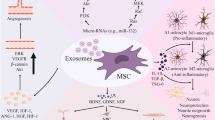
Abstract
Neuroinflammation is an important pathogenesis of neurological diseases and causes a series of physiopathological changes, such as abnormal activation of glial cells, neuronal degeneration and death, and disruption of the blood‒brain barrier. Therefore, modulating inflammation may be an important therapeutic tool for treating neurological diseases. Mesenchymal stem cells (MSCs), as pluripotent stem cells, have great therapeutic potential for neurological diseases due to their regenerative ability, immunity, and ability to regulate inflammation. However, recent studies have shown that MSC-derived exosomes (MSC-Exos) play a major role in this process and play a key role in neuroprotection by regulating neuroglia. This review summarizes the recent progress made in regulating neuroinflammation by focusing on the mechanisms by which MSC-Exos are involved in the regulation of glial cells through signaling pathways such as the TLR, NF-κB, MAPK, STAT, and NLRP3 pathways to provide some references for subsequent research and therapy.
Graphical Abstract
Exosomes derived from MSCs exhibit neuroprotective effects by regulating signaling pathways and mitigating neuroinflammation triggered by glial cells.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroinflammation refers to the inflammatory response that arises when central nervous system (CNS) damage is induced by either endogenous or exogenous stimuli and is accompanied by the activation of neuroglia, particularly microglia and astrocytes (Kwon and Koh 2020; Leng and Edison 2021). In the initial phase of CNS injury, inflammation exerts a protective effect by eliminating harmful substances. However, a sustained inflammatory response continuously stimulates neuroglia, releasing inflammatory factors, and mediators. This activation prompts neuronal degeneration, impairs the blood‒brain barrier (BBB), and exacerbates brain damage via various mechanisms (Liddelow and Barres 2017; Rodriguez-Gomez et al. 2020). Neuroinflammation significantly contributes to the initiation and progression of neurodegenerative conditions and acute CNS ailments (Stephenson et al. 2018; Novoa et al. 2022; Liu et al. 2022b). Consequently, targeting neuroinflammation has emerged as a promising intervention strategy.
MSCs are pluripotent stem cells that can be isolated from various tissues, such as the umbilical cord, placenta, and bone marrow; they have the ability to undergo osteogenic, lipogenic, and chondrogenic differentiation. In recent years, MSCs have attracted much attention for use in cell therapy (Levy et al. 2020). MSCs exhibit a regulatory influence on the immune response by dispensing anti-inflammatory mediators, cytokines, and immunosuppressive factors (da Silva Meirelles et al. 2006; Shi et al. 2018). MSCs, known to inhibit neuroinflammation, actively stimulate neuronal differentiation and promote neural axon growth; they also enhance damaged nerve functions (Skok 2021; Huang et al. 2022; Bagheri-Mohammadi 2021b; Ba et al. 2022). Although MSCs are known to possess therapeutic effects, these effects are believed to be primarily induced through paracrine mechanisms, and sufficient evidence supports this claim (Ha et al. 2020). Extracellular vesicles secreted by MSCs possess a bilayer lipid membrane structure and are termed MSC-Exos (Harrell et al. 2019; Qiu et al. 2019; Palmulli and van Niel 2018). These vesicles contain proteins, lipids, and nucleic acids and can be used for tissue regeneration, immunomodulation, and inflammation modulation. MSC-Exos also play a vital role in cellular transmission (Tang et al. 2021). The significant inflammatory regulatory capacity of MSC-Exos has garnered considerable interest from researchers investigating neurological disorders (Losurdo et al. 2020).
Glial Cells and Neuroinflammation
Neuroglia, pivotal in the CNS, include microglia, astrocytes, and oligodendrocytes. These cells actively participate in the immune response within the CNS, fostering neuronal nourishment and ensuring synaptic homeostasis (Schirmer et al. 2021; Liu et al. 2023). The role of microglia and astrocytes in neuroinflammation is a subject of growing interest (Hashioka et al. 2021).
Microglia, which are derived from the embryonic yolk sac, are innate immune cells that dominate the CNS (Rodriguez-Gomez et al. 2020; Bagheri-Mohammadi 2021a). These immune cells, which dwell in the CNS, play vital roles in pathogen defense and damage repair (Subhramanyam et al. 2019). Microglia, which are indispensable for maintaining CNS homeostasis, are activated by diverse pathological stimuli. This activation gives rise to two distinct types of M1 macrophages: classical M1 macrophages and selective M2 macrophages. Proinflammatory cytokines, chemokines, and neurotoxic factors such as tumor necrosis factor-alpha (TNF-α), nitric oxide (NO), prostaglandin E2 (PGE2), interleukin-1β (IL-1β), and IL-6, which are generally secreted by M1 microglia, contribute to damage of the CNS (Zavatti et al. 2022). M2 macrophages secrete anti-inflammatory and neuroprotective factors such as IL-4, IL-10, arginase-1 (Arg-1), and chitinase 3-like 3 (Ym1), which promote nerve repair and regeneration to maintain CNS homeostasis (Zong et al. 2021). Chronically activated M1 macrophages lead to the excessive release of inflammatory mediators, intensifying neuroinflammation and exacerbating neuronal damage (Guo et al. 2022; Cowan and Petri 2018); this highlights the importance of understanding the balance between M1 and M2 microglia. Modulating microglia could thus serve as a potent intervention method to regulate neuroinflammation.
Astrocytes, abundant in the brain, are instrumental in numerous physiological processes. These processes include blood flow regulation, BBB preservation, synaptogenesis facilitation, CNS homeostasis maintenance, and neuronal function regulation (Giovannoni and Quintana 2020). Astrocytes, which share similarities with microglia, serve dual functions as proinflammatory and neuroprotective agents. When exposed to constant pathological stimuli, these cells secrete proinflammatory cytokines such as IL-1β and TNF-α. Consequently, this action elevates reactive oxygen species (ROS) production, and this escalation leads to neurodegeneration (Hasel and Liddelow 2021; Linnerbauer et al. 2020). The release of inflammatory factors by activated microglia can trigger proinflammatory astrocytes, resulting in secondary inflammatory responses (Liddelow et al. 2017). Astrocytes, which possess neuroprotective traits, generate anti-inflammatory cytokines such as IL-4 and IL-10, contributing to nerve regeneration (Li et al. 2022b). The role of neuroinflammation mediated by astrocytes in CNS disorders is significant and cannot be overlooked.
Despite being distinct cell types, microglia and astrocytes are interconnected in their response to CNS injury; they participate in complex mechanisms that regulate neuroinflammation, and their role in the CNS is double-edged (Rueda-Carrasco et al. 2021). It is crucial to regulate glial cells and exert a protective effect on them (Fig. 1).
The Role of MSC-Exos in CNS Disorders
CNS studies revealed that MSC-Exos exhibit neuroprotective effects by harnessing their ability to regulate inflammation, adjust neuroglial activity, and boost the functionality of damaged neural tissue (Jin et al. 2021; Liu et al. 2022a; Cui et al. 2022). As a promising, innovative therapeutic tool, MSC-Exos can be a potential game changer for neurological disorders, offering fresh hope to the medical community (Joo et al. 2020; Guo et al. 2020) (Table 1).
