Abstract
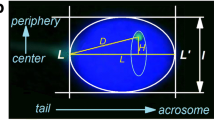
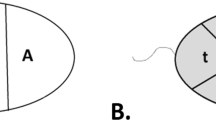
It is well established that chromosomes occupy distinct positions within the interphase nuclei, conferring a potential functional implication to the genome. In addition, alterations in the nuclear organisation patterns have been associated with disease phenotypes (e.g. cancer or laminopathies). The human sperm is the smallest cell in the body with specific DNA packaging and the mission of delivering the paternal genome to the oocyte during fertilisation. Studies of nuclear organisation in the sperm have postulated nonrandom chromosome position and have proposed a chromocentre model with the centromeres facing toward the interior and the telomeres toward the periphery of the nucleus. Most studies have assessed the nuclear address in the sperm longitudinally predominantly using centromeric or telomeric probes and to a lesser extent with whole chromosome paints. To date, studies investigating the radial organisation of human sperm have been limited. The purpose of this study was to utilise whole chromosome paints for six clinically important chromosomes (18, 19, 21, 22, X, and Y) to investigate nuclear address by assessing their radial and longitudinal nuclear organisation. A total of 10,800 sperm were analysed in nine normozoospermic individuals. The results have shown nonrandom chromosome position for all chromosomes using both methods of analysis. We present novel radial and polar analysis of chromosome territory localization within the human sperm nucleus. Specifically, a hierarchical organisation was observed radially with chromosomes organised from the interior to the periphery (chromosomes 22, 21, Y, X, 19, and 18 respectively) and polar organisation from the sperm head to tail (chromosomes X, 19, Y, 22, 21, and 18, respectively). We provide evidence of defined nuclear organisation in the human sperm and discuss the function of organisation and potential possible clinical ramifications of these results in regards to male infertility and early human development.




Similar content being viewed by others
Abbreviations
- CTs:
-
Chromosome territories
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- DNA:
-
Deoxyribonucleic acid
- DTT:
-
Dithiothreitol
- FISH:
-
Fluorescence in situ hybridization
- HCl:
-
Hydrochloric acid
- ICSI:
-
Intracytoplasmic sperm injection
- NaCl:
-
Sodium chloride
- PBS:
-
Phosphate-buffered Saline
- Rpm:
-
Rotations per minute
- SSC:
-
Saline sodium citrate
- TRITC:
-
Tetramethyl rhodamine isothiocyanate
- Tris:
-
2-Amino-2-hydroxymethyl-propane-1,3-diol
- WCP:
-
Whole chromosome paint
- 2D:
-
Two dimensional
- 3D:
-
Three dimensional
References
Barratt CL, Aitken RJ, Bjorndahl L et al (2010) Sperm DNA: organization, protection and vulnerability: from basic science to clinical applications—a position report. Hum Reprod 25(4):824–838
Bjorndahl L, Kvist U (2009) Human sperm chromatin stabilization—a proposed model including zinc bridges. Mol Hum Reprod 16(1):23–29
Bolzer A, Kreth G, Solovei I et al (2005) Three-dimensional maps of all chromosomes in human male fibroblast nuclei and prometaphase rosettes. PLoS Biol 3:e157
Boyle S, Gilchrist S, Bridger JM et al (2001) The spatial organization of human chromosomes within the nuclei of normal and emerin-mutant cells. Hum Mol Genet 10:211–219
Bridger JM, Boyle S, Kill IR, Bickmore WA (2000) Re-modelling of nuclear architecture in quiescent and senescent human fibroblasts. Curr Biol 10:149–152
Carrell DT, Hammoud SS (2009) The human sperm epigenome and its potential role in embryonic development. Mol Hum Reprod 16(1):37–47
Cavalli G (2007) Chromosome kissing. Curr Opin Genet Dev 17:443–450
Cremer T, Cremer C (2001) Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat Rev Genet 2:292–301
Croft JA, Bridger JM, Boyle S et al (1999) Differences in the localization and morphology of chromosomes in the human nucleus. J Cell Biol 145:1119–1131
Elcock LS, Bridger JM (2010) Exploring the relationship between interphase gene positioning, transcriptional regulation and the nuclear matrix. Biochem Soc Trans 38:263–267
Federico C, Cantarella CD, Di Mare P, Tosi S, Saccone S (2008) The radial arrangement of the human chromosome 7 in the lymphocyte cell nucleus is associated with chromosomal band gene density. Chromosoma 117:399–410
