Abstract
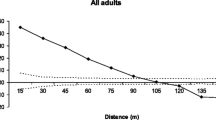
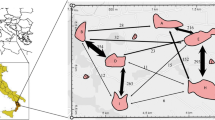
This study examines the levels of gene flow, the distance and the patterns of pollen and seed dispersal, the intra-population spatial genetic structure (SGS) and the effective population size of a spatially isolated Myracrodruon urundeuva population using five microsatellite loci. The study was carried out in the Paulo de Faria Ecological Station, São Paulo State, Brazil and included the sampling and mapping of 467 adult-trees and 149 juveniles. Open-pollinated seeds (514) from 29 seed-trees were also sampled and genotyped. Significant SGS was detected in both adult (S p = 0.0269) and juveniles trees (S p = 0.0246), indicating short-distance seed dispersal. Using maternity analysis, all juveniles had the mother-tree assigned within the stand. A father-tree within the stand was also assigned for 97.3% of the juveniles and 98.4% of offspring. The average pollen dispersal distance measured in juveniles \( \left( {\hat{\delta } = 1 3 8\pm 1 6 9 {\text{ m}},{\text{ mean}} \pm {\text{SD}}} \right) \) and offspring \( \left( {\hat{\delta } = 2 5 2\pm 20 4 {\text{ m}}} \right) \) were higher than the average seed dispersal distance measured in juveniles \( \left( {\hat{\delta } = 1 2 4\pm 1 50{\text{ m}}} \right) \). About 70% of the pollen from juveniles and 51% from offspring traveled less than 200 m and, 72% of the seeds traveled less than 50 m. The effective population size of the studied sample indicates that the 467 adult-trees and 145 juveniles correspond respectively to 335 and 63 individuals that are neither inbred nor relatives. The results are discussed in relation to their impact on seed collection practices and genetic conservation.





Similar content being viewed by others
References
Aguilar R, Quesada M, Ashworth L, Herrerias-Diego Y, Loco J (2008) Genetic consequences of habitat fragmentation in plant populations: susceptible signals in plant traits and methodological approaches. Mol Ecol 17:5177–5188. doi:j.1365-294X.2008.03971.x
Aldrich PR, Hamrick JL (1998) Reproductive dominance of pasture trees in a fragmented tropical forest mosaic. Science 281:103–105. doi:10.1126/science.281.5373.103
Bacles CFE, Lowe A, Ennos R (2006) Effective seed dispersal across a fragmented landscape. Science 311:628. doi:10.1126/science.1121543
Baleroni CRS, Alves PF, Santos EBR, Cambuim J, Andrade JAC, Moraes MLT (2003) Variação genética em populações naturais de aroeira em dois sistemas de plantio. Rev Inst Flor 15:125–136
Barcha SF, Arid FM (1971) Estudo de evapotranspiração na região norte-ocidental do Estado de São Paulo. Rev Ciências 1:99–122
Bawa KS (1994) Pollinators of tropical dioecious angiosperms: a reassessment? No, not yet. Am J Bot 81:456–460
Bittencourt JM, Sebbenn AM (2007) Patterns of pollen and seed dispersal in a small fragmented population of a wind pollinated Araucaria angustifolia in southern Brazil. Heredity 99:580–591. doi:10.1038/sj.hdy.6801019
Bittencourt JM, Sebbenn AM (2008) Pollen movement in a continuous forest of Araucaria angustifolia, inferred from paternity and TwoGener analysis. Conserv Genet 9:855–868. doi:10.1007/s10592-007-9411-2
Burczyk J, Adams WT, Shimizu JY (1996) Mating patterns and pollen dispersal in a natural knobcone pine (Pinus attenuata Lemmon) stand. Heredity 77:251–260. doi:10.1038/hdy.1996.139
Byrne M, Elliott CP, Yates CJ, Coates DJ (2008) Maintenance of high pollen dispersal in Eucalyptus wandoo a dominant tree of the fragmented agricultural region in Western Australia. Conserv Genet 9:97–105. doi:10.1007/s10592-007-9311-5
