Abstract
A promising method for cancer therapy is the coating of magnetic nanoparticles with carboxy methylcellulose. In a research project, hydroalcoholic extract of Cinnamomum camphora leaves was used to demonstrate the production of magnetic nanoparticles (MNPs); MNPs were coated with carboxymethyl cellulose to form carboxymethyl cellulose-coated magnetic nanoparticles (CMNPs)were formed. Preliminary phytochemical screening of C. camphora confirmed the presence of flavonoids, carbohydrates, phenolic compounds, and proteins. Phenolics 280.59 (mg/g), flavonoids 15.46 (mg/g), proteins 1.9 (mg/mL) and total carbohydrates 293.80 (mg/g) were all quantified. To confirm the formation of MNPs and CMNPs, UV–visible (UV–vis) spectroscopy, Fourier transform infrared (FT-IR) spectroscopy, X-ray diffraction (XRD), transmission electron microscopy (TEM), and X-ray photoelectron spectroscopy (XPS) were used. Peaks were observed at 232 nm and 240 nm, respectively. The largest absorption peaks were observed in MNPs and CMNPs, respectively. The particles were spherical in shape and less than 10 (nm) in diameter. The potential scavenging activity of biosynthesized MNPs and CMNPs was evaluated by the ABTS and DPPH assays, and the inhibition values IC50 were 141.3 ± 3.0 and 61.67 ± 2.5 (µg/mL) for ABTS and 176.1 ± 4.0 and 70.92 ± 3.0 (µg/mL) for DPPH, respectively (p ≤ 0.05). Furthermore, the cytotoxicity test results showed that the HCT-116 human colon cancer cell line had the lowest IC50 value of 20 (µg/mL) for CMNP, followed by the HepG2 hepatocellular carcinoma cell line with an IC50 value of 33 (µg/mL) for CMNP, indicating that the cytotoxic effect on colon cancer cells is stronger than on liver cancer cells. Molecular docking studies have revealed that CMNPs target and bind to apoptotic protein, enhancing their bioactivity and cytotoxic effects on cancer cells. Furthermore, our findings suggest that the induction of apoptosis may be responsible for the anticancer effects of CMNPs.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cancer treatment could benefit greatly from the application of nanotechnology. Chemotherapeutic drugs can be administered directly to cancer cells, thereby reducing damage to healthy tissue. A relatively new and highly interdisciplinary area that holds great promise for in vitro and in vivo therapeutic and diagnostic testing is the application of nanoparticles for medical reasons, such as anti-biofilm and antibacterial properties (El-Sayyad et al. 2022). Magnetic nanoparticles (MNPs) can be produced using several techniques; biological approaches are preferred over conventional methods for MNP synthesis because the production of MNPs involves the use of potentially harmful substances. Therefore, biological methods are not only environmentally safe and economical, but they are also rapid, single-step processes that can be easily scaled up. Furthermore, it does not require the use of energy, pressure, high temperatures, or potentially hazardous materials (Dhar et al. 2021; Yew et al. 2016; Abd El-Aziz et al. 2023; Eldeeb et al. 2023). Biological methods for nanoparticle synthesis are based on algae, bacteria, fungi, actinomycetes, yeasts, and plants (El-Rafie et al. 2016, 2023; Abd El-Aziz et al. 2023; Rai et al. 2009; Kalishwaralal et al. 2010; Saratale et al. 2018; Roy et al. 2019). C. camphora (camphor-tree) has high aromatic potential in the pharmaceutical and food industries (Lee et al. 2022). It has blood purifying properties, digestive properties, astringent, stimulant, antipyretic, antimicrobial, antinutritional, antiviral, and antifungal properties, as well as blood sugar and cholesterol-lowering properties. Although medicinal plants are important in new drug development, standardization of bioactive extracts should be done on active ingredient basis (Lee et al. 2006; Singh and Jawaid 2012; Pragadheesh et al. 2013; Marasini et al. 2015; Cardoso-Ugarte et al. 2016). Compounds derived from camphor have been found to from have