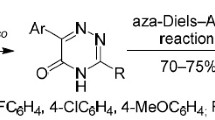
The reaction of 3,6-bis(het)aryl-1,2,4-triazines (hetaryl ≠ 2-pyridyl) with aryne intermediates generated in situ was studied. As a result, novel 1,4-bis(het)arylisoquinolines were synthesized with yields of up to 45%. The main rules of the reactions were studied, and the obtained experimental data were compared with those described in the literature.


Similar content being viewed by others
References
(a) Heterocycles in Natural Product Synthesis; Majumdar, K. C.; Chattopadhyay, S. K., Eds.; Wiley-VCH: Weinheim, 2011. (b) The Chemistry of Heterocyclic Compounds; Coppola, G. M.; Schuster, H. F.; Wiley: New York, 1981, Vol. 38, Part 3, p. 552.
Merck, G. Ann. Chem. Pharm. 1848, 66, 125.
(a) Handley, D. A.; Van Valen, R. G.; Melden, M. K.; Houlihan, W. J.; Saunders, R. N. J. Pharmacol. Exp. Ther. 1988, 247, 617. (b) Houlihan, W. J.; Cheon, S. H.; Parrino, V. A.; Handley, D. A.; Larson, D. A. J. Med. Chem. 1993, 36, 3098. (c) Scholz, D.; Schmidt, H.; Prieschl, E. E.; Csonga, R.; Scheirer, W.; Weber, V.; Lembachner, A.; Seidl, G.; Werner, G.; Mayer, P.; Baumruker, T. J. Med. Chem. 1998, 41, 1050. (d) Griffin, R. J.; Fontana, G.; Golding, B. T.; Guiard, S.; Hardcastle, I. R.; Leahy, J. J. J.; Martin, N.; Richardson, C.; Rigoreau, L.; Stockley, M.; Smith, G. C. M. J. Med. Chem. 2005, 48, 569.
(a) Iwasa, K.; Moriyasu, M.; Tachibana, Y.; Kim, H. S.; Wataya, Y.; Wiegrebe, W.; Bastow, K. F.; Cosentino, L. M.; Kozuka, M.; Lee, K. H. Bioorg. Med. Chem. 2001, 9, 2871. (b) Miller, J. F.; Gudmundsson, K. S.; D'Aurora Richardson, L.; Jenkinson, S.; Spaltenstein, A.; Thomson, M.; Wheelan, P. Bioorg. Med. Chem. Lett. 2010, 20, 3026. (c) Kashiwada, Y.; Aoshima, A.; Ikeshiro, Y.; Chen, Y.-P.; Furukawa, H.; Itoigawa, M.; Fujioka, T.; Mihashi, K.; Cosentino, L. M.; Morris-Natschke, S. L.; Lee, K. H. Bioorg. Med. Chem. 2005, 13, 443.
(a) Mukherjee, A.; Dutta, S.; Sanyal, U. J. Cancer Res. Ther. 2013, 9, 442. (b) Fontana, A.; Cavaliere, P.; Wahidulla, S.; Naik, C. G.; Cimino, G. Tetrahedron2000, 56, 7305.
(a) Jiang, Y.; Kong, W.; Shen, Y.; Wang, B. Tetrahedron2015, 71, 5584. (b) Kho, Y.-M.; Shin, E. J. Molecules2017, 22, 1569.
(a) Halder, S.; Ghosh, P.; Hazra, A.; Banerjee, P.; Roy, P. New J. Chem. 2018, 42, 8408. (b) Ma, Y.; Hao, Li, H.; Peng, S.; Wang, L. Anal. Chem. 2012, 84, 8415. (с) Zyryanov, G. V.; Kopchuk, D. S.; Kovalev, I. S.; Nosova, E. V.; Rusinov, V. L.; Chupakhin, O. N. Russ. Chem. Rev. 2014, 83, 783. [Usp. Khim.2014, 83, 783.]
(a) Kumar, N. S.; Rao, L. C.; Babu, N. J.; Meshram, H. M. RSC Adv. 2015, 5, 95539. (b) Roya, B.; Hazraab, P. J. Mol. Liq. 2018, 261, 520. (c) Zhao, Y.; Zhang, G.; Liu, Z.; Guo, C.; Peng, C.; Pei, M.; Li, P. J. Photochem. Photobiol., A2016, 314, 52. d Li, G.; Zhu, D.; Xue, L.; Jang, H. Org. Lett. 2013, 15, 5020.