Caetano S, Silveira P, Spichiger R, Naciri-Graven Y (2005) Identification of microsatellite markers in a neotropical seasonally dry forest tree, Astronium urundeuva (Anacardiaceae). Mol Ecol Notes 5:21–23. doi:10.1111/j.1471-8286.2004.00814.x
Carneiro FS, Degen B, Kanashiro M, Lacerda AEB, Sebbenn AM (2009) High levels of pollen dispersal in Symphonia globulifera in a dense Brazilian Amazon forest revealed by paternity analysis. Forest Ecol Manag 258:1260–1266. doi:10.1016/j.foreco.2009.06.019
Carvalho PER (2003) Espécies arbóreas brasileiras. Embrapa Informação Tecnológica, Brasília
Cockerham CC (1969) Variance of gene frequencies. Evolution 23:72–84
Dick CW, Etchelecu G, Austerlitz F (2003) Pollen dispersal of Neotropical trees (Dinizia excelsa: Fabaceae) by native insects and Africa honeybees in pristine and fragmented Amazonian rainforest. Mol Ecol 12:753–764. doi:10.1046/j.1365-294X.2003.01760.x
Dick CW, Hardy OJ, Jones FA, Petit RJ (2008) Spatial scales of pollen and seed-mediated gene flow in tropical rain forest trees. Tropic Plant Biol 1:20–33. doi:10.1007/s12042-007-9006-6
Dow BD, Ashley MV (1996) Microsatellite analysis of seed dispersal and parentage of sampling in bur oak, Quercus macrocarpa. Mol Ecol 5:615–627. doi:10.1111/j.1365-294X.1996.tb00357.x
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Dyer RJ, Sork LJ (2001) Pollen pool heterogeneity in shortleaf pine, Pinus schinata Mill. Mol Ecol 10:859–866. doi:10.1046/j.1365-294X.2001.01251.x
El Mousadik A, Petit RJ (1996) High level of genetic differentiation for allelic richness among populations of the argan tree [Argania spinosa (L.) Skeels] endemic to Morocco. Theor Appl Genet 92:832–839. doi:10.1007/BF00221895
Ghazoul J (2005) Pollen and seed dispersal among dispersed plants. Biol Rev 80:413–443. doi:10.1017/S1464793105006731
Goudet J (1995) Fstat. (Version 2.9.3.2.): a computer program to calculate F-statistics. J Heredity 86:485–486
Gribel R, Gibbs PE (2002) High outbreeding as a consequence of selfed ovule mortality and single vector bat pollination in the Amazonian tree Pseudobombax munguba (Bombacaceae). Int J Plant Sci 163:1035–1043. doi:10.1086/342518
Hamilton MB (1999) Tropical tree gene flow and seed dispersal. Nature 401:129–130. doi:10.1038/43597
Hardesty BD, Hubbell S, Bermingham E (2006) Genetic evidence of frequent long-distance recruitment in a vertebrate-dispersed tree. Ecol Lett 9:516–525. doi:10.1111/j.1461-0248.2006.00897.x
Hardy O, Vekemans X (2002) SPAGeDI: a versatile computer program to analyze spatial genetic structure at the individual or population levels. Mol Ecol Notes 2:618–620. doi:10.1046/j.1471-8286.2002.00305.x
Hufford KM, Hamrick JL (2003) Variability selection at three early life stages of the tropical tree Platypodium elegans (Fabaceae, Papilonoidae). Evolution 57:518–526
Idury RM, Cardon LR (1997) A simple method for automated allele binning in microsatellite markers. Genome Res 11:104–1109. doi:10.1101/gr.7.11.1104
Jones FA, Chen J, Weng G-J, Hubbell SP (2005) A genetic evaluation of seed dispersal in the Neotropical tree, Jacaranda copaia (Bignonianaceae). Am Nat 166:543–555. doi:10.1086/491661
Kalinowski ST, Taper ML, Marshall TC (2007) Revising how computer program Cervus accommodates genotyping error increase success in paternity assignment. Mol Ecol 16:1099–1106. doi:10.1111/j.1365-294X.2007.03089.x
Kamm U, Rotach P, Gugerli F, Siraky M, Edwards P, Holderegger R (2009) Frequent long-distance gene flow in a rare temperate forest tree (Sorbus domestica) at the landscape scale. Heredity. doi:10.1038/hdy.2009.70
Kramer AT, Ison JL, Ashley MV, Howe HF (2008) The paradox of forest fragmentation genetics. Conserv Biol 22:878–885. doi:10.1111/j.1523-1739.2008.00944.x