MSC-Exos and Alzheimer’s Disease
Slow progressive memory loss and cognitive impairment are the main clinical symptoms of Alzheimer’s disease (AD) (Monteiro et al. 2023). The pathological signatures of this disease typically involve excessive accumulation of extracellular Aβ and neurofibrillary tangles (NFTs) (Ratan et al. 2023; Ba et al. 2022). Glial-induced neuroinflammation has been found to significantly contribute to the acceleration of Aβ accumulation (Singh 2022; Huang et al. 2019). In contrast, MSC-Exos inhibited microglial and astrocyte activation, thereby reducing hippocampal inflammation and Aβ and tau deposits. Furthermore, it enhances synaptic function and increases brain-derived neurotrophic factor (BDNF), effectively mitigating cognitive dysfunction in AD mice (Liu et al. 2022a). In mice with amyloid precursor protein/progerin 1 (APP/PS1) mutations, MSC-Exos enhanced YM-1 and Arg-1 expression. This improvement was accompanied by superior spatial learning and memory function during water maze testing (Ding et al. 2018). MSC-Exos significantly decreased Aβ deposition in the brains of APP/PS1 mice, which is consistent with previous observations (Ding et al. 2018). MSC-Exos significantly attenuated Aβ-induced neuroinflammation by inhibiting the nuclear factor kappa B (NF-κB) signaling pathway and suppressing signal transducer and activator of transcription 3 (STAT3). This intervention also markedly improved the neurological function and locomotor ability of AD mice (Nakano et al. 2020; Cui et al. 2018). In vitro investigations demonstrated that MSC-Exos suppress the increase in proinflammatory agents such as TNF-α and NO induced by Aβ aggregation (Kaniowska et al. 2022). Furthermore, MSC-Exos protect Aβ-induced PC12 cells by reducing inflammatory factor release and attenuating PC12 cell apoptosis; this occurs through the inhibition of the nucleotide-binding oligomerization domain, leucine-rich repeat, pyrin domain-containing 3 (NLRP3), and caspase-1 (Zhai et al. 2021). Studies indicate that MSC-Exos can regulate glial cells, thereby modulating neuroinflammatory responses in AD.
MSC-Exos and Parkinson’s Disease
PD, a neurodegenerative disorder, is the second most common disorder. Dopamine depletion is characterized by the degeneration of dopaminergic neurons, which leads to motor and nonmotor impairments (Jankovic and Tan 2020). PD has a complex pathogenesis, often caused by neuroinflammation (Wang et al. 2022). MSC-Exos play a significant role in PD treatment (Chen et al. 2020a). Administering MSC-derived conditioned medium (MSC-CM) effectively reduced Iba-1 and CD4 levels, inhibited alpha-synuclein production, increased tyrosine hydroxylase levels in the striatum, and improved motor deficits in rats with rotenone-induced Parkinson’s disease. This finding highlights the potential of MSC-CM in Parkinson’s disease therapy (Chen et al. 2020c). In a PD in vitro model, MSC-generated conditioned medium effectively reduced neuroinflammation, oxidative stress, and apoptosis in MPP+-induced SH-SY5Y neuroblastoma cells (Li et al. 2019). MSC-Exos significantly reduced NLRP3-induced inflammation and cytosolic protein kinase 5 (CDK5)-related nigrostriatal autophagy; moreover, they decreased dopaminergic neuronal apoptosis and inflammation while inhibiting α-synuclein aggregation in PD mice (Li et al. 2021). MSC-Exos effectively modulate Parkinson’s disease-related neuroinflammation, reduce dopaminergic neuron apoptosis, and enhance PD motor symptoms.
MSC-Exos and Traumatic Brain Injury
Traumatic brain injury (TBI) is a severe central nervous system disorder that is associated with high mortality and disability rates (Jacquens et al. 2022). A study revealed a significant link between TBI and neurodegenerative diseases (Brett et al. 2022). Neuroinflammation mediated by glial cell overactivation is the leading cause of brain damage secondary to TBI (Karve et al. 2016). In a study of TBI mice, after injection of MSC-Exos, TNF-α and IL-1β expression decreased. Simultaneously, inducible nitric oxide synthase (iNOS) expression was downregulated, and Arg-1 was upregulated. This manipulation shifts the conversion of microglia from proinflammatory to anti-inflammatory, thereby reducing the neuroinflammatory effects of TBI (Ni et al. 2019). After 14 days of TBI, MSC-Exos significantly reduced the size of the brain lesions. This was attributed to their role in regulating inflammation and their capacity to alleviate secondary brain damage (Ni et al. 2019). Intervention with MSC-Exos led to a decrease in activated astrocytes following TBI, and its ability to modulate inflammation was positively correlated with the duration of administration (Zhang et al. 2020). Enriched miRNA-17-92 MSC-Exos exhibit considerable promise in restoring sensory-motor and cognitive abilities in mouse models of TBI (Zhang et al. 2021). Several studies have demonstrated that the HMGB1/NF-κB pathway can be inhibited by exosomes from MSCs enriched with miR-216a-5p, thereby reducing TBI-induced neuroinflammation (Xu et al. 2020). MSC-Exos interrupt TLR4 signaling, inhibit NF-κB and MAPK phosphorylation, and mitigate neuroinflammation triggered by microglial overactivation in rats with brain injury (Thomi et al. 2019). MSC-Exos exert inhibitory neuroinflammatory and neuroprotective effects by regulating glial cells and improving the microenvironment in regions of brain injury.
MSC-Exos and Other Neurological Disorders
MSC-Exos, a novel therapy, significantly improve cognitive function in mice suffering from sustained epilepsy (SE) (Liu et al. 2021a). By regulating glial cell activation, reducing hippocampal inflammation, and enhancing neuronal protection, MSC-Exos prevent SE-induced cognitive memory deficits and decrease cognitive activity. In a study investigating the beneficial impact of MSC-Exos on middle cerebral artery occlusion, it was discovered that MSC-Exos effectively suppressed microglial activation, simultaneously diminishing neutrophil factor and chemokine accumulation (Pathipati et al. 2021). The inhibition of MAPK11-involved astrocyte inflammation and the enhancement of antioxidant capacity are essential mechanisms by which MSC-Exos improve the prognosis of amyotrophic lateral sclerosis (ALS) (Provenzano et al. 2022). MSC-Exos exhibit immunomodulatory effects, effectively regulating microglial cell activation in multiple sclerosis (MS). Diminishing Th1 and Th17 expression enhances motility and fosters myelin regeneration (Laso-García et al. 2018). The efficacy of MSC-Exos in mitigating numerous neurological ailments is strongly connected to their function in regulating neuroinflammation, a factor that cannot be overlooked.
Mechanisms by Which MSC-Exos Regulate Neuroinflammation
Recent research has revealed that MSC-Exos possess neuroprotective effects under neurological conditions, with neuroinflammation being a critical pathogenic mechanism. Neuroglial activation initiates neuroinflammation, prompting astrocyte activation and the release of inflammatory agents. These factors contribute to neuronal damage and exacerbate neuroinflammation (Liddelow et al. 2017; Norden et al. 2016). The regulation of glial cells has been identified as a vital link in neuroinflammation. Numerous studies have demonstrated that MSC-Exos aid in transforming microglia from a proinflammatory state to an anti-inflammatory state, suppressing activated astrocytes, and providing neuroprotection (Go et al. 2020; Garcia-Contreras and Thakor 2021; Cui et al. 2022; Xian et al. 2019). The following question then arises: how do MSC-Exos regulate glial cells?