biological activity as antioxidants, fungicides, antifungals, and anti-inflammatory agents. Camphor wood and leaves are a readily available and inexpensive resources in Egypt. The combination of biodegradable polymers and nanomaterials (NMs) is a current research topic. Polysaccharides are non-toxic, readily biodegradable, widely dispersible, and biocompatible. Common reduction stabilizers of mono-, bi-, and tri-metallic nanoparticles are polysaccharides (Abd El-Aziz et al. 2023; Alam et al. 2020). This is because polysaccharides are environmentally benign and easily transformed into different types of hydrogels. Biopolymers are now be employed in more applications because nanoparticles (NPs) improve their thermal, mechanical, and barrier properties (Berradi et al. 2023). One of the most promising nano-reinforces is cellulose, which has a large surface area, flexibility in surface functionalization, good barrier properties, biodegradability, and biocompatibility (Wang et al. 2022a, b; Hazra et al. 2024; Schiavi et al. 2024). Interesting properties of cellulose include its high wet strength, low density, and large water retention capacity (Musino et al. 2021, Joshi and Manjare 2024). One of the most widely used cellulose derivatives, Carboxymethyl cellulose (CMC), has applications in a variety of industries, including the food industry, paper industry, textile industry, pharmaceutical industry, and biomedical engineering. In addition to being renewable, biodegradable, and non-hazardous to human health, CMCs are widely used in wastewater treatment, power generation, and quality maintenance of agricultural products (Spiliopoulos et al. 2021; Saberi Riseh et al 2023; Tang et al. 2024). CMCs are water soluble polymers with carboxymethyl groups attached to some of the three monomers that make up the cellulose backbone (Jabareen et al. 2021; Salem et al. 2022). Cellulose was used to develop different types of NPs, which are adsorbents that can efficiently remove anionic and cationic dyes (Domingues et al. 2022; Wang et al. 2022a, b). Due to their long cellulose chains, they were also used to stabilize nanoparticles. (He et al 2022). CMCs have frequently been used as suitable reducing agents and stabilizers in the production of NPs in an aqueous media (Aguilera et al. 2018; El-Naggar et al. 2022). Researchers are working hard to develop low-cost and environmentally friendly production techniques that will lower production costs and allow the use of safe materials such as CMCs for pharmaceutical applications. Therefore, the aim of this study was to evaluate the favorable effects of biogenic MNPs and CMNPs on the proliferation of HEPG-2 and HCT-116 cells (Hazra et al. 2020; Elbagory et al. 2021).
Materials and methods
Collection of C. camphora leaves
C. camphora leaves were collected from Helwan district, Egypt, and properly washed with tap water; C. camphora leaves were dried in the shade, and then placed in oven trays, and heated overnight at a temperature of 30–35 °C. After complete drying, plant samples were ground to a fine powder and stored in sealed bottles before use for extraction.
Preparation of C. camphora leaf extract
Thirty grams of C. camphora leaves were boiled in 150 mL of double-distilled water for 15 min. The plant extract was then filtered and dried at 40 °C, and stored for MNP synthesis.
C. camphora quantitative and qualitative screening for phytochemicals
Phytochemical qualitative reactions of typical plant secondary metabolites were performed on hydroalcoholic extract of C. camphora. Extracts were screened to determine the type and amount of flavonoids, carbohydrates, phenolic compounds, and proteins. Qualitative and quantitative phytochemical constituents were determined from color intensity and precipitate formation due to the presence of these metabolites (Parekh and Chanda 2007; Prabhu et al 2011; Shinde et al. 2012). Total protein, carbohydrate, phenolic, and flavonoid content were determined by standard methods (Kiranmai et al. 2011).
