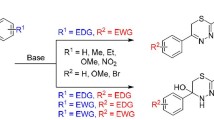
A number of previously undescribed derivatives of 2-amino-5-(2-aryl-2-oxoethyl)thiazol-4(5H)-one containing electron-donating substituents in the aromatic ring were synthesized by the reaction of (E)-4-aryl-4-oxobut-2-enoic acids with thiourea. Reduction of the reaction products with NaBH4 yielded diastereomeric alcohols, whereas bromination in AcOH was accompanied by elimination of HBr and the formation of (Z)-2-amino-5-(2-aryl-2-oxoethylidene)thiazol-4(5H)-ones.




Similar content being viewed by others
References
Manjal, S. R.; Kaur, R.; Bratia, R.; Kumar, K.; Signh, V.; Shankar, R.; Kaur, R.; Raval, R. K. Вioorg. Chem. 2017, 75, 406.
Kaminskyy, D.; den Hartog, G. J. M.; Wojtyra, M.; Lelyukh, M.; Gzella, A.; Bast, A.; Lesyk, R. Eur. J. Med. Chem. 2016, 112, 180.
Tripathi, A. C.; Gupta, S. J.; Fatima, G. N.; Sonar, P. K.; Verma, A.; Saraf, S. K. Eur. J. Med. Chem. 2014, 72, 52.
Alwiswasy, R. M.; Jameel, R. M.; Hameed, B. J. Int. J. Res. Pharm. Sci. (Madurai, India) 2019, 10, 1763.
Bayoumi, W. A.; Abdel-Rhman, S. H.; Shaker, M. E. Open Chem. J. 2014, 1, 33.
Saeed, A.; Abbas, N.; Flörke, U. J. Braz. Chem. Soc. 2007, 18, 559.
Liu, H.-L.; Li, Z.; Anthonsen, T. Molecules 2000, 5, 1055.
Zarghi, A.; Najafnia, L.; Daraee, B.; Dadrass, O. G.; Hedayati, M. Bioorg. Med. Chem. Lett. 2007, 17, 5634.
Appalanaidu, K.; Kotcherlakota, R.; Dadmal, T. L.; Bollu, V. S.; Kumbhare, R. M.; Patra, C. R. Bioorg. Med. Chem. Lett. 2016, 26, 5361.
Küçükgüzel, İ.; Satılmış, G.; Gurukumar, K. R.; Basu, A.; Tatar, E.; Nichols, D. B.; Talele, T. T.; Kaushik-Basu, N. Eur. J. Med. Chem. 2013, 69, 931.
Porshamsian, K.; Montazeri, N.; Rad-Moghadam, K.; Ali- Asgari, S. J. Heterocycl. Chem. 2010, 47, 1439.
Metwally, M. A.; Farahat, A. A.; Abdel-Wahab, B. F. J. Sulfur Chem. 2010, 31, 315.
Samala, G.; Madhuri, C.; Sridevi, P. J.; Nallangi, R.; Perumal, Y.; Dharmarajan, S. Eur. J. Chem. 2014, 5, 550.
Haouas, B.; Sbei, N.; Ayari, H.; Benkhoud, M. L.; Batanero, B. New J. Chem. 2018, 42, 11776.
Bougault, J.; Chabrier, P. Compt. Rend. 1947, 224, 656.
El-Sakka, S. S.; Soliman, M. H.; Abdullah, S. R. J. Chem. Sci. 2014, 1883.
El-Hashash, M. A.; Essawy, A.; Fawzy, A. S. Adv. Chem. 2014, 619749.
Abdel-Ghani, E. J. Chem. Res., Synop. 1999, 174.
Salem, M. S.; Guirguis, D. B.; El-Helw, E. A. E.; Ghareeb, M. A.; Derbala, H. A. Y. Int. J. Sci. Res. 2014, 3(5), 1274.
El-Aasar, N. K.; Saied, K. J. Heterocycl. Chem. 2008, 45, 645.