MSC-Exos with TLRs and NF-κB
Toll-like receptors (TLRs), including TLR2 and TLR4, serve as pattern recognition receptors (PRRs) capable of identifying pathogen-associated molecular patterns (PAMPs). TLRs are crucial in modulating CNS inflammation by regulating cytokines and chemokines, particularly in microglial activation (Sloane et al. 2010; Fiebich et al. 2018; Huang et al. 2017). CD14, a GPI-anchored protein expressed on myeloid cells, is activated by TLR4, which produces proinflammatory cytokines via MyD88-dependent and TRIF-independent signaling pathways (Ciesielska et al. 2021). Research has revealed that MSC-Exos can inhibit LPS binding to TLR4 via the TLR4/CD14 complex, manipulate IκBα and AP-1 transcription, and minimize inflammatory factor release (Thomi et al. 2019). MSC-Exos exhibit direct inhibitory effects on neuroinflammation driven by the TLR4 signaling pathway, explicitly targeting HMGB1 (Xiong et al. 2020). Additionally, research has revealed that MSC-Exos can suppress the TLR2/NF-κB signaling pathway, thereby reducing the inflammatory response by inhibiting IRAK1 expression (Zhang et al. 2022).
NF-κB, a nuclear transcription factor (Sun et al. 2022), initiates the expression of genes regulating inflammatory responses and proinflammatory factors when stimulated (Yu et al. 2020). The hippocampus of APP/PS1 mice exhibited increased NF-κB expression. However, upon treatment with MSC-Exos, TNF-α, TRAF6, and NF-κB expression decreased. This reduction attenuated the inflammatory response activated by astrocytes and mitigated the cognitive deficits characteristic of AD (Nakano et al. 2020). MSC-Exos also inhibit IRAK1 expression in astrocytes and intervene in NF-κB-mediated neuroinflammatory responses (Lai et al. 2022). Research in an LPS-induced neuroinflammation model utilizing RAW264.7 cells revealed that MSC-Exos effectively targeted tumor necrosis factor-stimulated gene-6 (TSG-6). This intervention inhibits the NF-κB/NLRP3 signaling pathway and modulates macrophage phenotypic transformation (Li et al. 2022a). NF-κB also regulates histone deacetylase 3 (HDAC3) expression, and MSC-Exos inhibit p65 phosphorylation, NF-κB transcriptional activity, HDAC3 expression, and neuroinflammation in subarachnoid hemorrhage (Lai et al. 2020). This study indicated that MSC-Exos can potentially reduce neuroinflammation and serve a neuroprotective function by hindering TLR/NF-κB activation.
MSC-Exos with MAPK
MAPKs, as members of the serine/threonine protein kinase family, include extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK)/stress-activated protein kinase, and p38 MAPK. These kinases activate MAPK from the extracellular region to the nucleus, where they function in the immune response, cell proliferation, and apoptosis (Behl et al. 2022; Guan et al. 2020). MSC-Exos inhibit neuroinflammation in brain injury by effectively regulating LPS- and oxygen–glucose deprivation (OGD)-induced microglial activation, which occurs through suppression of the phosphorylation of P38MAPK, JNK, ERK1/2, P65, IKKαβ, and NF-κB inhibitor alpha (IKBα), ultimately decreasing the activation of these proteins (Shu et al. 2022; Chen et al. 2020b). It has been observed that MSC-Exos, which contain miR-467f and miR-466q, effectively target Map3k8 and Mk2, inhibiting p38 MAPK. This intervention has been shown to be involved in regulating neuroinflammation induced by microglial activation (Giunti et al. 2021). Research has revealed that MSC-Exos exert regulatory effects on astrocyte activation through the MAPK pathway; simultaneously, they stimulate the nuclear translocation of nuclear factor erythroid2-related Factor 2 (Nrf2), amplifying antioxidant effects and diminishing neurotoxicity (Provenzano et al. 2022). MSC-Exos exhibit anti-inflammatory and antioxidant effects by regulating neuroinflammation through MAPK signaling and synergistically collaborating with Nrf2.
MSC-Exos with JAK/STAT
The Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway is constructed of three primary elements: the tyrosine kinase-related receptor, JAK, and STAT. Upon phosphorylation by JAK, STAT converts into a dimer that permeates the nuclear membrane, ultimately substantially impacting cell survival, inflammation, and immune regulation (Khera et al. 2022; Xin et al. 2020). MSC-Exos effectively safeguard microglia from inflammatory reactions triggered by OGD stimulation. This effect is achieved by inhibiting STAT3 phosphorylation, downregulating proinflammatory factor expression, and moderating inflammatory responses (Xin et al. 2022). STAT3 activation and interaction with p38MAPK occur, while MSC-Exos reduce STAT3 and p38MAPK phosphorylation; it also inhibits the overexpression of the inflammatory mediators cyclooxygenase-2 (COX-2), monocyte chemoattractant protein-1 (MCP-1), and iNOS, leading to neuroprotective effects in subarachnoid hemorrhage (Liu et al. 2021c). Interestingly, the TBI study revealed that MSC-Exos effectively inhibited neuroinflammation by stimulating STAT3 phosphorylation, which potentially occurs because activated STAT3 further elevates IL-10 expression, initiating an autocrine feedback loop that amplifies its anti-inflammatory properties (Wen et al. 2022). However, the JAK/STAT pathway has opposite regulatory effects on various diseases, indicating that MSC-Exos could contribute to neuroinflammation by controlling the JAK/STAT pathway.
MSC-Exos with NLRP3
The NLRP3 inflammasome, a complex consisting of NLRP3, ASC, and caspase-1, plays a crucial role in innate immunity; it regulates caspase-1-induced GSDMD-dependent pyroptosis and the release of IL-1β and IL-18. This inflammasome triggers cell death in response to infections, pathological stress, and various stimuli (Huang et al. 2021). MSC-Exos demonstrated robust potential for reducing Aβ-induced neuroinflammation while improving memory and locomotor abilities in APP/PS1 mice. The effectiveness of these agents stems from their ability to inhibit NLRP3 and caspase-1 expression (Zhai et al. 2021). In vitro studies revealed that MSC-Exos are effective inhibitors of the TSG-6/NF-κB/NLRP3 pathway, fostering the conversion of microglia to the M2 anti-inflammatory phenotype (Li et al. 2022a). MSC-Exos exhibit neuroprotective effects against ischemia‒reperfusion injury by efficiently suppressing NLRP3-induced neuronal death and regulating microglial activity (Liu et al. 2021b). Studies have shown that MSC-Exos boost FOXO3a expression, resulting in decreased inflammatory factor release and, simultaneously, decreased NLRP3, caspase-1, and GSDMD expression (Hu et al. 2021). Furthermore, MSC-Exos exhibit a more significant inhibitory effect on NLRP3 under hypoxic pretreatment (Kang et al. 2021). MSC-Exos robustly inhibited PDCD4 expression, thereby restraining NLRP3 and inhibiting inflammatory factors. This process mitigates neuroinflammation and brain damage after cerebral hemorrhage (Ding et al. 2021). MSC-Exos exhibit excellent potential to attenuate neuroinflammation by modulating NLRP3 inflammatory vesicles.