Lacerda EBL, Sebbenn AM, Kanashiro M (2008) Long-pollen movement and deviation of random mating in a low-density continuous population of Hymenaea courbaril in the Brazilian Amazon. Biotropica 40:462–470. doi:10.1111/j.1744-7429.2008.00.402.x
Levin DA (1988) The paternity pool plants. Am Nat 132:309–317
Lindgren D, Mullin TJ (1998) Relatedness and status number in seed orchard crops. Can J For Res 28:276–283. doi:10.1139/cjfr-28-2-276
Lindgren D, Gea L, Jefferson P (1996) Loss of genetic diversity by status number. Silva Genet 45:52–59
Loiselle BA, Sork VL, Nason J, Graham C (1995) Spatial genetic structure of a tropical understory shrub, Psychotria officinalis (Rubiaceae). Am J Bot 82:1420–1425
Lowe AJ, Boshier D, Ward M, Bacles CFE, Navarro C (2005) Genetic resource impacts of habitat loss and degradation; reconciling empirical evidence and predicted theory for Neotropical trees. Heredity 95:255–273. doi:10.1038/sj.hdy.6800725
Marshall TC, Slate J, Kruuk LEB, Pemberton JM (1998) Statistical confidence for likelihood-based paternity inference in natural populations. Mol Ecol 7:639–655. doi:10.1046/j.1365-294x.1998.00374.x
Meagher TR (1986) Analysis of paternity within a natural population of Chamaelirium luteum. 1. Identification of most-likely male parents. Am Nat 128:199–215
Meagher TR, Thompson E (1987) Analysis of parentage for naturally established seedlings of Chamaelirim luteum (Liliaceae). Ecology 68:803–812. doi:10.2307/1938351
Moraes MLT, Kageyama PY, Siqueira ACMF, Kano NK, Cambuim J (1992) Variação genética em duas populações de aroeira (Astronium urundeuva—Fr. All.—Engl.—Anacardiaceae). Rev Inst Flor 4:1241–1245
Naito Y, Konuma A, Iwata H, Suyama Y, Seiwa K, Okudo T, Lee SL, Muhammad N, Tsumura Y (2005) Selfing and inbreeding depression in seeds and seedlings of Neobalanocarpus heimii (Dipterocarpaceae). J Plant Res 118:423–430. doi:10.1007/s10265-005-0245-z
Nunes YRF, Fagundes M, Almeida HS, Veloso MDM (2008) Aspectos ecológicos da aroeira (Myracrodruon urundeuva Allemão- Anacardiaceae): fenologia e germinação de sementes. Rev Árv 32:233–243. doi:10.1590/S0100-67622008000200006
Nunney L, Campbell KA (1993) Assessing minimum viable population size: demography meets population genetics. Tree 8:234–239. doi:10.1016/0169-5347(93)90197-W
Ribeiro MC, Metzger JP, Martensen AC, Ponzoni FJ, Hirato MM (2009) The Brazilian Atlantic Forest: how much is left, and how is the remaining forest distributed? Implictions for conservation. Biol Conserv 142:1141–1153. doi:10.1016/j.biocon.2009.02.021
Ritland K (2002) Extensions of models for the estimation of mating systems using n independent loci. Heredity 88:221–228. doi:10.1038/sj.hdy.6800029
Robledo-Arnuncio JJ, Smouse PE, Gil L, Alía R (2004) Pollen movement under alternative silvicultural practices in native population of Scots pine (Pinus sylvestris L.) in central Spain. Forest Ecol Manag 197:245–255. doi:10.1016/j.foreco.2004.05.016
Savolainen O, Kärkkäinen K (1992) Effect of forest management on gene pools. New Forest 6:329–345
Sebbenn AM (2002) Número de árvores matrizes e conceitos genéticos na coleta de sementes para reflorestamentos com espécies nativas. Rev Inst Flor 14:115–132
Sebbenn AM, Carvalho ACM, Freitas MLM, Moraes SMB, Gaiano APSC, Silva JM, Jolivet C, Moraes MLT (2009) Low level of realized seed and pollen gene flow and strong spatial genetic structure in a small, isolated and fragmented population of the tropical tree Copaifera langsdorffii Desf. Heredity (in press)
Sezen UU, Chazdon RL, Holsinger KE (2005) Genetic consequences of tropical second-growth forest regeneration. Science 307:891. doi:10.1126/science.1105034
Silva MB, Kanashiro M, Ciampi AY, Tompson I, Sebbenn AM (2008) Genetic effects of selective logging and pollen gene flow in a low-density population of the dioecious tropical tree Bagassa guianensis in the Brazilian Amazon. Forest Ecol Manag 255:1548–1558. doi:10.1016/j.foreco.2007.11.012
