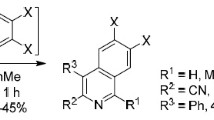
A new variant of Hantzsch reaction was developed for the synthesis of 1,2,3,4,5,6,7,8-octahydroquinolines on the basis of regioselective cascade recyclization of N-arylitaconimides in reactions with 3-aminocyclohex-2-enones. The mechanism of this domino process included С-nucleophilic addition of enaminone to the activated multiple bond of itaconimide and intramolecular transamidation with simultaneous recyclization of the intermediate.


Similar content being viewed by others

References
(a) Sato, T.; Yoritate, M.; Tajima, H; Chida, N. A. Org. Biomol. Chem. 2018, 16, 3864. (b) Shang, X. F.; Moris-Natschke, S. L.; Liu, Y.-Q.; Guo, X.; Xu, X.-S.; Goto, M.; Li, J.-C.; Yu, Y.; Yang, G.-Z.; Lee, K.-H. Med. Res. Rev. 2018, 38, 775. (c) Piccichè, M.; Pinto, A.; Griera, R.; Bosch, J.; Amat, M. Org. Lett. 2017, 19, 6654. (d) Bosch, C.; Fiser, B.; Gómez-Bengoa, E.; Bradshaw, B.; Bonjoch, J. Org. Lett. 2015, 17, 5084. (e) Dickson, E.; Pilkington, L. I.; Brimble, M. A.; Barker, D. A. Tetrahedron 2016, 72, 400. (f) Siengalewicz, P.; Mulzer, J.; Rinner, U. In The Alkaloids; Knolker, H.-J., Ed.; Elsevier: San Diego, 2013, Vol. 72, p. 1.
(a) Daly, J. W.; Nishizawa, Y.; Padgett, W.; Tokuyama, T.; McCloskey, P. J.; Waykole, L.; Schultz, A. G.; Aronstam, R. S. Neurochem. Res. 1991, 16, 1207. (b) Tsuneki, H.; You, Y.; Toyooka, N.; Sasaoka, T.; Nemoto, H.; Dani, J. A.; Kimura, I. Biol. Pharm. Bull. 2005, 28, 611. (c) Guthmann, H.; Conole, D.; Wright, E.; Körber, K.; Barker, D.; Brimble, M. A. Eur. J. Org. Chem. 2009, 1944.
Wright, A. D.; Goclik, E.; Konig, G. M.; Kaminsky, R. J. Med. Chem. 2002, 45, 3067.
Kubanek, J.; Williams, D. E.; De Silva, E. D.; Allen, T.; Andersen, R. J. Tetrahedron Lett. 1995, 36, 6189.
(a) Maiti, S.; Mendez, J. C. Chem. Commun. 2011, 47, 10554. (b) Akashi, M.; Sato Y.; Mori, M. J. Org. Chem. 2001, 66, 7873.
Procopiou, G.; Aggarwal P.; Newton, A. F.; Richards, D.; Mellor, J. R.; Harbottle, G.; Stockman, R. A. Chem. Commun. 2014, 50, 15355.
(a) Pelss, A.; Koskinen, A. M. P. Chem. Heterocycl. Compd. 2013, 49, 226. [Khim. Geterotsikl. Soedin. 2013, 249.] (b) Pu, X.; Ma, D. J. Org. Chem. 2006, 71, 6562.
(a) Nainwal, L. M.; Tasneem, S.; Akhtar, W.; Verma, G.; Khan, M. F.; Parvez, S.; Shaquiquzzaman, M.; Akhter, M.; Alam, M. M. Eur. J. Med. Chem. 2019, 121. (b) Nikoofar, K.; Yielzoleh, F. M. J. Saud. Chem. Soc. 2018, 715. (c) El Ashrya, El S. H.; Awada L. F.; El Kilanyc, Y.; Ibrahim, E. Adv. Hetеrocycl. Chem. 2009, 98, 1.
(a) Vill, J. J.; Steadman, T. R.; Godfrey, J. J. J. Org. Chem. 1964, 29, 2780. (b) Hickmott, P. W.; Rae, B. S. Afr. J. Chem. 1988, 41, 85. (c) Chelucci, G.; Cossu, S.; Scano, G.; Soccolini, F. Heterocycles 1990, 31, 1397.
(a) Mahajan, J. R.; Ferreira, G. A. L.; Araujo, H. C.; Nunes, B. Synthesis 1976, 112. (b) Campbell, A. D.; Stevens D. R. J. Chem. Soc. 1956, 959. (c) Reinshagen, H. Angew. Chem., Int. Ed. 1964, 3, 807. (d) Greenhill, J. V.; Mohamed, M. I. J. Chem. Soc., Perkin Trans. 1 1979, 1411.
Rai, A.; Singh, A. K.; Singh, P.; Yadav, L. D. S. Tetrahedron Lett. 2011, 52, 1354.
(a) Enders, D.; Demir, A. S. Tetrahedron Lett. 1987, 28, 3795. (b) Paulvannan, K.; Stille, J. R. Tetrahedron Lett. 1993, 34, 6673. (c) Strozhev, M. F.; Lielbriedis, I. É. Chem. Heterocycl. Compd. 1993, 29, 1048. [Khim. Geterotsikl. Soedin. 1993, 1227.] (d) Yao, C.; Jiao, W.; Xiao, Z.; Liu, R.; Li, T.; Yu, C. Tetrahedron 2013, 69, 1133.