Others:
(1) BACE1, an Aβ precursor protein cleaving enzyme in microglia, exacerbates AD by promoting Aβ production and inflammatory responses (Singh et al. 2022). Nonetheless, MSC-EVs that deliver miR-29c-3p can potentially inhibit BACE1, mitigate Aβ accumulation, and ameliorate neuroinflammation and neuronal apoptosis in AD mouse models. This efficacy is derived from activating the Wnt/β-catenin pathway (Sha et al. 2021). (2) Neutrophil gelatinase-associated lipid transport protein 2 (LCN2), secreted by activated astrocytes, serves as a critical mediator of neuroinflammation and neurodegeneration (Kim et al. 2022), and MSC-Exos containing miR-138-5p effectively downregulate LCN2 and inhibit neuroinflammation induced by astrocyte activation (Deng et al. 2019).
In essence, the genesis and progression of neuroinflammation rely heavily on signaling pathways. These pathways are interconnected, forming a regulatory network that influences one another. MSC-Exos can potentially alleviate neuroinflammation by adjusting these signaling pathways, opening up a new avenue for treating neurological disorders (Fig. 2).
MSC-Exos Carry miRNAs that Play a Role in Neuroinflammation
MSC-Exos, abundant in miRNAs, significantly contribute to cellular regulation (Schulz-Siegmund and Aigner 2021). These 22-nucleotide noncoding RNA molecules bind to the 30 untranslated regions (UTRs) or open reading frames (ORFs of target mRNAs, dictating mRNA degradation or translation inhibition, ultimately affecting protein expression (Das and Rao 2022). Research has demonstrated that MSC-Exos carry miRNAs capable of affecting CNS disorders (Iranifar et al. 2019). miR-216a-5p (Xu et al. 2020), miR-193b-3p (Lai et al. 2020), miR-21a-5p (Xin et al. 2022), miR-26b-5p (Liu et al. 2021c), miR-181b (Wen et al. 2022), and miR-183-5p (Ding et al. 2021) trigger the transformation of microglia into an anti-inflammatory state, diminish proinflammatory factors, and thereby achieve neuroprotective outcomes in acute CNS injuries. MSC-Exos, which are enriched in miRNA-17-92, effectively promote the recovery of sensory-motor and cognitive functions in TBI mice compared to those in mice not loaded with exosomes (Zhang et al. 2021). MiR-22 (Zhai et al. 2021) can regulate GSDMD-induced focal death, inhibit inflammation, and improve AD motor and memory abilities. Moreover, a study confirmed that MSC-Exos containing miR-146a (Nakano et al. 2020), miR-21 (Cui et al. 2018), and miR-29c-3p (Sha et al. 2021) interfered with neuroinflammation induced by Aβ stimulation and attenuated neuronal apoptosis. In addition, MSC-Exos enriched with miR-188-3p can inhibit inflammatory vesicles and ameliorate PD nigrostriatal dopamine neuronal damage by suppressing excessive autophagy (Li et al. 2021). In ALS studies, miR-466q and miR-467f in MSC-Exos were shown to downregulate Mapk11, miR-466 m-5p, and miR-466i-3p to promote the nuclear translocation of Nrf2, and miRNAs regulate inflammatory responses and oxidative stress in astrocytes through anti-inflammatory and antioxidant activities (Provenzano et al. 2022). Moreover, the role of miR-138-5p in astrocyte activation-mediated neuroinflammation has been identified (Deng et al. 2019). Taken together, these findings indicate that miRNA-rich MSC-Exos play a significant role in regulating neuroinflammation (Table 2).
Conclusion
MSC-Exos exhibit low immunogenicity and efficiently cross the BBB, allowing them to reach lesion sites easily. The miRNAs they carry influence the differentiation of glial cells and regulate neuroinflammation through signaling pathways. This process triggers the release of cytokines and inflammatory mediators, enhances apoptosis resistance, and has neuroprotective effects. Uncovering the mechanism of MSC-Exos in neurological diseases is thus crucial. Previous studies have shown that various components of MSC-Exos might collaborate to mitigate neuroinflammation through multiple cellular processes.
Nonetheless, the specific role of each MSC-Exo component in reducing neuroinflammation remains to be elucidated. Enhancing the targeting efficiency of MSC-Exos is also a crucial aspect to consider for future research. Despite their broad potential in treating neurological disease, MSC-Exos face several challenges; these include overcoming the barriers associated with MSC-Exo extraction technology, establishing standardized quality control measures, and optimizing the clinical benefits of these materials. Furthermore, few clinical studies on MSC-Exos exist, necessitating numerous basic and clinical investigations to unravel the underlying mechanism of MSC-Exos, particularly their role in neuroinflammation regulation and clinical safety, which will ultimately facilitate the early use of MSC-Exos in neurological disease treatment.
Data Availability
Not applicable.
References
Ba Z, Shi S, Huang N, Li Y, Huang J, You C, Yang X, Liu D, Yu C, He Y, Luo Y (2022) Mesenchymal stem cells after the proprocessing of tanshinone IIA attenuate cognitive deficits and oxidative stress injury in an amyloid β-peptide (25–35)-induced rodent model of Alzheimer’s disease. Neuroreport 33(2):61–71. https://doi.org/10.1097/wnr.0000000000001755
Bagheri-Mohammadi S (2021) Microglia in Alzheimer’s disease: the role of stem cell-microglia interaction in brain homeostasis. Neurochem Res 46(2):141–148. https://doi.org/10.1007/s11064-020-03162-4
Bagheri-Mohammadi S (2021) Protective effects of mesenchymal stem cells on ischemic brain injury: therapeutic perspectives of regenerative medicine. Cell Tissue Bank 22(2):249–262. https://doi.org/10.1007/s10561-020-09885-6
Behl T, Rana T, Alotaibi GH, Shamsuzzaman M, Naqvi M, Sehgal A, Singh S, Sharma N, Almoshari Y, Abdellatif AAH, Iqbal MS, Bhatia S, Al-Harrasi A, Bungau S (2022) Polyphenols inhibiting MAPK signalling pathway mediated oxidative stress and inflammation in depression. Biomed Pharmacother 146:112545. https://doi.org/10.1016/j.biopha.2021.112545
Brett BL, Gardner RC, Godbout J, Dams-O’Connor K, Keene CD (2022) Traumatic brain injury and risk of neurodegenerative disorder. Biol Psychiatry 91(5):498–507. https://doi.org/10.1016/j.biopsych.2021.05.025
