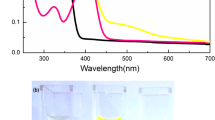
Abstract
The fluorescent moieties coumarin and xanthene (R6GCP) combined in a single molecule was designed and synthesized. The colorimetric and fluorescent variation of the probe towards the copper and mercury ions sensing is examined. With the added copper/mercury ions to the solution of R6GCP in DMF:H2O (2:8, v/v), the probe showed deep red color from yellow color. The probe showed turn-off and turn-on fluorescence for copper and mercury ion respectively. In the presence of other competing metal ions, the probe showed better sensitivity towards copper and mercury ions. The probe’s detection limit found to be 5.29 × 10–6 M and 1.24 × 10–5 M for Cu2+ and Hg2+ ion respectively by the UV–visible spectral measurement. Fluorescence measurement, the detection limit for the Cu2+ and Hg2+ ions detection by this probe is 1.91 × 10–7 M, and 1.32 × 10–8 M respectively. 1:1 binding stoichiometry was confirmed between the probe and Cu2+/Hg2+ ions from jobs plot by UV–visible spectral technique. Moreover, R6GCP combined filter paper were prepared. These test paper containing probe could detect Cu2+/Hg2+ ions in real-time with a spontaneous color change.
Graphical Abstract














Similar content being viewed by others
Availability of Data and Material
All data available.
Code Availability
Not applicable.
References
Garcia-Beltran O, Mena N, Friedrich LC, Netto-Ferreira JC, Vargas V, Quina FH, Nunez MT, Cassels BK (2012) Design and synthesis of a new coumarin-based ‘turn-on’ fluorescent probe selective for Cu+2. Tetrahedron Lett 53(39):5280–5283. https://doi.org/10.1016/j.tetlet.2012.07.082
Dujols V, Ford F, Czarnik AW (1997) A long-wavelength fluorescent chemodosimeter selective for Cu(II) ion in water. J Am Chem Soc 119(31):7386–7387. https://doi.org/10.1021/ja971221g
Angupillai S, Hwang JY, Lee JY, Rao BA, Son YA (2015) Efficient rhodamine-thiosemicarbazide-based colorimetric/fluorescent ‘turn-on’ chemodosimeters for the detection of Hg2+ in aqueous samples. Sens Actuators B 214(31):101–110. https://doi.org/10.1016/j.snb.2015.02.126
Sanchez AJ, Farfan N, Santillan R (2013) A reversible fluorescent–colorimetric Schiff base sensor for Hg2+ ion. Tetrahedron Lett 54(39):5279–5283. https://doi.org/10.1016/j.tetlet.2013.07.072
Yuan Y, Jiang S, Miao Q, Zhang J, Wang M, An L, Cao Q, Guan Y, Zhang Q, Liang G (2014) Fluorescent switch for fast and selective detection of mercury(II) ions in vitro and in living cells and a simple device for its removal. Talanta 125(1):204–209. https://doi.org/10.1016/j.talanta.2014.02.063
Yari A, Papi F (2011) Ultra trace mercury (II) detection by a highly selective new optical sensor. Sens Actuators B 160(1):698–704. https://doi.org/10.1016/j.snb.2011.08.051
Li G, Gao G, Cheng J, Chen X, Zhao Y, Ye Y (2016) Two new reversible naphthalimide-based fluorescent chemosensors for Hg2+. Luminescence 31:992–996. https://doi.org/10.1002/bio.3063
Zhou Q, Wu Z, Huang X, Zhong F, Cai Q (2015) A highly selective fluorescent probe for in vitro and in vivo detection of Hg2+. Analyst 140:6720–6726. https://doi.org/10.1039/C5AN00452G
Ni J, Li B, Zhang L, Zhao H, Jiang H (2015) A fluorescence turn-on probe based on rhodamine derivative and its functionalized silica material for Hg2+-selective detection. Sens Actuators B 215:174–180. https://doi.org/10.1016/j.snb.2015.03.057
Wang M, Wen J, Qin Z, Wang H (2015) A new coumarin–rhodamine FRET system as an efficient ratiometric fluorescent probe for Hg2+ in aqueous solution and in living cells. Dyes Pigm 120:208–212. https://doi.org/10.1016/j.dyepig.2015.04.013