(a) Gu, X.; Georg, H. I. Tetrahedron 2013, 69, 9406. (b) Strozhev, M. F.; Lielbriedis, I. É.; Neiland, O. Y. Chem. Heterocycl. Compd. 1990, 26, 655. [Khim. Geterotsikl. Soedin. 1990, 786.] (c) Wang, X.-S.; Zhang, M.-M.; Jiang, H.; Yao, C.-S.; Wang, J.; Tu, S.-J. Tetrahedron 2007, 63, 4439. c Jiang, B.; Liang, Y.-B.; Kong, L.-F.; Tu, X.-J.; Hao, W.-J.; Ye, Q.; Tu, S.-J. RSC Adv. 2014, 4, 54480. d Vereshchagin, A. N.; Elinson, M. N.; Anisina, Y. E.; Karpenko, K. A.; Goloveshkin, A. S.; Zlotin, S. G.; Egorov, M. P. Mol. Diversity 2018, 22, 627. e Zadsirjan, V.; Mandizadeh, S. J.; Heravi, M. M.; Heydari, M. Can. J. Chem. 2018, 96, 1071.
(a) Azzam, S. H. S.; Siddekha, A.; Pasha, M. A. Tetrahedron Lett. 2012, 53, 6306. (b) Suárez, M.; Ochoa, E.; Verdecia, Y.; Pita, B.; Morán, L.; Martín, N.; Quinteiro, M.; Seoane, C.; Soto, J.; Novoa, H.; Blaton, N.; Peters, O. M. Tetrahedron 1999, 55, 875. (c) Tu, S.; Zhu, X.; Zhang, J.; Xu, Z.; Zhang, Y.; Wang, Q.; Jia, R.; Jiang, B.; Zhang, J.; Yao, C. Bioorg. Med. Chem. Lett. 2006, 16, 2925. (d) Ziarani, G. M.; Asadi, S.; Badiei, A.; Mousavi, S.; Gholamzadeh, P. Res. Chem. Intermed. 2015, 41, 637. (e) Guoyong, S.; Bo, W.; Xiaoyin, W.; Yuru, K.; Liming, Y. Synth. Commun. 2005, 35, 2875.
(a) Shah, K. R.; Blanton, C. D. Jr. J. Org. Chem. 1982, 47, 502. (b) Rudenko, R. V.; Komykhov, S. A.; Desenko, S. M.; Sen'ko, Y. V.; Shishkin, O. V.; Konovalova, I. S.; Shishkina, S. V.; Chebanov, V. A. Synthesis 2011, 3161. (c) Vandyshev, D. Y.; Shikhaliev, K. S.; Potapov, A. Y.; Krysin, M. Yu. Chem. Heterocycl. Compd. 2015, 51, 829. [Khim. Geterotsikl. Soedin. 2015, 51, 829.] (d) Rudenko, R. V.; Komykhov, S. A.; Musatov, V. I.; Konovalova, I. S.; Shishkin, O. V.; Desenko, S. M. J. Heterocycl. Chem. 2011, 48, 888. d Havrylyuk, D.; Zimenkovsky, B.; Lesyk, R. Phosphorus, Sulfur Silicon Relat. Elem. 2009, 184, 638. e Lesyk, R.; Vladzimirska, O.; Holota, S.; Zaprutko, L.; Gzella, A. Eur. J. Med. Chem. 2007, 42, 641. f Hahn, H.-G.; Nam, K. D.; Mah, H. Heterocycles 2001, 55, 1283.
Vandyshev, D. Y.; Shikhaliev, K. S.; Kokonova, A. V.; Potapov, A. Y.; Kolpakova, M. G.; Sabynin, A. L.; Zubkov, F. I. Chem. Heterocycl. Compd. 2016, 52, 493. [Khim. Geterotsikl. Soedin. 2016, 52, 493.]
Vandyshev, D. Y.; Shikhaliev, K. S.; Potapov, A. Y.; Krysin, M. Y.; Zubkov, F. I.; Sapronova, L. V. Beilstein J. Org. Chem. 2017, 13, 2561.
(a) Stout, D. M.; Meyers, A. I. Chem. Rev. 1982, 82, 223. (b) Simon, C.; Constantieux, T.; Rodriguez, J. Eur. J. Org. Chem. 2004, 4957.
Huang, Y.; Hartmann, R. W. Synth. Commun. 1998, 28, 1197.
Abdel-Naby, A. S. J. Appl. Polym. Sci. 2011, 121, 169.
This work was performed with financial support from the Russian Science Foundation (contract No. 18-74-10097).
The results of this study were obtained in part by using the scientific equipment at the Collective Use Center of Voronezh State University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2019, 55(8), 748–754
Electronic supplementary material
ESM 1
(PDF 26777 kb)
Rights and permissions
About this article
Cite this article
Kovygin, Y.A., Shikhaliev, K.S., Krysin, M.Y. et al. Cascade recyclization of N-arylitaconimides as a new approach to the synthesis of polyfunctional octahydroquinolines. Chem Heterocycl Comp 55, 748–754 (2019). https://doi.org/10.1007/s10593-019-02530-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-019-02530-5