Chen HX, Liang FC, Gu P, Xu BL, Xu HJ, Wang WT, Hou JY, Xie DX, Chai XQ, An SJ (2020) Exosomes derived from mesenchymal stem cells repair a Parkinson’s disease model by inducing autophagy. Cell Death Dis 11(4):288. https://doi.org/10.1038/s41419-020-2473-5
Chen Y, Li J, Ma B, Li N, Wang S, Sun Z, Xue C, Han Q, Wei J, Zhao RC (2020) MSC-derived exosomes promote recovery from traumatic brain injury via microglia/macrophages in rat. Aging 12(18):18274–18296. https://doi.org/10.18632/aging.103692
Chen YR, Lai PL, Chien Y, Lee PH, Lai YH, Ma HI, Shiau CY, Wang KC (2020) Improvement of impaired motor functions by human dental exfoliated deciduous teeth stem cell-derived factors in a rat model of Parkinson’s disease. Int J Mol Sci. https://doi.org/10.3390/ijms21113807
Ciesielska A, Matyjek M, Kwiatkowska K (2021) TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol Life Sci: CMLS 78(4):1233–1261. https://doi.org/10.1007/s00018-020-03656-y
Cowan M, Petri WA Jr (2018) Microglia: immune regulators of neurodevelopment. Front Immunol 9:2576. https://doi.org/10.3389/fimmu.2018.02576
Cui GH, Wu J, Mou FF, Xie WH, Wang FB, Wang QL, Fang J, Xu YW, Dong YR, Liu JR, Guo HD (2018) Exosomes derived from hypoxia-preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. FASEB J 32(2):654–668. https://doi.org/10.1096/fj.201700600R
Cui L, Luo W, Jiang W, Li H, Xu J, Liu X, Wang B, Wang J, Chen G (2022) Human umbilical cord mesenchymal stem cell-derived exosomes promote neurological function recovery in rat after traumatic brain injury by inhibiting the activation of microglia and astrocyte. Regen Ther 21:282–287. https://doi.org/10.1016/j.reth.2022.07.005
da Silva ML, Chagastelles PC, Nardi NB (2006) Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci 119(Pt 11):2204–2213. https://doi.org/10.1242/jcs.02932
Das K, Rao LVM (2022) The role of microRNAs in inflammation. Int J Mol Sci. https://doi.org/10.3390/ijms232415479
Deng Y, Chen D, Gao F, Lv H, Zhang G, Sun X, Liu L, Mo D, Ma N, Song L, Huo X, Yan T, Zhang J, Miao Z (2019) Exosomes derived from microRNA-138–5p-overexpressing bone marrow-derived mesenchymal stem cells confer neuroprotection to astrocytes following ischemic stroke via inhibition of LCN2. J Biol Eng 13:71. https://doi.org/10.1186/s13036-019-0193-0
Ding M, Shen Y, Wang P, Xie Z, Xu S, Zhu Z, Wang Y, Lyu Y, Wang D, Xu L, Bi J, Yang H (2018) Exosomes isolated from human umbilical cord mesenchymal stem cells alleviate neuroinflammation and reduce amyloid-beta deposition by modulating microglial activation in Alzheimer’s disease. Neurochem Res 43(11):2165–2177. https://doi.org/10.1007/s11064-018-2641-5
Ding H, Jia Y, Lv H, Chang W, Liu F, Wang D (2021) Extracellular vesicles derived from bone marrow mesenchymal stem cells alleviate neuroinflammation after diabetic intracerebral hemorrhage via the miR-183–5p/PDCD4/NLRP3 pathway. J Endocrinol Invest 44(12):2685–2698. https://doi.org/10.1007/s40618-021-01583-8
Fiebich BL, Batista CRA, Saliba SW, Yousif NM, de Oliveira ACP (2018) Role of microglia TLRs in neurodegeneration. Front Cell Neurosci 12:329. https://doi.org/10.3389/fncel.2018.00329
Garcia-Contreras M, Thakor AS (2021) Human adipose tissue-derived mesenchymal stem cells and their extracellular vesicles modulate lipopolysaccharide activated human microglia. Cell Death Discovery. https://doi.org/10.1038/s41420-021-00471-7
Giovannoni F, Quintana FJ (2020) The role of astrocytes in CNS inflammation. Trends Immunol 41(9):805–819. https://doi.org/10.1016/j.it.2020.07.007
Giunti D, Marini C, Parodi B, Usai C, Milanese M, Bonanno G, de Rosbo NK, Uccelli A (2021) Role of miRNAs shuttled by mesenchymal stem cell-derived small extracellular vesicles in modulating neuroinflammation. Sci Rep. https://doi.org/10.1038/s41598-021-81039-4
Go V, Bowley BGE, Pessina MA, Zhang ZG, Chopp M, Finklestein SP, Rosene DL, Medalla M, Buller B, Moore TL (2020) Extracellular vesicles from mesenchymal stem cells reduce microglial-mediated neuroinflammation after cortical injury in aged Rhesus monkeys. Geroscience 42(1):1–17. https://doi.org/10.1007/s11357-019-00115-w
Guan S, Liu Q, Gu H, Zhang YY, Wei PL, Qi YF, Liu J, Wang Z (2020) Pluripotent anti-inflammatory immunomodulatory effects of papaverine against cerebral ischemic-reperfusion injury. J Pharmacol Sci 144(2):69–75. https://doi.org/10.1016/j.jphs.2020.07.008
Guo M, Yin Z, Chen F, Lei P (2020) Mesenchymal stem cell-derived exosome: a promising alternative in the therapy of Alzheimer’s disease. Alzheimers Res Ther 12(1):109. https://doi.org/10.1186/s13195-020-00670-x
Guo S, Wang H, Yin Y (2022) Microglia polarization from M1 to M2 in neurodegenerative diseases. Front Aging Neurosci 14:815347. https://doi.org/10.3389/fnagi.2022.815347
Ha DH, Kim H-k, Lee J, Kwon HH, Park G-H, Yang SH, Jung JY, Choi H, Lee JH, Sung S, Yi YW, Cho BS (2020) Mesenchymal stem/stromal cell-derived exosomes for immunomodulatory therapeutics and skin regeneration. Cells. https://doi.org/10.3390/cells9051157
Harrell CR, Jovicic N, Djonov V, Arsenijevic N, Volarevic V (2019) Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells. https://doi.org/10.3390/cells8121605
Hasel P, Liddelow SA (2021) Astrocytes. Curr Biol 31(7):R326–R327. https://doi.org/10.1016/j.cub.2021.01.056
Hashioka S, Wu Z, Klegeris A (2021) Glia-driven neuroinflammation and systemic inflammation in Alzheimer’s disease. Curr Neuropharmacol 19(7):908–924. https://doi.org/10.2174/1570159x18666201111104509
Hu Z, Yuan Y, Zhang X, Lu Y, Dong N, Jiang X, Xu J, Zheng D (2021) Human umbilical cord mesenchymal stem cell-derived exosomes attenuate oxygen-glucose deprivation/reperfusion-induced microglial pyroptosis by promoting FOXO3a-dependent mitophagy. Oxid Med Cell Longev 2021:6219715. https://doi.org/10.1155/2021/6219715
Huang NQ, Jin H, Zhou SY, Shi JS, Jin F (2017) TLR4 is a link between diabetes and Alzheimer’s disease. Behav Brain Res 316:234–244. https://doi.org/10.1016/j.bbr.2016.08.047
Huang N, Li Y, Zhou Y, Zhou Y, Feng F, Shi S, Ba Z, Luo Y (2019) Neuroprotective effect of tanshinone IIA-incubated mesenchymal stem cells on Aβ(25–35)-induced neuroinflammation. Behav Brain Res 365:48–55. https://doi.org/10.1016/j.bbr.2019.03.001
Huang Y, Xu W, Zhou R (2021) NLRP3 inflammasome activation and cell death. Cell Mol Immunol 18(9):2114–2127. https://doi.org/10.1038/s41423-021-00740-6