Yang YK, Yook KJ, Tae J (2005) A Rhodamine-based fluorescent and colorimetric chemodosimeter for the rapid detection of Hg2+ ions in aqueous media. J Am Chem Soc 127(48):16760–16761. https://doi.org/10.1021/ja054855t
Huang K, Yue Y, Jiao X, Liu C, Wang Q, He S, Zhao L, Zeng X (2017) Fluorescence regulation of 4-aminobenzofluoran and its applications for Cu2+-selective fluorescent probe and bioimaging. Dyes Pigm 143:379–386. https://doi.org/10.1016/j.dyepig.2017.04.064
Maity D, Govindaraju T (2011) Highly Selective Visible and Near-IR Sensing of Cu2+ Based on Thiourea-Salicylaldehyde Coordination in Aqueous Media. Chem Eur J 17:1410–1414. https://doi.org/10.1002/chem.201002570
Liu C, Jiao X, He S, Zhao L, Zeng X (2017) A highly selective and sensitive fluorescent probe for Cu2+ based on a novel naphthalimide–rhodamine platform and its application in live cell imaging. Org Biomol Chem 15:3947–3954. https://doi.org/10.1039/C7OB00538E
Yamini Y, Alizadeh N, Shamsipur M (1997) Solid phase extraction and determination of ultra trace amounts of mercury(II) using octadecyl silica membrane disks modified by hexathia-18-crown-6-tetraone and cold vapour atomic absorption spectrometry. Anal Chim Acta 355(1):69–74. https://doi.org/10.1016/S0003-2670(97)81613-3
Thomas F, Maslon A, Bottero JY, Rouiller J, Montlgny F, Genévrlere F (1993) Aluminum(III) Speciation with Hydroxy Carboxylic Acids. 27AI NMR Study. Environ Sci Technol 27(12):2511–2516
Zheng F, Hu B (2008) Novel bimodal porous N-(2-aminoethyl)-3-aminopropyltrimethoxysilane-silica monolithic capillary microextraction and its application to the fractionation of aluminum in rainwater and fruit juice by electrothermal vaporization inductively coupled plasma mass spectrometry. Spectrochim Acta Part B 63(1):9–18. https://doi.org/10.1016/j.sab.2007.10.034
Bobrowski A, Nowak K, Zarebski J (2005) Application of a bismuth film electrode to the Voltammetric determination of trace iron using a Fe(III)-TEA-Br O3- catalytic system. Anal Bioanal Chem 382(7):1691–1697. https://doi.org/10.1007/s00216-005-3313-2
Harrington CF, Merson SA, D’Silva TMD (2004) Method to reduce the memory effect of mercury in the analysis of fish tissue using inductively coupled plasma mass spectrometry. Anal Chim Acta 505(2):247–254. https://doi.org/10.1016/j.aca.2003.10.046
Ferreira SLC, Queiroz AS, Fernandes MS, dos Santos HC (2002) Application of factorial designs and Doehlert matrix in optimization of experimental variables associated with the preconcentration and determination of vanadium and copper in seawater by inductively coupled plasma optical emission spectrometry. Spectrochim Acta B 57(12):1939–1950. https://doi.org/10.1016/S0584-8547(02)00160-X
Zioła-Frankowska A, Frankowski M, Siepak J (2009) Development of a new analytical method for online simultaneous qualitative determination of aluminium (free aluminium ion, aluminium-fluoride complexes) by HPLC-FAAS. Talanta 78(2):623–630. https://doi.org/10.1016/j.talanta.2008.12.028
Bayindir S (2019) A simple rhodanine-based fluorescent sensor for mercury and copper: The recognition of Hg2+ in aqueous solution, and Hg2+/Cu2+ in organic solvent. J Photochem Photobiol A 372:235–244. https://doi.org/10.1016/j.jphotochem.2018.12.021
Mohanasundaram D, Bhaskar R, Sankarganesh M, Nehru K, Kumar GGV, Rajesh J (2022) A simple pyridine based fluorescent chemosensor for selective detection of copper ion. Spectrochim Acta A Mol Biomol Spectrosc 265:120395. https://doi.org/10.1016/j.saa.2021.120395