GC/MS analysis of the hydroalcoholic extract of C. camphora
The C. camphora hydroalcoholic extract was analyzed on a fused silica capillary column, TG-5MS capillary column, helium flow rate 1 mL/min as carrier gas, injection temperature250°C, oven temperature 80 °C, 0.0 min, ramp 10 °C/min to 320 °C. The analysis was performed by GC/MS (GC/MS GC TRACE 1300/ISQ 7000).
Synthesis of MNPs using C. camphora extract
Ferrous sulfate (10 mL) and ferric chloride (20 mL) were mixed and heated at 80 °C for 40 min, then 10 mL of hydroalcoholic extract of C. camphora was added and stirred for 5 min. The pH was then raised to 11 using 1 M NaOH; after 30 min of continued stirring, a black precipitate of magnetic nanoparticles formed. The MNPs were separated using a magnet and washed with distilled water. Finally, the MNPs were dried at 95 °C.
Carboxymethyl cellulose
This study used carboxymethyl cellulose (CMC), a white or slightly yellowish hygroscopic solid that was odorless, tasteless, non-toxic, and easy to ferment. Carboxymethyl cellulose (cellulose gum) is obtained from wood by treating it with alkali and monochloroacetic acid, or its sodium salt. With water, CMC (CMC from sigma-aldrich company) average molecular weight (Mw) of ~ 250,000, a degree of substitution of 0.7, and a grade of 97–100%.
Coating process
10 mL of distilled water was used to disperse 100 mg of dried and finely milled magnetic nanoparticles, which were then sonicated for 10 min. 10 mg of carboxymethyl cellulose (CMC) was dissolved 10 mL of DI and physically swirled until the CMC was completely dissolved, then the CMC solution was added dropwise to the magnetic nanoparticle suspension and stirred at 500 rpm for 10 h (Aguilera et al. 2018).
Characterization of biosynthesized MNPs and CMNPs
UV–vis spectroscopy
Samples were suspended in water at a concentration of 1 mg/mL. The pH of the sample was adjusted to 7.0 using 0.1 M HCl or 0.1 M NaOH. Samples were diluted 1:3 with distilled water, sonicated for 10 min, and subjected to measurement. A UVS-260D spectrophotometer was used for UV–vis measurements. The following parameters were utilized: scale up (top 3.0), bottom (0), wavelength start 200 nm, wavelength end 800 nm (Takai et al. 2019).
Zeta size and potential analysis
Samples were suspended in water at a concentration of 1 mg/mL. The pH of the sample was adjusted to 7.0 using 0.1 M HCl or 0.1 M NaOH. Samples were diluted with distilled water (1:3) and sonicated for 10 min. The potential and size of MNPs and CMNPs were measured at the Center for Nanotechnology, Helwan University, Egypt, using a Zeta Sizer Nano ZS from Malvern Instruments, Malvern, UK. Using a 150 mV electric field and water as a dispersant, were used to determine the potential surface charge of the particles. The RI of the dispersant was 1.330, the viscosity (cp) was 0.8872, and the dispersant constant of the dispersant was 7.5. The measurements were repeated three times, and the standard deviations were calculated (Fan et al 2013).
Transmission electron microscopy analysis
Samples were prepared by drop-casting a suspension of MNPs or CMNPs onto a carbon-coated copper grid and drying at room temperature. To measure the size and shape of MNPs and CMNPs, a JEOL electron microscope (TEM, JEOL JEM 2100, Japan) was used. The grids were examined at various magnifications of 100, 50, and 10 nm at TEM 120 kV at the Nanotechnology Center, Petroleum Refining Department, Egyptian Petroleum Research Institute (EPRI) (HV = 120 kV; magnification = 1500000X; print magnification at 211 mm = 371000x; TEM mode: Imaging (Poole and Owens 2003).