Huang J, Yang F, Ji Z, Lin J, Weng Z, Tsang LL, Merson TD, Ruan YC, Wan C, Li G, Jiang X (2022) Human pluripotent stem cell-derived ectomesenchymal stromal cells promote more robust functional recovery than umbilical cord-derived mesenchymal stromal cells after hypoxic-ischaemic brain damage. Theranostics 12(1):143–166. https://doi.org/10.7150/thno.57234
Iranifar E, Seresht BM, Momeni F, Fadaei E, Mehr MH, Ebrahimi Z, Rahmati M, Kharazinejad E, Mirzaei H (2019) Exosomes and microRNAs: new potential therapeutic candidates in Alzheimer disease therapy. J Cell Physiol 234(3):2296–2305. https://doi.org/10.1002/jcp.27214
Jacquens A, Needham EJ, Zanier ER, Degos V, Gressens P, Menon D (2022) Neuro-inflammation modulation and post-traumatic brain injury lesions: from bench to bed-side. Int J Mol Sci. https://doi.org/10.3390/ijms231911193
Jankovic J, Tan EK (2020) Parkinson’s disease: etiopathogenesis and treatment. J Neurol Neurosurg Psychiatry 91(8):795–808. https://doi.org/10.1136/jnnp-2019-322338
Jin Q, Wu P, Zhou X, Qian H, Xu W (2021) Extracellular vesicles: novel roles in neurological disorders. Stem Cells Int 2021:6640836. https://doi.org/10.1155/2021/6640836
Joo HS, Suh JH, Lee HJ, Bang ES, Lee JM (2020) Current knowledge and future perspectives on mesenchymal stem cell-derived exosomes as a new therapeutic agent. Int J Mol Sci. https://doi.org/10.3390/ijms21030727
Kang X, Jiang L, Chen X, Wang X, Gu S, Wang J, Zhu Y, Xie X, Xiao H, Zhang J (2021) Exosomes derived from hypoxic bone marrow mesenchymal stem cells rescue OGD-induced injury in neural cells by suppressing NLRP3 inflammasome-mediated pyroptosis. Exp Cell Res 405(1):112635. https://doi.org/10.1016/j.yexcr.2021.112635
Kaniowska D, Wenk K, Rademacher P, Weiss R, Fabian C, Schulz I, Guthardt M, Lange F, Greiser S, Schmidt M, Braumann UD, Emmrich F, Koehl U, Jaimes Y (2022) Extracellular vesicles of mesenchymal stromal cells can be taken up by microglial cells and partially prevent the stimulation induced by beta-amyloid. Stem Cell Rev Rep 18(3):1113–1126. https://doi.org/10.1007/s12015-021-10261-4
Karve IP, Taylor JM, Crack PJ (2016) The contribution of astrocytes and microglia to traumatic brain injury. Br J Pharmacol 173(4):692–702. https://doi.org/10.1111/bph.13125
Khera R, Mehan S, Kumar S, Sethi P, Bhalla S, Prajapati A (2022) Role of JAK-STAT and PPAR-gamma signalling modulators in the prevention of autism and neurological dysfunctions. Mol Neurobiol 59(6):3888–3912. https://doi.org/10.1007/s12035-022-02819-1
Kim JH, Kwon O, Bhusal A, Lee J, Hwang EM, Ryu H, Park JY, Suk K (2022) Neuroinflammation induced by transgenic expression of lipocalin-2 in astrocytes. Front Cell Neurosci 16:839118. https://doi.org/10.3389/fncel.2022.839118
Kwon HS, Koh SH (2020) Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener 9(1):42. https://doi.org/10.1186/s40035-020-00221-2
Lai NS, Wu DG, Liang TY, Pan PJ, Yuan GQ, Li X, Li HY, Shen HT, Wang Z, Chen G (2020) Systemic exosomal miR-193b-3p delivery attenuates neuroinflammation in early brain injury after subarachnoid hemorrhage in mice. J Neuroinflamm. https://doi.org/10.1186/s12974-020-01745-0
Lai X, Wang Y, Wang X, Liu B, Rong L (2022) miR-146a-5p-modified hUCMSC-derived exosomes facilitate spinal cord function recovery by targeting neurotoxic astrocytes. Stem Cell Res Ther 13(1):487. https://doi.org/10.1186/s13287-022-03116-3
Laso-García F, Ramos-Cejudo J, Carrillo-Salinas FJ, Otero-Ortega L, Feliú A, Gómez-de Frutos M, Mecha M, Díez-Tejedor E, Guaza C, Gutiérrez-Fernández M (2018) Therapeutic potential of extracellular vesicles derived from human mesenchymal stem cells in a model of progressive multiple sclerosis. PLoS ONE 13(9):e0202590. https://doi.org/10.1371/journal.pone.0202590
Leng F, Edison P (2021) Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat Rev Neurol 17(3):157–172. https://doi.org/10.1038/s41582-020-00435-y
Levy O, Kuai R, Siren EMJ, Bhere D, Milton Y, Nissar N, De Biasio M, Heinelt M, Reeve B, Abdi R, Alturki M, Fallatah M, Almalik A, Alhasan AH, Shah K, Karp JM (2020) Shattering barriers toward clinically meaningful MSC therapies. Sci Adv 6(30):6884. https://doi.org/10.1126/sciadv.aba6884
Li H, Yahaya BH, Ng WH, Yusoff NM, Lin J (2019) Conditioned medium of human menstrual blood-derived endometrial stem cells protects against MPP(+)-induced cytotoxicity in vitro. Front Mol Neurosci 12:80. https://doi.org/10.3389/fnmol.2019.00080
Li Q, Wang Z, Xing H, Wang Y, Guo Y (2021) Exosomes derived from miR-188-3p-modified adipose-derived mesenchymal stem cells protect Parkinson’s disease. Mol Ther Nucleic Acids 23:1334–1344. https://doi.org/10.1016/j.omtn.2021.01.022
Li C, Li X, Shi Z, Wu P, Fu J, Tang J, Qing L (2022) Exosomes from LPS-preconditioned bone marrow MSCs accelerated peripheral nerve regeneration via M2 macrophage polarization: involvement of TSG-6/NF-κB/NLRP3 signaling pathway. Exp Neurol. https://doi.org/10.1016/j.expneurol.2022.114139
Li J, Wei Y, Zhou J, Zou H, Ma L, Liu C, Xiao Z, Liu X, Tan X, Yu T, Cao S (2022) Activation of locus coeruleus-spinal cord noradrenergic neurons alleviates neuropathic pain in mice via reducing neuroinflammation from astrocytes and microglia in spinal dorsal horn. J Neuroinflam 19(1):123. https://doi.org/10.1186/s12974-022-02489-9
Liddelow SA, Barres BA (2017) Reactive astrocytes: production, function, and therapeutic potential. Immunity 46(6):957–967. https://doi.org/10.1016/j.immuni.2017.06.006
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung W-S, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541(7638):481–487. https://doi.org/10.1038/nature21029
Linnerbauer M, Wheeler MA, Quintana FJ (2020) Astrocyte crosstalk in CNS inflammation. Neuron 108(4):608–622. https://doi.org/10.1016/j.neuron.2020.08.012
Liu K, Cai GL, Zhuang Z, Pei SY, Xu SN, Wang YN, Wang H, Wang X, Cui C, Sun MC, Guo SH, Jia KP, Wang XZ, Cai GF (2021) Interleukin-1 beta-treated mesenchymal stem cells inhibit inflammation in hippocampal astrocytes through exosome-activated Nrf-2 signaling. Int J Nanomed 16:1423–1434. https://doi.org/10.2147/ijn.S289914