Finch KA, Fonseka G, Ioannou D et al (2008a) Nuclear organisation in totipotent human nuclei and its relationship to chromosomal abnormality. J Cell Sci 121:655–663
Finch KA, Fonseka KG, Abogrein A et al (2008b) Nuclear organization in human sperm: preliminary evidence for altered sex chromosome centromere position in infertile males. Hum Reprod 23:1263–1270
Foster HA, Bridger JM (2005) The genome and the nucleus: a marriage made by evolution. Genome organisation and nuclear architecture. Chromosoma 114:212–229
Gao S, Chung YG, Parseghian MH et al (2004) Rapid H1 linker histone transitions following fertilization or somatic cell nuclear transfer: evidence for a uniform developmental program in mice. Dev Biol 266:62–75
Greaves IK, Rens W, Ferguson-Smith MA, Griffin D, Marshall Graves JA (2003) Conservation of chromosome arrangement and position of the X in mammalian sperm suggests functional significance. Chromosome Res 11:503–512
Haaf T, Ward DC (1995) Higher order nuclear structure in mammalian sperm revealed by in situ hybridization and extended chromatin fibers. Exp Cell Res 219:604–611
Habermann FA, Cremer M, Walter J et al (2001) Arrangements of macro- and microchromosomes in chicken cells. Chromosome Res 9:569–584
Hammoud SS, Nix DA, Zhang H et al (2009) Distinctive chromatin in human sperm packages genes for embryo development. Nature 460:473–478
Hann MC, Lau PE, Tempest HG (2011) Meiotic recombination and male infertility: from basic science to clinical reality? Asian J Androl 13:212–218
Harton GL, Tempest HG (2012) Chromosomal disorders and male infertility. Asian J Androl 14:32–39
Hassold T, Hunt P (2001) To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet 2:280–291
Hazzouri M, Rousseaux S, Mongelard F et al (2000) Genome organization in the human sperm nucleus studied by FISH and confocal microscopy. Mol Reprod Dev 55:307–315
Ioannou D, Griffin DK (2011) Male fertility, chromosome abnormalities, and nuclear organization. Cytogenet Genome Res 133:269–279
Ioannou D, Meershoek EJ, Christopikou D et al (2011) Nuclear organisation of sperm remains remarkably unaffected in the presence of defective spermatogenesis. Chromosome Res 19:741–753
Ioannou D, Fonseka KG, Meershoek EJ et al (2012) Twenty-four chromosome FISH in human IVF embryos reveals patterns of post-zygotic chromosome segregation and nuclear organisation. Chromosome Res 20:447–460
Kuroda M, Tanabe H, Yoshida K et al (2004) Alteration of chromosome positioning during adipocyte differentiation. J Cell Sci 117:5897–5903
Luetjens CM, Payne C, Schatten G (1999) Non-random chromosome positioning in human sperm and sex chromosome anomalies following intracytoplasmic sperm injection. Lancet 353:1240
Lukasova E, Kozubek S, Kozubek M, Falk M, Amrichova J (2002) The 3D structure of human chromosomes in cell nuclei. Chromosome Res 10:535–548
Manuelidis L (1990) A view of interphase chromosomes. Science 250:1533–1540
Marella NV, Bhattacharya S, Mukherjee L, Xu J, Berezney R (2009) Cell type specific chromosome territory organization in the interphase nucleus of normal and cancer cells. J Cell Physiol 221:130–138
Martin C, Brochard V, Migne C et al (2006) Architectural reorganization of the nuclei upon transfer into oocytes accompanies genome reprogramming. Mol Reprod Dev 73:1102–1111
Meaburn KJ, Misteli T (2007) Cell biology: chromosome territories. Nature 445:379–781
Meaburn KJ, Newbold RF, Bridger JM (2008) Positioning of human chromosomes in murine cell hybrids according to synteny. Chromosoma 117(6):579–591
Meistrich ML, Mohapatra B, Shirley CR, Zhao M (2003) Roles of transition nuclear proteins in spermiogenesis. Chromosoma 111:483–488
Meyer-Ficca M, Muller-Navia J, Scherthan H (1998) Clustering of pericentromeres initiates in step 9 of spermiogenesis of the rat (Rattus norvegicus) and contributes to a well defined genome architecture in the sperm nucleus. J Cell Sci 111(Pt 10):1363–1370
Misteli T (2005) Concepts in nuclear architecture. Bioessays 27:477–487
Mora L, Sanchez I, Garcia M, Ponsa M (2006) Chromosome territory positioning of conserved homologous chromosomes in different primate species. Chromosoma 115:367–375
Moskovtsev SI, Willis J, White J, Mullen JB (2010) Disruption of telomere–telomere interactions associated with DNA damage in human spermatozoa. Syst Biol Reprod Med 56:407–412
Mudrak O, Tomilin N, Zalensky A (2005) Chromosome architecture in the decondensing human sperm nucleus. J Cell Sci 118:4541–4550