FTIR spectroscopy
Samples were prepared by grinding the MNP and CMNP, C. camphora extract, and CMC with KBr and extruding the mixture into pellets using a KBr Beam Divider. MIR TGS detector (4000–450 cm−1). We used the PerkinElmer ATR Sample Base Plate DIAMOND Spectrometer was used to analyze FTIR spectra with the following parameters: instrument mode (Spectrum Two), instrument serial number (109,979), software revision (NIOS2 Main 00.02.0091), number of scans (4)), resolution (4), detector (MIR TGS), scan speed (0.2), and UATR crystal combination (Diamond) were used (Zhang et al. 2011).
XRD analysis
Samples were prepared by finely grinding the MNPs and CMNPs. Target Cu, voltage 40 kV, current 30 mA, divergence slit 1.00000°, scatteringslit 0.00000°, receiving slit 0.30000 mm, ribaxis: θ-2θ,scan range 4.0000—90.0000°, scan mode: continuous scan, Scanning speed: 8.0000°/min, Sampling pitch: 0.0200°, after 600 °C ignition (Sotiriou et al. 2012).
Assessment of carboxymethyl cellulose’s computational absorptivity
The magnetic nanoparticle adsorbent material’s structure was obtained in a (mol) file format from the BIOVIA Materials Studio Wizard tool, while the carboxymethyl cellulose adsorbate’s mol file was obtained from PubChem. The “Optimize Geometry” tool in BIOVIA Materials Studio was utilized to optimize the structure for simulation. The “Forcite” module of BIOVIA Materials Studio was used to perform classical molecular dynamics simulations for the magnetic adsorption simulation. One chitosan structure was housed in a 20 × 20 × 20 Å cubic simulation cell that was assembled. The “Builder” tool was utilized to insert the adsorbate molecule, in this case insulin, into the simulation cell and place it inside the chitosan structure. After that, water molecules were added to the system. Constant numbers of atoms, pressure, and temperature were set as the simulation parameters for an NPT ensemble. An NVT ensemble (constant number of atoms, volume, and temperature) was used to equilibrate the system for one nanosecond after it was first heated to 300 K. Magnetic interactions were induced by applying a 10 T magnetic field perpendicular to the simulation cell’s xy-plane. The simulation had a time step of 1 fs and ran for 5 ns. Temperature, pressure, and energy were tracked until convergence. The “Analysis” module in Materials Studio was used to examine simulation results by calculating and analyzing adsorption energies and other relevant parameters in an effort to identify possible applications.
Antioxidant activity of MNPs and CMNPs
The ABTS and DPPH assays, respectively, were used to measure the radical scavenging activity of MNPs and CMNPs. The assay used the methods of Benzie and Strain (1996) and Arnao et al. (2001) technique. We performed the measurements three times and calculated the standard deviation after each time.
Anticancer activity of MNPs and CMNPs
Cell lines
HCT 116, HepG2, and WI-38 are human cell lines that indicate normal lung fibroblasts, colon cancer, and liver cancer, respectively. In a 5 (%) CO2 incubator at 37 °C, they were cultivated in RPMI-1640, Dulbico 10 (%) fetal bovine serum supplemented medium, and 1 (%) Pen/Strep solution.
Cell viability assay
Cell survival was assessed by conducting the MTT test (Mosmann 1983; Ohno and Abe 1991). HCT-116 and HepG2 cells were plated and allowed to grow in 96-well plates for 24 h. Different concentrations of MNPs and CMNPs (100, 50, 25, 12.5, and 6.5 (µg/mL)) were added to the media. After 48 h, 50 μL of MTT solution (5 mg/mL in PBS) was added to the wells and incubated at 37 °C for 4 h. The media was discarded after the treatment period of 48 h, and the cells were washed twice with PBS. then 50 µl of DMSO was then added to dissolve the formazan crystals, resulting in purple color. Cell survival percentage was determined by reading the absorbance at 570 nm using a Biotek (ELX-800) plate reader after incubating the plates at 37 °C for 10 min. Each measurement was performed three times, and the standard deviation between results was calculated.