Isaad J, Achari AE (2022) Water-soluble coumarin based sequential colorimetric and fluorescence on-off chemosensor for copper(II) and cyanide ions in water. Opt Mater 127:112275. https://doi.org/10.1016/j.optmat.2022.112275
Sasan S, Chopra T, Gupta A, Tsering D, Kapoor KK, Parkesh R (2022) Fluorescence “Turn-Off” and Colorimetric Sensor for Fe2+, Fe3+, and Cu2+ Ions Based on a 2,5,7-Triarylimidazopyridine Scaffold. ACS Omega 7(13):11114–11125. https://doi.org/10.1021/acsomega.1c07193
Zhu H, Fan J, Wang B, Peng X (2015) Fluorescent, MRI, and colorimetric chemical sensors for the first-row d-block metal ions. Chem Soc Rev 44:4337–4366. https://doi.org/10.1039/C4CS00285G
Lee MH, Kim JS, Sessler JL (2015) Small molecule-based ratiometric fluorescence probes for cations, anions, and biomolecules. Chem Soc Rev 44:4185–4191. https://doi.org/10.1039/C4CS00280F
Yang Y, Zhao Q, Feng W, Li F (2013) Luminescent chemodosimeters for bioimaging. Chem Rev 113(1):192–270. https://doi.org/10.1021/cr2004103
Chen X, Pradhan T, Wang F, Kim JS, Yoon J (2012) Fluorescent chemosensors based on spiroring-opening of xanthenes and related derivatives. Chem Rev 112(3):1910–1956. https://doi.org/10.1021/cr200201z
Zhang X, Shiraishi Y, Hirai T (2007) Cu(II)-selective green fluorescence of a rhodamine−diacetic acid conjugate. Org Lett 9(24):5039–5042. https://doi.org/10.1021/ol7022714
Garcia-Beltran O, Cassels BK, Perez C, Mena N, Nunez MT, Martinez NP, Pavez P, Aliaga ME (2014) Coumarin-based fluorescent probes for dual recognition of copper(II) and iron(III) ions and their application in bio-imaging. Sensors 14(1):1358–1371. https://doi.org/10.3390/s140101358
Yeh JT, Chen WC, Liu SR, Wu SP (2014) A coumarin-based sensitive and selective fluorescent sensor for copper(II) ions. New J Chem 38:4434–4439. https://doi.org/10.1039/C4NJ00695J
Huang L, Cheng J, Xie K, Xi P, Hou F, Li Z, Xie G, Shi Y, Liu H, Bai D, Zeng Z (2011) Cu2+-selective fluorescent chemosensor based on coumarin and its application in bioimaging. Dalton Trans 40:10815–10817. https://doi.org/10.1039/C1DT11123J
Kumar M, Kumar N, Bhalla V, Sharma PR, Kaur T (2012) Highly selective fluorescence turn-on chemodosimeter based on rhodamine for nanomolar detection of copper ions. Org Lett 14(1):406–409. https://doi.org/10.1021/ol203186b
Chen X, Jia J, Ma H, Wang S, Wang X (2009) Characterization of rhodamine B hydroxylamide as a highly selective and sensitive fluorescence probe for copper(II). Anal Chim Acta 632(1):9–14. https://doi.org/10.1016/j.aca.2007.08.025
Wang Y, Chang HQ, Wu WN, Peng WB, Yan YF, He CM, Chen TT, Zhao XL, Xu ZQ (2016) Rhodamine 6G hydrazone bearing pyrrole unit: Ratiometric and selective fluorescent sensor for Cu2+ based on two different approaches. Sens Actuators B Chem 228:395–400. https://doi.org/10.1016/j.snb.2016.01.052
Wang E, Zhou Y, Huang Q, Pang L, Qiao H, Yu F, Gao B, Zhang J, Min Y, Ma T (2016) 5-Hydroxymethylfurfural modified rhodamine B dual-function derivative: Highly sensitive and selective optical detection of pH and Cu2+. Spectrochim Acta A Mol Biomol Spectrosc 152:327–335. https://doi.org/10.1016/j.saa.2015.07.090
Yu M, Yuan R, Shi C, Zhou W, Wei L, Li Z (2013) 1,8-Naphthyridine and 8-hydroxyquinoline modified Rhodamine B derivatives: “Turn-on” fluorescent and colorimetric sensors for Al3+ and Cu2+. Dyes Pigm 99(3):887–894. https://doi.org/10.1016/j.dyepig.2013.07.030
Tian MZ, Hu MM, Fan JL, Peng XJ, Wang JY, Sun SG, Zhang R (2013) Rhodamine-based ‘turn-on’ fluorescent probe for Cu2+ and its fluorescence imaging in living cells. Bioorg Med Chem Lett 23(10):2916–2919. https://doi.org/10.1016/j.bmcl.2013.03.052