Liu X, Zhang M, Liu H, Zhu R, He H, Zhou Y, Zhang Y, Li C, Liang D, Zeng Q, Huang G (2021) Bone marrow mesenchymal stem cell-derived exosomes attenuate cerebral ischemia-reperfusion injury-induced neuroinflammation and pyroptosis by modulating microglia M1/M2 phenotypes. Exp Neurol 341:113700. https://doi.org/10.1016/j.expneurol.2021.113700
Liu ZW, Wang B, Guo QH (2021) MiR-26b-5p-modified hUB-MSCs derived exosomes attenuate early brain injury during subarachnoid hemorrhage via MAT2A-mediated the p38 MAPK/STAT3 signaling pathway. Brain Res Bull 175:107–115. https://doi.org/10.1016/j.brainresbull.2021.07.014
Liu S, Fan M, Xu JX, Yang LJ, Qi CC, Xia QR, Ge JF (2022) Exosomes derived from bone-marrow mesenchymal stem cells alleviate cognitive decline in AD-like mice by improving BDNF-related neuropathology. J Neuroinflamm 19(1):35. https://doi.org/10.1186/s12974-022-02393-2
Liu TW, Chen CM, Chang KH (2022) Biomarker of neuroinflammation in Parkinson’s disease. Int J Mol Sci. https://doi.org/10.3390/ijms23084148
Liu Y, Shen X, Zhang Y, Zheng X, Cepeda C, Wang Y, Duan S, Tong X (2023) Interactions of glial cells with neuronal synapses, from astrocytes to microglia and oligodendrocyte lineage cells. Glia 71(6):1383–1401. https://doi.org/10.1002/glia.24343
Long Q, Upadhya D, Hattiangady B, Kim DK, An SY, Shuai B, Prockop DJ, Shetty AK (2017) Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc Natl Acad Sci USA 114(17):E3536-e3545. https://doi.org/10.1073/pnas.1703920114
Losurdo M, Pedrazzoli M, D’Agostino C, Elia CA, Massenzio F, Lonati E, Mauri M, Rizzi L, Molteni L, Bresciani E, Dander E, D’Amico G, Bulbarelli A, Torsello A, Matteoli M, Buffelli M, Coco S (2020) Intranasal delivery of mesenchymal stem cell-derived extracellular vesicles exerts immunomodulatory and neuroprotective effects in a 3 × Tg model of Alzheimer’s disease. Stem Cells Transl Med 9(9):1068–1084. https://doi.org/10.1002/sctm.19-0327
Monteiro AR, Barbosa DJ, Remião F, Silva R (2023) Alzheimer’s disease: insights and new prospects in disease pathophysiology, biomarkers and disease-modifying drugs. Biochem Pharmacol 211:115522. https://doi.org/10.1016/j.bcp.2023.115522
Nakano M, Kubota K, Kobayashi E, Chikenji TS, Saito Y, Konari N, Fujimiya M (2020) Bone marrow-derived mesenchymal stem cells improve cognitive impairment in an Alzheimer’s disease model by increasing the expression of microRNA-146a in hippocampus. Sci Rep. https://doi.org/10.1038/s41598-020-67460-1
Ni H, Yang S, Siaw-Debrah F, Hu J, Wu K, He Z, Yang J, Pan S, Lin X, Ye H, Xu Z, Wang F, Jin K, Zhuge Q, Huang L (2019) Exosomes derived from bone mesenchymal stem cells ameliorate early inflammatory responses following traumatic brain injury. Front Neurosci 13:14. https://doi.org/10.3389/fnins.2019.00014
Norden DM, Trojanowski PJ, Villanueva E, Navarro E, Godbout JP (2016) Sequential activation of microglia and astrocyte cytokine expression precedes increased Iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia 64(2):300–316. https://doi.org/10.1002/glia.22930
Novoa C, Salazar P, Cisternas P, Gherardelli C, Vera-Salazar R, Zolezzi JM, Inestrosa NC (2022) Inflammation context in Alzheimer’s disease, a relationship intricate to define. Biol Res 55(1):39. https://doi.org/10.1186/s40659-022-00404-3
Palmulli R, van Niel G (2018) To be or not to be secreted as exosomes, a balance finely tuned by the mechanisms of biogenesis. Essays Biochem 62(2):177–191. https://doi.org/10.1042/EBC20170076
Pathipati P, Lecuyer M, Faustino J, Strivelli J, Phinney DG, Vexler ZS (2021) Mesenchymal stem cell (MSC)-derived extracellular vesicles protect from neonatal stroke by interacting with microglial cells. Neurotherapeutics 18(3):1939–1952. https://doi.org/10.1007/s13311-021-01076-9
Provenzano F, Nyberg S, Giunti D, Torazza C, Parodi B, Bonifacino T, Usai C, Kerlero de Rosbo N, Milanese M, Uccelli A, Shaw PJ, Ferraiuolo L, Bonanno G (2022) Micro-RNAs shuttled by extracellular vesicles secreted from mesenchymal stem cells dampen astrocyte pathological activation and support neuroprotection in in-vitro models of ALS. Cells. https://doi.org/10.3390/cells11233923
Qiu G, Zheng G, Ge M, Wang J, Huang R, Shu Q, Xu J (2019) Functional proteins of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res Ther 10(1):359. https://doi.org/10.1186/s13287-019-1484-6
Ratan Y, Rajput A, Maleysm S, Pareek A, Jain V, Pareek A, Kaur R, Singh G (2023) An insight into cellular and molecular mechanisms underlying the pathogenesis of neurodegeneration in Alzheimer’s disease. Biomedicines. https://doi.org/10.3390/biomedicines11051398
Rodriguez-Gomez JA, Kavanagh E, Engskog-Vlachos P, Engskog MKR, Herrera AJ, Espinosa-Oliva AM, Joseph B, Hajji N, Venero JL, Burguillos MA (2020) Microglia: agents of the CNS pro-inflammatory response. Cells. https://doi.org/10.3390/cells9071717
Rueda-Carrasco J, Martin-Bermejo MJ, Pereyra G, Mateo MI, Borroto A, Brosseron F, Kummer MP, Schwartz S, López-Atalaya JP, Alarcon B, Esteve P, Heneka MT, Bovolenta P (2021) SFRP1 modulates astrocyte-to-microglia crosstalk in acute and chronic neuroinflammation. EMBO Rep 22(11):e51696. https://doi.org/10.15252/embr.202051696
Schirmer L, Schafer DP, Bartels T, Rowitch DH, Calabresi PA (2021) Diversity and function of glial cell types in multiple sclerosis. Trends Immunol 42(3):228–247. https://doi.org/10.1016/j.it.2021.01.005
Schulz-Siegmund M, Aigner A (2021) Nucleic acid delivery with extracellular vesicles. Adv Drug Delivery Rev 173:89–111. https://doi.org/10.1016/j.addr.2021.03.005
Sha S, Shen X, Cao Y, Qu L (2021) Mesenchymal stem cells-derived extracellular vesicles ameliorate Alzheimer’s disease in rat models via the microRNA-29c-3p/BACE1 axis and the Wnt/β-catenin pathway. Aging 13(11):15285–15306. https://doi.org/10.18632/aging.203088
Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C, Wang Y (2018) Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol 14(8):493–507. https://doi.org/10.1038/s41581-018-0023-5
Shu JP, Jiang L, Wang MQ, Wang R, Wang XY, Gao CL, Xia ZK (2022) Human bone marrow mesenchymal stem cells-derived exosomes protect against nerve injury via regulating immune microenvironment in neonatal hypoxic-ischemic brain damage model. Immunobiology. https://doi.org/10.1016/j.imbio.2022.152178
Singh D (2022) Astrocytic and microglial cells as the modulators of neuroinflammation in Alzheimer’s disease. J Neuroinflamm 19(1):206. https://doi.org/10.1186/s12974-022-02565-0