(a) Rotzoll, S.; Willy, B.; Schönhaber, J.; Rominger, F.; Müller, T. J. J. Eur. J. Org. Chem. 2010, 3516. (b) Woody, K. B.; Henry, E. M.; Jagtap, S.; Collard, D. M. Macromolecules2011, 44, 9118. (c) Schaffroth, M.; Lindner, B. D.; Vasilenko, V.; Rominger, F.; Bunz, U. H. F. J. Org. Chem. 2013, 78, 3142. (d) Kopchuk, D. S.; Khasanov, A. F.; Kim, G. A.; Nosova, E. V.; Zyryanov, G. V.; Kovalev, I. S.; Rusinov, V. L.; Chupakhin, O. N. Russ. Chem. Bull., Int. Ed. 2015, 64, 872. [Izv. Akad. Nauk, Ser. Khim.2015, 872.] (e) Moni, L.; Gers-Panther, C. F.; Anselmo, M.; Müller, T. J. J.; Riva, R. Chem.–Eur. J. 2016, 22, 2020.
(a) Pomeranz, C. Monatsh. Chem. 1893, 14, 116. (b) Bischler, A.; Napieralski, B. Ber. Dtsch. Chem. Ges. 1893, 26, 190. (c) Comprehensive Organic Name Reactions and Reagents; Wang, Z., Ed.; Wiley-Interscience: New Jersey, 2010, p. 2206. c Doi, S.; Shirai, N.; Sato, Y. J. Chem. Soc., Perkin Trans. 1997, 1, 2217. (e) Comprehensive Organic Name Reactions and Reagents; Wang, Z., Ed.; Wiley-Interscience: New Jersey, 2010, p. 544. d Loones, K. T. J.; Maes, B. U. W.; Dommisse, R. A.; Lemiere, G. L. F. Chem. Commun. 2004, 2466. e Sharon, A.; Pratap, R.; Maulik, P. R.; Ram, V. J. Tetrahedron2005, 61, 3781.
Kopchuk, D. S.; Kovalev, I. S.; Khasanov, A. F.; Zyryanov, G. V.; Slepukhin, P. A.; Rusinov, V. L.; Chupakhin, O. N. Mendeleev Commun. 2013, 23, 142.
(a) Kopchuk, D. S.; Chepchugov, N. V.; Khasanov, A. F.; Kovalev, I. S.; Santra, S.; Nosova, E. V.; Zyryanov, G. V.; Majee, A.; Rusinov, V. L.; Chupakhin, O. N. Tetrahedron Lett. 2016, 57, 3862. (b) Kopchuk, D. S.; Nikonov, I. L.; Zyryanov, G. V.; Nosova, E. V.; Kovalev, I. S.; Slepukhin, P. A.; Rusinov, V. L.; Chupakhin, O. N. Mendeleev Commun. 2015, 25, 13. (c) Kopchuk, D. S.; Nikonov, I. L.; Zyryanov, G. V.; Kovalev, I. S.; Taniya, O. S.; Rusinov, V. L.; Chupakhin, O. N. Russ. J. Org. Chem. 2015, 51, 1170. [Zh. Org. Khim.2015, 51, 1189.] (d) Nikonov, I. L.; Kopchuk, D. S.; Kovalev, I. S.; Zyryanov, G. V.; Khasanov, A. F.; Slepukhin, P. A.; Rusinov, V. L.; Chupakhin, O. N. Tetrahedron Lett. 2013, 54, 6427.
(a) Kopchuk, D. S.; Nikonov, I. L.; Zyryanov, G. V.; Kovalev, I. S.; Rusinov, V. L.; Chupakhin, O. N. Chem. Heterocycl. Compd. 2014, 50, 907. [Khim. Geterotsikl. Soedin.2014, 983.] (b) Kopchuk, D. S.; Krinochkin, A. P.; Khasanov, A. F.; Kovalev, I. S.; Slepukhin, P. A.; Starnovskaya, E. S.; Mukherjee, A.; Rahman, M.; Zyryanov, G. V.; Majee, A.; Rusinov, V. L.; Chupakhin, O. N.; Santra, S. Synlett2018, 483. (с) Kopchuk, D. S.; Nikonov, I. L.; Krinochkin, A. P.; Kovalev, I. S.; Zyryanov, G. V.; Rusinov, V. L.; Chupakhin, O. N. Russ. J. Org. Chem. 2017, 53, 959. [Zh. Org. Khim.2017, 53, 942.]
Kopchuk, D. S.; Chepchugov, N. V.; Taniya, O. S.; Khasanov, A. F.; Giri, K.; Kovalev, I. S.; Santra, S.; Zyryanov, G. V.; Majee, A.; Rusinov, V. L.; Chupakhin, O. N. Tetrahedron Lett. 2016, 57, 5639.
Kopchuk, D. S.; Nikonov, I. L.; Khasanov, A. F.; Giri, K.; Santra, S.; Kovalev, I. S.; Nosova, E. V.; Gundala, S.; Venkatapuram, P.; Zyryanov, G. V.; Majee, A.; Chupakhin, O. N. Org. Biomol. Chem. 2018, 16, 5119.
