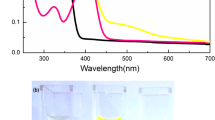
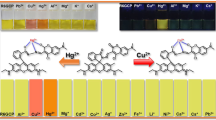
A 3-hydroxyflavone derivative as fluorescence chemosensor for copper and zinc ions has been synthesized. Its recognition properties for copper and zinc ions in aqueous solution were investigated based on UV-vis absorption and fluorescence spectra change. The compound exhibits color change from colorless to yellow and quenching of green fluorescence in the presence of copper ions in aqueous solution due to coordination reaction. In turn, the addition of zinc ions induces the color change from colorless to green and fluorescence enhancement of the compound. The complex ratios between the sensor molecule and copper(zinc) ions are 1:1 and 1:2, respectively, based on the Job's plots and in situ mass spectra. Thus, this compound can selectively recognize copper and zinc ions, and the recognition also can be observed by naked eye.





Similar content being viewed by others
References
Jia, J.; Wu, A.; Luan, S. J. Colloid. Surf., A 2014, 449, 1.
Wang, F.; Lu, X.; Li, X.-Y. J. Hazard. Mater. 2016, 308, 75.
Zhou, Y.; Tang, L.; Zeng, G.; Zhang, C.; Xie, X. Sens. Actuators, B 2016, 223, 280.
Wei, Z.; Sandron, S.; Townsend, A. T.; Nesterenko, P. N.; Paull, B. Talanta 2015, 135, 155.
Li, Y.; Hu, Y.; Zhao, Y.; Shi, G.; Deng, L.; Hou, Y. B.; Qu, L. T. Adv. Mater. 2011, 23, 776.
Huang, D. W.; Niu, C.; Ruan, M.; Wang, X.; Zeng, G.; Deng, C. Environ. Sci. Technol. 2013, 47, 4392.
Yao, J.; Zhang, K.; Zhu, H.; Ma, F.; Sun, M.; Yu, H.; Sun, J.; Wang, S. Anal. Chem. 2013, 85, 6461.
Wang, M.; Yan, F.; Zou, Y.; Chen, L.; Yang, N.; Zhou, X. Sens. Actuators, B 2014, 192, 512.
Zhang, G.; Li, H.; Bi, S.; Song, L.; Lu, Y.; Zhang, L.; Yu, J.; Wang, L. Analyst 2013, 138, 6163.
Hapuarachchige, S.; Bryant, B. K.; Arterburn, J. B. Chem. Heterocycl. Compd. 2014, 50, 254. [Khim. Geterotsikl. Soedin. 2014, 280.]
Yang, Y. M.; Zhao, Q.; Feng, W.; Li, F. Chem. Rev. 2013, 113, 192.
Liu, S. J.; Zhou, N.; Chen, Z.; Wei, H.; Zhu, Y.; Guo, S.; Zhao, Q. Opt. Lett. 2017, 42, 13.
Yuan, M.-S.; Wang, Q.; Wang, W.; Wang, D.-E.; Wang, J.; Wang, J. Analyst 2014, 139, 1541.
Yu, X.; Wang, K.; Cao, D.; Wu, Q.; Guan, R.; Xu, Y.; Sun, Y.; Liu, Z. Chem. Heterocycl. Compd. 2017, 53, 42. [Khim. Geterotsikl. Soedin. 2017, 53, 42.]
Ha, C. H. H.; Fatima, A.; Gaurav, A. Adv. Bioinf. 2015, 1.
Jin, X.; Liu, C.; Wang, X.; Huang, H.; Zhang, X.; Zhu, H. Sens. Actuators, B 2015, 216, 141.
Ghosh, D.; Ahamed, G.; Batuta, S.; Begum, N. A.; Mandal, D. J. Photochem. Photobiol., A 2016, 328, 77.
Chen, S.; Hou, P.; Zhou, B.; Song, X.; Wu, J.; Zhang, H.; Foley, J. W. RSC Adv. 2013, 3, 11543.
Feng, W.; Wang, Y.; Chen, S.; Wang, C.; Wang, S.; Li, S.; Li, H.; Zhou, G.; Zhang, J. Dyes Pigm. 2016, 131, 145.
Wu, Q.; Wang, K.; Wang, Z.; Sun, Y.; Cao, D.; Liu, Z.; Guan, R.; Zhao, S.; Yu, X. Talanta 2018, 181, 118.
Gunduz, S.; Goren, A. C.; Ozturk, T. Org. Lett. 2012, 14, 1576.
Wu, Q.; Wang, Z.; Li, J.; Qiu, S.; Cao, D.; Liu, Z.; Guan, R. RSC Adv. 2016, 6, 72698.
Ashok, D.; Ravi, S.; Lakshmi, B. V.; Ganesh, A. J. Serb. Chem. Soc. 2015, 80, 1361.
Zhu, M.; Yuan, M.; Liu, X.; Xu, J.; Lv, J.; Huang, C.; Liu, H.; Li, Y.; Wang, S.; Zhu, D. Org. Lett. 2008, 10, 1481.
Isaad, J.; Achari, A. E. Tetrahedron 2011, 67, 5678.
Hibbert, D. B.; Thordarson, P. Chem. Commun. 2016, 52, 12792.
Ulatowski, F.; Dąbrowa, K.; Balakier, T.; Jurczak, J. J. Org. Chem. 2016, 81, 1746.
Wang, F.; Wang, L.; Chen, X.; Yoon, J. Chem. Soc. Rev. 2014, 43, 4312.
Li, Z.; Lou, X.; Yu, H.; Li, Z.; Qin, J. Macromolecules 2008, 41, 7433.
Anbu, S.; Ravishankaran, R.; Guedes da Silva, M. F. C.; Karande, A. A.; Pombeiro, A. J. L. Inorg. Chem. 2014, 53, 6655.
Grazul, M.; Budzisz, E. Coord. Chem. Rev. 2009, 253, 2588.
This work is supported by Shandong wall materials innovation and building energy saving research and development project (2012QG007), Shandong science and technology project of department of housing and urban rural construction (2013RK031), Shandong science and technology project of department of housing and urban rural construction (2016).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary information file containing UV-vis absorption, mass, and IR spectra of compound 1 is available at the journal website at http://link.springer.com/journal/10593.
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2018, 54(2), 125–129
Electronic supplementary material
ESM 1
(PDF 646 kb)
Rights and permissions
About this article
Cite this article
Gao, H., Wu, X. A 3-hydroxyflavone derivative as fluorescence chemosensor for copper and zinc ions. Chem Heterocycl Comp 54, 125–129 (2018). https://doi.org/10.1007/s10593-018-2243-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-018-2243-9