Singh N, Das B, Zhou J, Hu X, Yan R (2022) Targeted BACE-1 inhibition in microglia enhances amyloid clearance and improved cognitive performance. Sci Adv 8(29):3610. https://doi.org/10.1126/sciadv.abo3610
Skok M (2021) Mesenchymal stem cells as a potential therapeutic tool to cure cognitive impairment caused by neuroinflammation. World J Stem Cells 13(8):1072–1083. https://doi.org/10.4252/wjsc.v13.i8.1072
Sloane JA, Batt C, Ma Y, Harris ZM, Trapp B, Vartanian T (2010) Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through TLR2. Proc Natl Acad Sci USA 107(25):11555–11560. https://doi.org/10.1073/pnas.1006496107
Stephenson J, Nutma E, van der Valk P, Amor S (2018) Inflammation in CNS neurodegenerative diseases. Immunology 154(2):204–219. https://doi.org/10.1111/imm.12922
Subhramanyam CS, Wang C, Hu Q, Dheen ST (2019) Microglia-mediated neuroinflammation in neurodegenerative diseases. Semin Cell Dev Biol 94:112–120. https://doi.org/10.1016/j.semcdb.2019.05.004
Sun E, Motolani A, Campos L, Lu T (2022) The pivotal role of NF-kB in the pathogenesis and therapeutics of Alzheimer’s disease. Int J Mol Sci. https://doi.org/10.3390/ijms23168972
Tang Y, Zhou Y, Li HJ (2021) Advances in mesenchymal stem cell exosomes: a review. Stem Cell Res Ther 12(1):71. https://doi.org/10.1186/s13287-021-02138-7
Thomi G, Surbek D, Haesler V, Joerger-Messerli M, Schoeberlein A (2019) Exosomes derived from umbilical cord mesenchymal stem cells reduce microglia-mediated neuroinflammation in perinatal brain injury. Stem Cell Res Ther 10(1):105. https://doi.org/10.1186/s13287-019-1207-z
Wang T, Shi C, Luo H, Zheng H, Fan L, Tang M, Su Y, Yang J, Mao C, Xu Y (2022) Neuroinflammation in Parkinson’s disease: triggers, mechanisms, and immunotherapies. Neurosci: Rev J Bring Neurobiol Neurol Psychiatry 28(4):364–381. https://doi.org/10.1177/1073858421991066
Wen L, Wang YD, Shen DF, Zheng PD, Tu MD, You WD, Zhu YR, Wang H, Feng JF, Yang XF (2022) Exosomes derived from bone marrow mesenchymal stem cells inhibit neuroinflammation after traumatic brain injury. Neural Regen Res 17(12):2717–2724. https://doi.org/10.4103/1673-5374.339489
Xian P, Hei Y, Wang R, Wang T, Yang J, Li J, Di Z, Liu Z, Baskys A, Liu W, Wu S, Long Q (2019) Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics 9(20):5956–5975. https://doi.org/10.7150/thno.33872
Xin P, Xu X, Deng C, Liu S, Wang Y, Zhou X, Ma H, Wei D, Sun S (2020) The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int Immunopharmacol 80:106210. https://doi.org/10.1016/j.intimp.2020.106210
Xin DQ, Zhao YJ, Li TT, Ke HF, Gai CC, Guo XF, Chen WQ, Liu DX, Wang Z (2022) The delivery of miR-21a-5p by extracellular vesicles induces microglial polarization via the STAT3 pathway following hypoxia-ischemia in neonatal mice. Neural Regen Res 17(10):2238–2246. https://doi.org/10.4103/1673-5374.336871
Xiong LL, Sun LL, Zhang YX, Peng J, Yan JH, Liu XH (2020) Exosomes from bone marrow mesenchymal stem cells can alleviate early brain injury after subarachnoid hemorrhage through miRNA129–5p-HMGB1 pathway. Stem Cells Dev 29(4):212–221. https://doi.org/10.1089/scd.2019.0206
Xu H, Jia Z, Ma K, Zhang J, Dai C, Yao Z, Deng W, Su J, Wang R, Chen X (2020) Protective effect of BMSCs-derived exosomes mediated by BDNF on TBI via miR-216a-5p. Med Sci Monit 26:e920855. https://doi.org/10.12659/MSM.920855
Yu H, Lin L, Zhang Z, Zhang H, Hu H (2020) Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct Target Ther 5(1):209. https://doi.org/10.1038/s41392-020-00312-6
Zavatti M, Gatti M, Beretti F, Palumbo C, Maraldi T (2022) Exosomes derived from human amniotic fluid mesenchymal stem cells preserve microglia and neuron cells from A beta. Int J Mol Sci. https://doi.org/10.3390/ijms23094967
Zhai L, Shen H, Sheng Y, Guan Q (2021) ADMSC Exo-MicroRNA-22 improve neurological function and neuroinflammation in mice with Alzheimer’s disease. J Cell Mol Med 25(15):7513–7523. https://doi.org/10.1111/jcmm.16787
Zhang Y, Zhang Y, Chopp M, Zhang ZG, Mahmood A, Xiong Y (2020) Mesenchymal stem cell-derived exosomes improve functional recovery in rats after traumatic brain injury: a dose-response and therapeutic window study. Neurorehabilitation Neural Repair 34(7):616–626. https://doi.org/10.1177/1545968320926164
Zhang Y, Zhang Y, Chopp M, Pang H, Zhang ZG, Mahmood A, Xiong Y (2021) MiR-17-92 cluster-enriched exosomes derived from human bone marrow mesenchymal stromal cells improve tissue and functional recovery in rats after traumatic brain injury. J Neurotrauma 38(11):1535–1550. https://doi.org/10.1089/neu.2020.7575
Zhang J, Buller BA, Zhang ZG, Zhang Y, Lu M, Rosene DL, Medalla M, Moore TL, Chopp M (2022) Exosomes derived from bone marrow mesenchymal stromal cells promote remyelination and reduce neuroinflammation in the demyelinating central nervous system. Exp Neurol 347:113895. https://doi.org/10.1016/j.expneurol.2021.113895
Zong L, Huang P, Song Q, Kang Y (2021) Bone marrow mesenchymal stem cells-secreted exosomal H19 modulates lipopolysaccharides-stimulated microglial M1/M2 polarization and alleviates inflammation-mediated neurotoxicity. Am J Transl Res 13(3):935–951
Funding
This work is supported by the National Natural Science Foundation of China (82160858), the Science and Technology Department of Guizhou Province (ZK[2021]-570), the Zunyi Science and Technology Bureau (HZ-2022-45), and the Funds of The First People’s Hospital of Zunyi for Research and Experimental Development (R&D 2020-06).
Author information
Authors and Affiliations
Contributions
YG and JW designed the draft. YG, JW, LZ, QH, and YL drafted or revised the manuscript. All the authors read and approved the final manuscript for publication.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Ethical Approval
Not applicable.
Consent for Publication
All the authors have approved the publication of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ge, Y., Wu, J., Zhang, L. et al. A New Strategy for the Regulation of Neuroinflammation: Exosomes Derived from Mesenchymal Stem Cells. Cell Mol Neurobiol 44, 24 (2024). https://doi.org/10.1007/s10571-024-01460-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10571-024-01460-x