Apoptotic staining
The apoptotic damage was evaluated using the dual acridine orange/ethidium bromide (AO/EB) assay. HCT 116 and HepG2 cancer cells, as well as untreated cells, were stained with AO/EB following 48 h of customized treatments with MNPs and CMNPs at IC50. After the staining solution was added to the plates, they were washed three times in PBS. Living and dead cells were stained with AO, and cells whose membrane integrity had been compromised by EB were labeled (Pfeffer et al. 2018). Morphological changes were monitored using a fluorescent microscope (at 40 and 100 × magnifications).
In silico molecular docking and absorptivity test
The objective of the molecular docking study was to examine the binding affinities and interactions between doxorubicin, magnetic nanoparticles (MNPs), and three targets of apoptotic proteins: caspase 3, caspase 9, and Bcl-2-associated death promoter (BAD). With the following PDB IDs: 7Q16 for BAD, 3DEI for caspase 3, and 2AR9 for caspase 9, the protein structures utilized in the investigation were acquired from the RCSB Protein Data Bank. The MM2 forcefield in Chem3D of the ChemDraw software was used to optimize the ligand structures of doxorubicin, MNPs, and carboxymethyl cellulose MNPs (CMNPs) after they were built using Material Studio 2020. The Protein Preparation Wizard in Maestro was used to prepare the protein structures for docking. This included adding hydrogen atoms, determining bond orders, and reducing steric clashes. The active sites of each receptor were then surrounded by grids. After the proteins were ready, they were put through the Glide docking process in the Schrodinger suite for molecular docking. The binding poses were ascertained by analyzing the docking scores and interactions with significant amino acid residues. Additionally, absorptivity scores were computed in order to forecast the possible uptake of nanoparticles.
Statistics
The analysis was performed using Graph Pad Prism 8.0 software at a confidence level of 95 (%).
Results and discussion
Phytochemical analysis
Plants have many different metabolites, such as proteins, carbohydrates, phenols, and flavonoids, which contribute to their appealing characteristics. A wide range of phytochemicals were found in the hydroalcoholic extract of C. camphora. Table 1 illustrates that C. camphora leaves contain phenolics 280.59 mg/g, flavonoids 15.46 mg/g, proteins 1.9 mg/mL, and total carbohydrates 293.80 mg/g, all of which are highly concentrated in C. camphora. The main ingredients in the formation and stabilization of CMNPs were proteins, carbohydrates, phenolic compounds, and flavonoids (Huang et al. 2007). We repeated the measurements three times and calculated the standard deviations.
GC/MS analysis of the hydroalcoholic extract of C. camphora
The components of hydroalcoholic extracts of C. camphora were identified using the GC–MS technique (method), both, the functional groups that made up MNPs and C. camphora extract. The GC/MS analysis of the C. camphora hydroalcoholic extract, as represented by the total ion chromatogram in Fig. 1, and the results compiled in Table 2, demonstrated the identification of 22 compounds, which accounted for 90% of the hydroalcoholic extract’s total peak area. The major compounds identified in the extract were 15 (%), 13-Octadecenal (Z)-12.3 (%), 5-Octadecene (E)- 9.22(%), 13-Octadecenal (Z)- 7.25 (%), and 9-Octadecenoic acid (z)- methyl ester 5.5 (%). The identification of fifteen ketone and aldehyde molecules associated with C. camphora was confirmed through GC–MS analysis.