Kopchuk, D. S.; Chepchugov, N. V.; Gorbunov, E. B.; Zyryanov, G. V.; Kovalev, I. S.; Nosova, E. V.; Slepukhin, P. A.; Rusinov, V. L.; Chupakhin, O. N. J. Iran. Chem. Soc. 2017, 14, 1507.
Gonsalves, A. M. d’A. R.; Pinho e Melo, T. M. V. D.; Gilchrist, T. L. Tetrahedron1992, 48, 6821.
Dhar, R.; Hühnermann, W.; Kämpchen, T.; Overheu, W.; Seitz, G. Chem. Ber. 1983, 116, 97.
Himmelsbach, F.; Langkopf, E.; Eckhardt, M.; Maier, R.; Mark, M.; Tadayyon, M.; Lotz, R. WO Patent 2004/041820 A1.
Balog, J.; Riedl, Z.; Hajós, G.; Miskolczy, Z.; Biczók, L. ARKIVOC2012, (v), 109.
(a) Metal Free C–H Functionalization of Aromatics; Charushin, V. N.; Chupakhin, O. N., Eds.; Springer International Publishing: Cham, 2014, p. 283. a Chupakhin, O. N.; Charushin, V. N. Tetrahedron Lett. 2016, 57, 2665. b Chupakhin, O. N.; Charushin, V. N. Pure Appl. Chem. 2017, 89, 1195. c Chupakhin, O. N.; Postovskii, I. Ya. Russ. Chem. Rev.1976, 45, 454. [Usp. Khim.1976, 45, 908.]
Konno, S.; Sagi, M.; Takaharu, E.; Fujimura, S.; Hayashi, K.; Yamanaka, H. Chem. Pharm. Bull. 1988, 36, 1721.
Krinochkin, A. P.; Kopchuk, D. S.; Kovalev, I. S.; Zyryanov, G. V.; Rusinov, V. L.; Chupakhin, O. N. Russ. J. Org. Chem. 2019, 55, 266. [Zh. Org. Khim.2019, 55, 303.]
Kozhevnikov, D. N.; Kovalev, I. S.; Prokhorov, A. M.; Rusinov, V. L.; Chupakhin O. N. Russ. Chem. Bull., Int. Ed.2003, 52, 1588. [Izv. Akad. Nauk, Ser. Khim.2003, 1504.]
Prokhorov, A. M.; Mąkosza, M.; Chupakhin O. N. Tetrahedron Lett. 2009, 50, 1444.
Kozhevnikov, D. N.; Kozhevnikov, V. N.; Kovalev, I. S.; Rusinov, V. L.; Chupakhin, O. N.; Aleksandrov, G. G. Russ. J. Org. Chem. 2002, 38, 744. [Zh. Org. Khim.2002, 38, 780.]
Dubovtsev, A. Yu.; Dar'in, D. V.; Krasavin, M.; Kukushkin, V. Yu. Eur. J. Org. Chem. 2019, 1856.
Saraswathi, T. V.; Srinivasan, V. R. Tetrahedron1977, 33, 1043.
Kozhevnikov, V. N.; Shabunina, O. V.; Kopchuk, D. S.; Ustinova, M. M.; König B.; Kozhevnikov, D. N. Tetrahedron2008, 64, 8963.
(a) Kopchuk, D. S.; Khasanov, A. F.; Kovalev, I. S.; Zyryanov, G. V.; Rusinov, V. L.; Chupakhin, O. N. Mendeleev Commun. 2013, 23, 209. (b) Kopchuk, D. S.; Khasanov, A. F.; Krinochkin, A. P.; Kovalev, I. S.; Zyryanov, G. V.; Rusinov, V. L.; Chupakhin, O. N. Russ. J. Org. Chem. 2016, 52, 1036. [Zh. Org. Khim.2016, 52, 1041.] (с) Kopchuk, D. S.; Khasanov, A. F.; Chepchugov, N. V.; Kovalev, I. S.; Zyryanov, G. V.; Rusinov, V. L.; Chupakhin, O. N. Russ. J. Org. Chem. 2017, 53, 99. [Zh. Org. Khim.2017, 53, 103.]
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr.2008, A64, 112.
This work was supported by the Russian Science Foundation (grant 18-13-00365).
Elemental analysis was performed by the elemental analysis group of the Postovsky Institute of Organic Synthesis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2019, 55(10), 978–984
Rights and permissions
About this article
Cite this article
Kopchuk, D.S., Nikonov, I.L., Khasanov, A.F. et al. One-step synthesis of 1,4-bis(het)arylisoquinolines by the reaction of 1,2,4-triazines with arynes. Chem Heterocycl Comp 55, 978–984 (2019). https://doi.org/10.1007/s10593-019-02565-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-019-02565-8