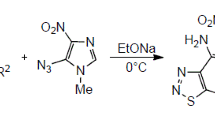
Novel 2-carboxamide-substituted 1,3,4-thiadiazines and 5,6-dihydro-4H-1,3,4-thiadiazin-5-ols were synthesized by the reaction of oxamic acid thiohydrazides with phenacyl bromides. This reaction was performed using an equimolar mixture of thiohydrazide and α-bromoacetophenones in methanol under basic conditions. The ratio of products obtained in the cyclocondensation was found to be strongly dependent on the substitution pattern and reaction conditions.


Similar content being viewed by others
References
Pfeiffer, W.-D.; Junghans, D.; Saghyan, A. S.; Langer, P. J. Heterocycl. Chem. 2014, 51, 1063.
Emelianov, V. V.; Ivanov, A. V.; Savateeva, E. A.; Sidorova, L. P.; Tseitler, T. A.; Gette, I. F.; Bulavintseva, T. S.; Danilova, I. G.; Maksimova, N. E.; Mochul'skaya, N. N.; Chupakhin, O. N.; Chereshnev, V. A. Russ. Chem. Bull., Int. Ed. 2017, 66, 1873. [Izv. Akad. Nauk, Ser. Khim. 2017, 1873.]
Sidorova, L. P.; Tseitler, T. A.; Perova, N. M.; Emel'yanov, V. V.; Savateeva, E. A.; Maksimova, N. E.; Mochul'skaya, N. N.; Chereshnev, V. A.; Chupakhin, O. N. Pharm. Chem. J. 2015, 49, 501. [Khim.-Farm. Zh. 2015, 49(8), 8.]
Shevelev, O. B.; Illarionova, N. B.; Petrovski, D. V.; Sarapultsev, A. P.; Chupakhin, O. N.; Moshkin, M. P. PLoS One 2017, 12(7), e0180739.
Almajan, G. L.; Barbuceanu, S.-F.; Saramet, I.; Draghici, C. Eur. J. Med. Chem. 2010, 45, 3191.
Abdel-Aziem, A. J. Heterocycl. Chem. 2015, 52, 251.
Prakash, O.; Aneja, D. K.; Hussain, K.; Lohan, P.; Ranjan, P.; Arora, S.; Sharma, C.; Aneja, K. R. Eur. J. Med. Chem. 2011, 46, 5065.
Hussein, M. A.; Shaker, R. M.; Ameen, M. A.; Mohammed, M. F. Arch. Pharm. Res. 2011, 34, 1239.
Sarıgüney, A. B.; Kocabaş, E.; Erci, F.; Torlak, E.; Coşkun, A. J. Heterocycl. Chem. 2018, 55, 2107.
Radini, I. A. M. Molecules 2018, 23, 2092.
Sibley, G. E. M.; Malström, L. J.; Larsson, J. M. WO Patent 2017009651.
Sagar, S. R.; Singh, D. P.; Panchal, N. B.; Das, R. D.; Pandya, D. H.; Sudarsanam, V.; Nivsarkar, M.; Vasu, K. K. ACS Chem. Neurosci. 2018, 9, 1663.
Fahmy, A. F. M.; Rizk, S. A.; Hemdan, M. M.; El-Sayed, A. A.; Hassaballah, A. I. J. Heterocycl. Chem. 2018, 55, 2545.
Čačić, M.; Pavić, V.; Molnar, M.; Šarkanj, B.; Has-Schön, E. Molecules 2014, 19, 1163.
Yang, Y.; Feng, Z.; Jiang, J.; Yang, Y.; Pan, X.; Zhang, P. Chem. Pharm. Bull. 2011, 59, 1016.
Ørstavik, Ø.; Ata, S.; Riise, J.; Dahl, C.; Andersen, G. Ø.; Levy, F.; Skomedal, T.; Osnes, J. B.; Qvigstad, E. Br. J. Pharmacol. 2014, 171, 5169.
Trines, S. A.; Smits, C. A.; van der Moer, J.; Slager, C. J.; Verdouw, P. D.; Krams, R. J. Cardiovasc. Pharmacol. 2002, 39, 61.
Duncker, D. J.; Haitsma, D. B.; Liem, D. A.; Heins, N.; Stubenitsky, R.; Verdouw, P. Br. J. Pharmacol. 2001, 134, 553.
Iradyan, M.; Iradyan, N.; Paronikyan, R.; Stepanyan, G. Pharm. Chem. J. 2010, 44, 413. [Khim.-Farm. Zh. 2010, 44(8), 11.]
Kumar, K.; Ung, P. M.-U.; Wang, P.; Wang, H.; Li, H.; Andrews, M. K.; Stewart, A. F.; Schlessinger, A.; DeVita, R. Eur. J. Med. Chem. 2018, 157, 1005.
Sarapultsev, A. P.; Chupakhin, O. N.; Sarapultsev, P. A.; Sidorova, L. P.; Tseitler, T. A. Pharmaceuticals 2016, 9, 27.
Nofal, Z. M.; Soliman, E. A.; Abd El-Karim, S. S.; El-Zahar, M. I.; Srour, A. M.; Sethumadhavan, S.; Maher, T. J. J. Heterocycl. Chem. 2014, 51, 1797.
Knak, S.; Pfeiffer, W. D.; Dollinger, H.; Saghyan, A. S.; Langer, P. J. Heterocycl. Chem. 2015, 52, 463.
Wolf, M.; Eggenweiler, H. DE Patent 10150517, 2003; Chem. Abstr. 2003, 297702.
Penta, S.; Vedula, R. R. J. Sulfur Chem. 2011, 32, 327.
Sarapultsev, A. P.; Vassiliev, P. M.; Sarapultsev, P. A.; Chupakhin, O. N.; Ianalieva, L. R.; Sidorova, L. P. Molecules 2018, 23, 1611.
Yarovenko, V. N.; Shirokov, A. V.; Krupinova, O. N.; Zavarzin, I. V.; Krayushkin, M. M. Russ. J. Org. Chem. 2003, 39, 1133. [Zh. Org. Khim. 2003, 39, 1204.]
Pfeiffer, W.-D.; Bulka, E.; Miethchen, R. Z. Chem. 1987, 27, 296.
Acknowledgements
The research is supported by the Russian Science Foundation (grant 18-33-00913 мол_а).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary information file containing NMR spectra of compounds 5, 6, and 7, synthesis of starting materials, results of X-ray crystallographic analysis is available at the journal website at http://link.springer.com/journal/10593.
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2019, 55(7), 665–671
Electronic supplementary material
ESM 1
(PDF 851 kb)
Rights and permissions
About this article
Cite this article
Komendantova, A.S., Ivanova, K.A., Lyssenko, K.V. et al. Facile Synthesis of Carboxamide-Substituted 1,3,4-Thiadiazines and 5,6-Dihydro-4H-1,3,4-Thiadiazin-5-Ols. Chem Heterocycl Comp 55, 665–671 (2019). https://doi.org/10.1007/s10593-019-02514-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-019-02514-5