Kaminskyy, D.; Den Hartog, G. J. M.; Wojtyra, M.; Lelyukh, M.; Gzella, A.; Bast, A.; Lesyk, R. Eur. J. Med. Chem. 2016, 112, 180.
El-Mobayed, M. M.; Hussein, A. M.; Mohlhel, W. M. J. Heterocycl. Chem. 2010, 47, 534.
El-Hashash, M. A; Rizk, S. A.; Ahmed, E. A. Am. J. Chem. 2012, 2(1), 1.
Khachikyan, R. Dzh.; Atashyan, S. M.; Agbalyan, S. G. Arm. Khim. Zhurn. 1981, 34, 775.
Khachikyan, R. Dzh.; Grigoryan, R. T.; Atashyan, S. M.; Panosyan, G. А.; Agbalyan, S. G. Arm. Khim. Zhurn. 1984, 37, 237.
El-Desuky, S.; El-Deen, I. M.; Abdel-Megid, M. J. Indian Chem. Soc. 1992, 69, 340.
Kolos, N. N.; Beryozkina, T. V.; Orlov, V. D. Heterocycles 2003, 60, 2115.
Beryozkina, T. V.; Kolos, N. N.; Orlov, V. D.; Zubatyuk, R. I.; Shishkin, O. V. Phosphorus, Sulfur Silicon Relat. Elem. 2004, 179, 2153.
Kolos, N. N.; Kovalenko, L. U.; Borovskoy, V. A. Chem. Heterocycl. Compd. 2011, 47, 983 [Khim. Geterotsikl. Soedin. 2011, 1198.]
Yaseen, R.; Ekinci, D.; Senturk, M.; Hameed, A. D.; Ovais, S.; Rathore, P.; Samim, M.; Javed, K.; Supuran, C. T. Bioorg. Med. Chem. Lett. 2016, 26, 1337.
El-Hashash, M. A; Abdel-Rahman, T. M.; Soliman, F. M. Boll. Chim. Farm. 1998, 137, 87.
Ramsh, S. M.; Smorygo, V. K.; Ginak, A. I. Chem. Heterocycl. Compd. 1984, 20, 865 [Khim. Geterotsikl. Soedin. 1984, 1066.]
Espinosa, E.; Molins, E.; Lecomte, C. Chem. Phys. Lett. 1998, 285, 170.
Kolos, N. N.; Kovalenko, L. Yu.; Kulikov, A. Yu. Russ. Chem.Bull. 2009, 58, 1041 [Izv. Akad. Nauk, Ser. Khim. 2009, 1014.]
Wolinski, K.; Hinton. J. F.; Pulay, P. J. Am. Chem. Soc. 1990, 112, 8251.
(а) Onoue, K.; Shintou, T.; Zhang, C. S.; Itoh, I. Chem. Lett. 2006, 35, 22. a Sonye, J. P.; Koide, K. J. Org. Chem. 2006, 71, 6254. b Runcie, K. A.; Taylor, R. J. K. Chem. Commun. 2002, 974.
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J. A., Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Keith, T.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, O.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian 09, Revision B.01; Gaussian, Inc.: Wallingford, 2010.
Becke, A. D. J. Chem. Phys. 1993, 98, 5648.
Woon, D. E.; Dunning, T. H. J. Chem. Phys. 1993, 98, 1358.
Bader, R. F. W. Chem. Rev. 1991, 91, 893.
Bader, R. F. W. J. Phys. Chem. A 1998, 102, 7314.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, A64, 112.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2020, 56(9), 1202–1209
Rights and permissions
About this article
Cite this article
Kolos, N.N., Nazarenko, N.V., Shishkina, S.V. et al. Synthesis, study of the structure, and modification of the products of the reaction of 4-aryl-4-oxobut-2-enoic acids with thiourea. Chem Heterocycl Comp 56, 1202–1209 (2020). https://doi.org/10.1007/s10593-020-02798-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-020-02798-y