UV–visible spectroscopy
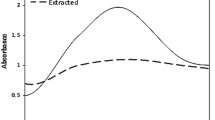
A UV–Vis device was used to characterize MNPs synthesized from the hydroalcoholic extract of C. camphora, Spectra with wavelengths between 200 and 800 (nm) were measured. The UV–visible spectra of CMC, MNPs, and CMNPs are shown In Fig. 2A. A peak at 232 (nm) was generated by MNP production using C. camphora, while a peak at 240 (nm) was generated by CMNP production. The shift in peak positions and intensity between the two samples indicates a change in the electronic structure of the nanoparticles, which is likely due to the presence of the CMC coating. Figure 2B (S1–S6) shows that MNPs (S1–S3) and CMNPs (S4–S6) were stable at different times, with S1 being MNPs at time zero, S2 at 3 months, and S3 at 6 months. On the other hand, S4 are CMNPs at time zero, S5 at 3 months, and S6 at 6 months (Yusefi et al. 2023).
Zeta size and potential analysis
Based on the data shown in Table 3, Figs. 3 and 4, The MNPs and CMNPs had an average diameter of 33 and 9.5 (d.nm), respectively, with a polydispersion index (PDI) of 0.503 and 0.121, respectively, according to the measurements. The surface potentials of CMC, MNPs, and CMNPs were −32.2, −22.7, and −52.6 (mV), respectively. Additional studies on the surface charge and size of the MNPs and CMNPs nanoparticles were carried out using Zeta Sizer. Based on the findings, CMNPs have a smaller particle size than MNPs, shown by the comparison between the two types of nanoparticles. Furthermore, the zeta potential of CMNPs is significantly lower than that of MNPs. Because CMC acts as a stabilizing agent (it contains a long chain), the particle size has decreased since the CMC coating was applied. This is because the coating increases stability and prevents the aggregating of MNPs (Abd El-Aziz et al. 2023). We repeated the measurements three times and calculated the standard deviations.
Transmission electron microscopy analysis
Monitoring the geometric shape and size is a crucial criterion for the characterization of nanoparticles. TEM is widely used in aquatic environments to accurately assess particle size, dispersion, and aggregation with high resolution. As shown in Fig. 5a, the CMC coating is not visible in the TEM images because CMC is as a thin layer on the surface of the nanoparticles. As shown in Fig. 5b, c, and d, transmission electron microscope (TEM) images show that both MNPs and CMNPs have a spherical shape, with an average diameter of 15 (nm) for MNPs and 10 (nm) for CMNPs. By TEM, the morphology of optimized MNPs and CMNPs was shown to be spherical, as shown in Fig. 5 (e) TEM of CMNPs measured at magnifications of 10 (nm) to illustrate the claim of CMC coating on MNPs (Eldeeb et al. 2023).
FT-IR spectroscopy
We verified the existence of functional groups using FTIR. The identification of biomolecules is a necessary step in the process of developing innovative techniques for producing nanoparticles. Figure 6 shows the Fourier transform infrared (FT-IR) analysis of C. camphora extract, its MNPs, and its CMNPs. FT-IR analysis includes (A) plant extract, (B) CMC, (C) MNPs, and (D) CMNPs. In Fig. 6A, and B, the broad peaks at 3276 and 3350 (cm−1) is assigned to (OH), and the peaks at 2921, 2933 (cm−1) are assigned to (C–H) stretching. The peak was observed at 1700, 1607 and 1587 (cm−1) confirming the presence of COO, which is attributed to the stretching of the carboxyl group. This band was important in determining complexation between MNPs and CMC, and is expected to shift or otherwise peak at 1300–1500 (cm−1) when complexes occur (Zaidi et al. 2011; Su et al. 2010), peaks from 1300–1500 (cm−1) are assigned to OH stretching in the plane and C–H stretching in the symmetry of CMC (AbouTaleb et al. 2009), 1000–1300 (cm−1) due to –(C–O) H stretching, (Chai and Isa 2013). Figures 6, C, and D, the disappearance of the broad peaks at 3276 and 3350 (cm−1), was observed, meaning that they were consumed in the formation and coating of MNPs, as were the peaks at around 3000 (cm−1), which were assigned to –C–H were shifted to a higher wavenumber, and the carboxyl group peaks were also shifted from 1587 to 1750 (cm−1) confirming the complexation between MNPs and CMC. After the formation of MNPs, these absorption bands shifted to higher wavenumbers with a loss of intensity, suggesting that these functional groups were used in the synthesis of MNPs (Rozali et al. 2016). The peaks at 500–630 (cm−1) are the stretching vibrations of the Fe–O bonds (Waldron 1955; Bordbar et al. 2014; Aslam et al. 2021; Masri et al. 2018).