Huang L, Chen F, Xi P, Xie G, Li Z, Shi Y, Xu M, Liu H, Ma Z, Bai D, Zeng Z (2011) A turn-on fluorescent chemosensor for Cu2+ in aqueous media and its application to bioimaging. Dyes Pigm 90(3):265–268. https://doi.org/10.1016/j.dyepig.2011.01.003
Tang L, Li F, Liu NR (2011) Single sensor for two metal ions: Colorimetric recognition of Cu2+ and fluorescent recognition of Hg2+. Spectrochim Acta A Mol Biomol Spectrosc 78(3):1168–1172. https://doi.org/10.1016/j.saa.2010.12.072
Huang L, Hou FP, Xi P, Bai D, Xu M, Li Z, Xie G, Shi Y, Liu H, Zeng Z (2011) A rhodamine-based “turn-on” fluorescent chemodosimeter for Cu2+ and its application in living cell imaging. J Inorg Biochem 105(6):800–805. https://doi.org/10.1016/j.jinorgbio.2011.02.012
Tang J, Ma S, Zhang D, Liu Y, Zhao Y, Ye Y (2016) Highly sensitive and fast responsive ratiometric fluorescent probe for Cu2+ based on a naphthalimide-rhodamine dyad and its application in living cell imaging. Sens Actuators B 236:109–115. https://doi.org/10.1016/j.snb.2016.05.144
Zhou Y, Wang F, Kim Y, Kim SJ, Yoon J (2009) Cu2+-selective ratiometric and “off-on” sensor based on the rhodamine derivative bearing pyrene group. Org Lett 11(19):4442–4445. https://doi.org/10.1021/ol901804n
Xu ZH, Wang HW, Hou XF, Xu WL, Xiang TC, Wu CZ (2014) A novel ratiometric colorimetric and NIR fluorescent probe for detecting Cu2+ with high selectivity and sensitivity based on rhodamine-appended cyanine. Sens Actuators B 201:469–474. https://doi.org/10.1016/j.snb.2014.05.026
Muthuraj B, Deshmukh R, Trivedi V, Iyer PK (2014) Highly selective probe detects Cu2+ and endogenous NO gas in living cell. ACS Appl Mater Interfaces 6(9):6562–6569. https://doi.org/10.1021/am501476w
Kar C, Adhikari MD, Ramesh A, Das G (2013) NIR-and FRET-based sensing of Cu2+ and S2- in physiological conditions and in live cells. Inorg Chem 52(2):743–752. https://doi.org/10.1021/ic301872q
Yuan L, Lin W, Zheng K, Zhu S (2013) FRET-based small-molecule fluorescent probes: rational design and bioimaging applications. Acc Chem Res 46(7):1462–1473. https://doi.org/10.1021/ar300273v
He G, Zhang X, He C, Zhao X, Duan C (2010) Ratiometric fluorescence chemosensors for copper(II) and mercury(II) based on FRET systems. Tetrahedron 66(51):9762–9768. https://doi.org/10.1016/j.tet.2010.09.043
Lopez MT, Munoz A, Ibeas S, Serna F, Garcia FC, Garcia JM (2016) Colorimetric detection and determination of Fe(III), Co(II), Cu(II) and Sn(II) in aqueous media by acrylic polymers with pendant terpyridine motifs. Sens Actuators B 226:118–126. https://doi.org/10.1016/j.snb.2015.11.116
Heo G, Manivannan R, Kim H, Kim MJ, Min KS, Son YA (2019) Developing an RGB - Arduino device for the multi-color recognition, detection and determination of Fe(III), Co(II), Hg(II) and Sn(II) in aqueous media by a terpyridine moiety. Sens Actuators, B 297:126723. https://doi.org/10.1016/j.snb.2019.126723
Georgiev NI, Dimitrova MD, Asiri AM, Alamry KA, Bojinov VB (2015) Synthesis, sensor activity and logic behaviour of a novel bichromophoric system based on rhodamine 6G and 1,8-naphthalimide. Dyes Pigm 115:172–180. https://doi.org/10.1016/j.dyepig.2015.01.001
Zeng X, Dong L, Wu C, Mu L, Xue SF, Tao Z (2009) Highly sensitive chemosensor for Cu(II) and Hg(II) based on the tripodal rhodamine receptor. Sens Actuators B 141(2):506–510. https://doi.org/10.1016/j.snb.2009.07.013
Wang L, Yan J, Qin W, Liu W, Wang R (2012) A new rhodamine-based single molecule multianalyte (Cu2+, Hg2+) sensor and its application in the biological system. Dyes Pigm 92(3):1083–1090. https://doi.org/10.1016/j.dyepig.2011.07.010