Olszewska M, Wiland E, Kurpisz M (2008) Positioning of chromosome 15, 18, X and Y centromeres in sperm cells of fertile individuals and infertile patients with increased level of aneuploidy. Chromosome Res 16:875–890
Parada L, Misteli T (2002) Chromosome positioning in the interphase nucleus. Trends Cell Biol 12:425–432
Petrova NV, Yakutenko II, Alexeevski AV et al (2007) Changes in chromosome positioning may contribute to the development of diseases related to X-chromosome aneuploidy. J Cell Physiol 213(1):278–283
Sbracia M, Baldi M, Cao D et al (2002) Preferential location of sex chromosomes, their aneuploidy in human sperm, and their role in determining sex chromosome aneuploidy in embryos after ICSI. Hum Reprod 17:320–324
Shah K, Sivapalan G, Gibbons N, Tempest H, Griffin DK (2003) The genetic basis of infertility. Reproduction 126:13–25
Sharbatoghli M, Valojerdi MR, Amanlou M, Khosravi F, Jafar-Abadi MA (2012) Relationship of sperm DNA fragmentation, apoptosis and dysfunction of mitochondrial membrane potential with semen parameters and ART outcome after intracytoplasmic sperm injection. Arch Gynecol Obstet 286(5):1315–1322
Skinner BM, Volker M, Ellis M, Griffin DK (2009) An appraisal of nuclear organisation in interphase embryonic fibroblasts of chicken, turkey and duck. Cytogenet Genome Res 126:156–164
Solovei I, Kreysing M, Lanctot C et al (2009) Nuclear architecture of rod photoreceptor cells adapts to vision in mammalian evolution. Cell 137:356–368
Solov’eva L, Svetlova M, Bodinski D, Zalensky AO (2004) Nature of telomere dimers and chromosome looping in human spermatozoa. Chromosome Res 12:817–823
Szczerbal I, Foster HA, Bridger JM (2009) The spatial repositioning of adipogenesis genes is correlated with their expression status in a porcine mesenchymal stem cell adipogenesis model system. Chromosoma 118:647–663
Takizawa T, Meaburn KJ, Misteli T (2008) The meaning of gene positioning. Cell 135:9–13
Tempest HG (2011) Meiotic recombination errors, the origin of sperm aneuploidy and clinical recommendations. Syst Biol Reprod Med 57:93–101
Tempest HG, Griffin DK (2004) The relationship between male infertility and increased levels of sperm disomy. Cytogenet Genome Res 107:83–94
Terada Y, Luetjens CM, Sutovsky P, Schatten G (2000) Atypical decondensation of the sperm nucleus, delayed replication of the male genome, and sex chromosome positioning following intracytoplasmic human sperm injection (ICSI) into golden hamster eggs: does ICSI itself introduce chromosomal anomalies? Fertil Steril 74:454–460
Tilgen N, Guttenbach M, Schmid M (2001) Heterochromatin is not an adequate explanation for close proximity of interphase chromosomes 1–Y, 9–Y, and 16–Y in human spermatozoa. Exp Cell Res 265:283–287
Ward WS, Coffey DS (1991) DNA packaging and organization in mammalian spermatozoa: comparison with somatic cells. Biol Reprod 44:569–574
World Health Organization (2010) WHO laboratory manual for the examination and processing of human semen. World Health Organization
Wykes SM, Krawetz SA (2003) The structural organization of sperm chromatin. J Biol Chem 278:29471–29477
Zalenskaya IA, Zalensky AO (2004) Non-random positioning of chromosomes in human sperm nuclei. Chromosome Res 12:163–173
Zalensky A, Zalenskaya I (2007) Organization of chromosomes in spermatozoa: an additional layer of epigenetic information? Biochem Soc Trans 35:609–611
Zalensky AO, Breneman JW, Zalenskaya IA, Brinkley BR, Bradbury EM (1993) Organization of centromeres in the decondensed nuclei of mature human sperm. Chromosoma 102:509–518
Zalensky AO, Allen MJ, Kobayashi A et al (1995) Well-defined genome architecture in the human sperm nucleus. Chromosoma 103:577–590
Acknowledgments
We would like to thank the following for their help in the success of this project: the patients who participated in this research study, IVF Florida Reproductive Associates staff, Michael Ellis (Digital Scientific UK), and Dr. Lakshmi Rao Kandukuri.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Conly Rieder.
Rights and permissions
About this article
Cite this article
Millan, N.M., Lau, P., Hann, M. et al. Hierarchical radial and polar organisation of chromosomes in human sperm. Chromosome Res 20, 875–887 (2012). https://doi.org/10.1007/s10577-012-9323-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-012-9323-y