Smouse PE, Dyer RJ, Westfall RD, Sork VL (2001) Two-generation analysis of pollen flow across a landscape. I. Male gamete heterogeneity among females. Evolution 55:260–271
Sokal RR, Rohlf FJ (1995) Biometry: principles and practices of statistics in biological research, 3rd edn. W.H. Freeman and Company, New York
Sork VL, Smouse PE, Apsit VJ, Dyer RJ, Westfall RD (2005) A Two-generation analysis of pollen pool genetic structure in flowering dogwood, Cornus florida (Cornaceae), in the Missouri Ozarks. Am J Bot 92:261–271
Sousa VA, Sebbenn AM, Hattemer H, Ziehe M (2005) Correlated mating in populations of a dioecious Brazilian conifer Araucaria angustifolia (Bert.) O. Ktze. For Genet 12:107–119
Stranghetti V, Ranga NT (1998) Levantamento florístico das espécies vasculares da floresta estacional mesófila semidecídua da Estação Ecológica de Paulo de Faria–SP. Rev Bras Bot 21:295–304. doi:10.1590/S0100-84041998000300008
Vekemans X, Hardy OJ (2004) New insights from fine-scale spatial genetic structure analysis in plant populations. Mol Ecol 13:921–935. doi:10.1046/j.1365-294X.2004.02076.x
Wang J, Ye Q, Kang M, Huang H (2007) Novel polymorphic microsatellite loci and patterns of pollen-mediated gene flow in an ex situ population of Eurycorymbus cavaleriei (Sampindaceae) as revealed by categorical paternity analysis. Conserv Genet 9:559–567. doi:10.1007/s10592-007-9369-0
White GM, Boshier DH, Powell W (2002) Increased pollen flow counteracts fragmentation in tropical dry forest: an example from Swietenia humilis Zuccarini. Proc Natl Acad Sci USA 99:2038–2042. doi:10.1073/pnas.042649999
Wilmer P, Gilbert F, Ghazoul J, Zalat S, Semida F (1994) A novel form to territoriality—daily paternal investment in an anthophorid bee. Anim Behav 48:535–549
Young AG, Boyle TJ (2000) Effects of logging and other forms of harvesting on genetic diversity in humid tropical forest. For Conserv Genet, pp 115–123
Young AG, Boyle T, Brown ADH (1996) The population genetic consequences of habitat fragmentation for plants. Trends Ecol Evol 11:413–418. doi:10.1016/0169.5347(96)10045-8
Acknowledgements
This study was supported financially by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP contract number: 06/53357-0). The article was part of the doctoral thesis of A.P.S.C. Gaino in the Faculdade de Engenharia de Ilha Solteira/UNESP, Ilha Solteira, São Paulo, Brazil. We would like to thank to José Cambuim for his assistance in sample collection and Selma M.B. Moraes, Juliana P. Moreira, and Laila T. Cardin for their lab work. A.P.S.C. Gaino would like to thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the support of a doctoral scholarship at Faculdade de Engenharia de Ilha Solteira/UNESP (Brazil). The author M.A. Moraes and P.F. Alves thank FAPESP for the support of a Graduate scholarship. The author A.M. Sebbenn and M.L.T. Moraes thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for providing each with a Research Fellowship. We are also grateful to Dr. André E.B. Lacerda and Dr. Evelyn Nimmo for their suggestions and English correction in a previous version of this manuscript. The authors also are very grateful to an anonymous reviewer for corrections, suggestions and constructive criticism of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gaino, A.P.S.C., Silva, A.M., Moraes, M.A. et al. Understanding the effects of isolation on seed and pollen flow, spatial genetic structure and effective population size of the dioecious tropical tree species Myracrodruon urundeuva . Conserv Genet 11, 1631–1643 (2010). https://doi.org/10.1007/s10592-010-0046-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-010-0046-3