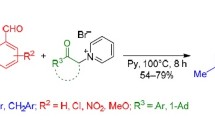
An efficient method for the synthesis of substituted 2-pyridones based on the aza-Diels–Alder reaction of 1,2,4-triazin-5-ones and dienophiles, 2,5-norbornadiene and 4-(cyclopent-1-en-1-yl)morpholine, is proposed.

Similar content being viewed by others
References
(а) Schultz, A. G. Chem. Rev.1973, 73, 385. a Teshima, Y.; Shin-Ya, K.; Shimazu, A.; Furihata, K.; Chul, H. S.; Furihata, K.; Hayakawa, Y.; Nagai, K.; Seto, H. J. Antibiot.1991, 44, 685.
Chong, D. J.; Lerman, A. M. Curr. Neurol. Neurosci. Rep.2016, 16, 39.
Niewerth, M.; Kunze, D.; Seibold, M.; Schaller, M.; Korting, H. C.; Hube, B. Antimicrob. Agents Chemother.2003, 47, 1805.
Koster, G.; Bekema, H. J.; Wetterslev, J.; Gluud, C.; Keus, F.; van der Horst, I. C. C. Intensive Care Med.2016, 42, 1322.
Wu, B.; Oesker, V.; Wiese, J.; Schmaljohann, R.; Imhoff, J. F. Mar. Drugs2014, 12, 1208.
(a) Morimoto, K.; Furusawa, H.; Terachi, T.; Nawamaki, T.; Watanabe, S.; Nakahira, K.; Noguchi, J. WO Patent 9857957; Chem. Abstr.1999, 130, 66511b. (b) TePaske, M. R.; Gloer, J. B.; Wicklow, D. T.; Dowd, P. F. Tetrahedron Lett.1991, 32, 5687.
Sellstedt, M.; Nyberg, A.; Rosenbaum, E.; Engström, P.; Wickström, M.; Gullbo, J.; Bergström, S.; Johansson, L. B.-Å; Almqvist, F. Eur. J. Org. Chem.2010, 6171.
Hagimori, M.; Mizuyama, N.; Tominaga, Y.; Mukai, T.; Saji, H. Dyes Pigm.2015, 113, 205.
Mizuyama, N.; Tominaga, Y.; Kohra, S.; Ueda, K.; Hirayama, S.; Shigemitsu, Y. Bull. Chem. Soc. Jpn. 2006, 79, 602.
(а) Torres, M.; Gil, S.; Parra, M. Curr. Org. Chem.2005, 9, 1757. a Heravi, M. M.; Hamidi, H. J. Iran. Chem. Soc.2013, 10, 265. b Hamama, W. S.; Waly, M.; El-Hawary, I.; Zoorob, H. H. Synth. Commun.2014, 44, 1730.
(а) Pabst, G. R.; Sauer, J. Tetrahedron Lett.1998, 39, 6687. a Rykowski, A.; Branowska, D.; Kielak, J. Tetrahedron Lett.2000, 41, 3657. b Kozhevnikov, V. N.; Shabunina, O. V.; Kopchuk, D. S.; Ustinova, M. M.; König, B.; Kozhevnikov, D. N. Tetrahedron2008, 64, 8963. c Kopchuk, D. S.; Kovalev, I. S.; Khasanov, A. F.; Zyryanov, G. V.; Slepukhin, P. A.; Rusinov, V. L.; Chupakhin, O. N. Mendeleev Commun.2013, 23, 142.
Taylor, E. C. Bull. Soc. Chim. Belg.1988, 97, 599.
(a) Taylor, E. C.; Pont, J. L.; Warner, J. C. J. Heterocycl. Chem.1988, 25, 1733. (b) Taylor, E. C.; Pont, J. L.; Van Engen, D.; Warner, J. C. J. Org. Chem.1988, 53, 5093.
Savchuk, M. I.; Starnovskaya, E. S.; Shtaitz, Y. K.; Kopchuk, D. S.; Nosova, E. V.; Zyryanov, G. V.; Rusinov, V. L.; Chupakhin, O. N. Russ. J. Gen. Chem.2018, 88, 2213. [Zh. Obshch. Khim.2018, 88, 1728.]
Shtaitz, Ya. K.; Savchuk, M. I.; Starnovskaya, E. S.; Krinochkin, A. P.; Kopchuk, D. S.; Santra, S.; Zyryanov, G. V.; Rusinov, V. L.; Chupakhin, O. N. AIP Conf. Proc.2019, 2063, 040050.
Fatykhov, R. F.; Savchuk, M. I.; Starnovskaya, E. S.; Bobkina, M. V.; Kopchuk, D. S.; Nosova, E. V.; Zyryanov, G. V.; Khalymbadzha, I. A.; Chupakhin, O. N.; Charushin, V. N.; Kartsev, V. G. Mendeleev Commun.2019, 29, 299.
(a) Kozhevnikov, V. N.; Kozhevnikov, D. N.; Nikitina, T. V.; Rusinov, V. L.; Chupakhin, O. N.; Zabel, M.; König, B. J. Org. Chem.2003, 68, 2882. (b) Kozhevnikov, D. N.; Kozhevnikov, V. N.; Kovalev, I. S.; Rusinov, V. L.; Chupakhin, O. N.; Aleksandrov, G. G. Russ. J. Org. Chem.2002, 38, 744. [Zh. Org. Khim.2002, 38, 780.]
(a) Rykowski, A.; Branowska, D.; Makosza, M.; Van Ly, P. J. Heterocycl. Chem.1996, 33, 1567. (b) Huang, J. J. J. Org. Chem.1985, 50, 2293. (c) Kozhevnikov, V. N.; Cowling, S. J.; Karadakov, P. B.; Bruce, D. W. J. Mater. Chem.2008, 18, 1703. (d) Chupakhin, O. N.; Egorov, I. N.; Rusinov, V. L.; Slepukhin, P. A. Russ. Chem. Bull., Int. Ed.2010, 59, 991. [Izv. Akad. Nauk, Ser. Khim.2010, 970.]
Kozhevnikov, V. N.; Ustinova, M. M.; Slepukhin, P. A.; Santoro, A.; Bruce, D. W.; Kozhevnikov, D. N. Tetrahedron Lett.2008, 49, 4096.
Chauhan, P.; Ravi, M.; Singh, S.; Prajapati, P.; Yadav, P. P. RSC Adv.2016, 6, 109.
Neunhoeffer, H.; Reichel, D.; Cullmann, B.; Rehn, I. Liebigs Ann. Chem.1990, 631.
This work was supported by the Russian Science Foundation (grant 18-13-00365).
Elemental analysis was performed by an elemental analysis group of the Postovsky Institute of Organic Synthesis of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2019, 55(10), 985–988
Rights and permissions
About this article
Cite this article
Savchuk, M.I., Shtaitz, Y.K., Kopchuk, D.S. et al. Efficient one-step synthesis of 3-aryl-2-pyridones from 6-aryl-1,2,4-triazin-5-ones. Chem Heterocycl Comp 55, 985–988 (2019). https://doi.org/10.1007/s10593-019-02566-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-019-02566-7