Jiao Y, Zhou L, He H, Yin J, Duan C (2017) A new fluorescent chemosensor for recognition of Hg2+ ions based on a coumarin derivative. Talanta 162:403–407. https://doi.org/10.1016/j.talanta.2016.10.004
Guha S, Lohar S, Hauli I, Mukhopadhyay SK, Das D (2011) Vanillin-coumarin hybrid molecule as an efficient fluorescent probe for trace level determination of Hg(II) and its application in cell imaging. Talanta 85(3):1658–1664. https://doi.org/10.1016/j.talanta.2011.06.073
Chang HQ, Zhao XL, Wu WN, Jia L, Wang Y (2017) A highly sensitive on-off fluorescent chemosensor for Cu2+ based on coumarin. J Lumin 182:268–273. https://doi.org/10.1016/j.jlumin.2016.10.041
Xu WJ, Qi DQ, You JZ, Hu FF, Bian JY, Yang CX, Huang J (2015) Coumarin-based ‘turn-off’ fluorescent chemosensor with high selectivity for Cu2+ in aqueous solution. J Mol Struct 1091:133–137. https://doi.org/10.1016/j.molstruc.2015.02.083
Yin GX, Niu TT, Gan YB, Yu T, Yin P, Chen HM, Zhang Y, Li HT, Yao SZ (2018) A multi-signal fluorescent probe with multiple binding sites for simultaneous sensing of cysteine, homocysteine, and glutathione. Angew Chem Int Ed 57:4991–4994. https://doi.org/10.1002/anie.201800485
Yu C, Chen L, Zhang J, Li J, Liu P, Wang W, Yan B (2011) “Off-On” based fluorescent chemosensor for Cu2+ in aqueous media and living Cells. Talanta 85(3):1627–1633. https://doi.org/10.1016/j.talanta.2011.06.057
Namita K, Nilanjan D, Santanu B (2014) Remarkable role of positional isomers in the design of sensors for the ratiometric detection of copper and mercury ions in water. RSC Adv 4(9):4230–4238. https://doi.org/10.1039/C3RA45054F
Moorthy S, Sandhya M, Sanjiv KM, Suresh E, Amal KM, Anupama S, Amitava D (2009) Resonance energy transfer approach and a new ratiometric probe for Hg2+ in aqueous media and living organism. Org Lett 11(13):2740–2743. https://doi.org/10.1021/ol900810q
Hong M, Lu X, Chen Y, Xu D (2016) A novel rhodamine-based colorimetric and fluorescent sensor for Hg2+ in water matrix and living cell. Sens Actuators B 232:28–36. https://doi.org/10.1016/j.snb.2016.03.125
Acknowledgements
The authors thank Acharya Nagarjuna University for providing lab facilities to conduct research work.
Author information
Authors and Affiliations
Contributions
Mahesh Gosi: Methodology, Investigation, Formal analysis, Visualization, Writing – original draft. Anitha C. Kumar: Investigation, Visualization. Yeturu Sunandamma: Conceptualization, Methodology, Investigation, Formal analysis, Writing—review & editing, Supervision.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Ethics Approval
Not applicable.
Consent to Participate
Informed consent obtained from all individual participants included in the study.
Consent for Publication
Not applicable.
Conflicts of Interest/Competing Interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• A coumarin-xanthene based optical probe to senses copper and mercury ion.
• The probe showed colorimetric response and fluorescence turn-on for Hg2+ and turn-off for Cu2+ ion.
• The test strip prepared can detect very effectively the Cu2+ and Hg2+ ion in aqueous solution.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gosi, M., Kumar, A.C. & Sunandamma, Y. Fluorescence Variation in Selective Sensing of Hg2+and Cu2+ Ions By Coumarin-xanthene Fused Optical Probe. J Fluoresc 32, 2379–2393 (2022). https://doi.org/10.1007/s10895-022-03030-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-022-03030-0