. E.; Dupplin, V.; Goberdhan, D. G. C.; Meakins, G. D. J. Chem. Soc., Perkin Trans. 1 1987, 3, 639. (c) Aissaoui, H.; Boss, C.; Gude, M.; Koberstein, R.; Lehmann, D.; Sifferlen, T.; Trachsel, D. WO Patent 2009016560A2.
(a) Gazieva, G. A.; Kravchenko, A. N. J. Heterocycl. Chem. 2015, 52, 1858. (b) Sigachev, A. S.; Kravchenko, A. N.; Lyssenko, K. A.; Belyakov, P. A.; Lebedev, O. V.; Makhova, N. N. Mendeleev Commun. 2003, 13, 190. (c) Gazieva, G. A.; Karpova, T. B.; Nechaeva, T. V.; Nelyubina, Yu. V.; Zanin, I. E.; Kravchenko, A. N. Synlett 2017, 858. (d) Gazieva, G. A.; Vasilevskii, S. V.; Belyakov, P. A.; Nelyubina, Yu. V.; Lubuzh, E. D.; Kravchenko, A. N. Mendeleev Commun. 2010, 20, 285.
Bruker. APEX-III; Bruker AXS Inc.: Madison, 2019.
Krause, L.; Herbst-Irmer, R.; Sheldrick, G. M.; Stalke, D. J. Appl. Crystallogr. 2015, 48, 3.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Adv. 2015, A71, 3.
Sheldrick, G. M. Acta Crystallogr., Sect. C: Struct. Chem. 2015, C71, 3.
This study was financially supported by the Grants Council of the President of the Russian Federation for the State support of young Russian scientists (project MK-2375.2022.1.3).
The X-ray structural analysis and registration of highresolution mass spectra were performed at the Department of Structural Research of N. D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences (Moscow).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2022, 58(10), 524–530
Supplementary Information
ESM 1
(PDF 3682 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vinogradov, D.B., Izmest’ev, A.N., Kravchenko, A.N. et al. A regioselective synthesis of imidazothiazolotriazines based on the cyclization of imidazotriazinethiones with phenacyl bromides. Chem Heterocycl Comp 58, 524–530 (2022). https://doi.org/10.1007/s10593-022-03123-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-022-03123-5