X-ray diffraction
In addition to providing information about unit cell dimensions, X-ray diffraction (XRD) is a rapid analytical technique commonly used for phase identification of crystalline materials. By examining the diffractograms of CMC, MNPs, and CMNPs, Fig. 7 shows the diffractograms of CMC, MNPs, and CMNPs. The diffraction profile of CMC showed no peaks. However, MNPs and CMNPs exhibit peaks at 2θ values of 30.2°, 36.5°, 42.91°, 57.3°, 64.21°, 65.21°, and 74.98°, which are the planes (220), (311), (400), (511), (440), (531), and (622) of their crystalline MNPs and CMNPs (Compeán et al. 2008, Chomchoey et al. 2010, Jia et al. 2016).
XPS analysis
We performed XPS analyzes to further characterize the surface chemical composition of the MNPs and CMNPs. Fig. S (1–4) and 8 (a–d) shows typical XPS spectra of MNPs and CMNPs. The XPS examination of MNPs (Fig. S1) shows that the peaks of Fe 2p3 and O 1s were observed in the hybrids and no other element peak was detected, showing that the MNPs mainly contained two elements of Fe and O. Figure 8a shows from the XPS study of CMNPs that the peaks of Fe 2p3, O 1s, and C 1s were observed in the hybrids, and no other element peak was detected, showing that the CMNPs mainly contain the three elements of Fe, O, and C. Figs. S2 and 8b show the Fe 2p spectrum of MNPs and CMNPs. There are two broad peaks at about 711.48 and 724.96 (eV) corresponding to Fe 2p 3/2 and Fe 2p 1/2, respectively, (Yamashita and Hayes 2008; Xie et al. 2017; Qi et al. 2017; Han et al. 2019). The O 1s XPS spectrum of MNPs Fig. S3, the peak positions of the three divided parts are 531.25 (Fe–O), 531.790 (eV) (C–O–Fe), 532.58 (eV) (Fe-OH). The O 1s XPS spectrum of CMNPs Fig. 8 (c), the peak positions of the three parts divided in are 530 (Fe–O), 531.21 (eV) (C-O-Fe), 532.65 (eV) (Fe-OH), and 534.71 (eV) (C–OH/C–O–C), indicating the linkage of MNPs with CMC via this C-O-Fe bond in CMNPs ( Han et al. 2019). The CMNPs' C 1s XPS spectrum of the CMNPs in Fig. 8d shows three main peaks with binding energies of 284.98, 286.73, and 288.51 (eV). These energies were consistent with the C–C/C = C, C-O, and O-C = O configurations, respectively (Han et al. 2019).
Measure of absorptivity of carboxymethyl cellulose
Table 4 shows the values of total energy: adsorption, rigid adsorption, deformation, and magnetic carboxymethyl cellulose for different configurations. We display the values for each structure in rows, and the different energies in columns. The results show that all structures have negative adsorption energies, indicating their significant ability to absorb certain compounds. Furthermore, the strain energy values are negative, indicating that the structures are generally stable, Fig. 9. The carboxymethyl cellulose values (Table 4.) can determine the magnetic properties of the structures, potentially leading to practical applications.
The results of the adsorption energy evaluations provide insight into the suitability of these adsorbent assemblies for various adsorption requirements. The negative adsorption energies and deformation energies suggest that these structures have the potential to efficiently adsorb substances while remaining stable. Furthermore, magnetic carboxymethyl cellulose values suggest that these structures may possess significant magnetic properties. To corroborate these results and further explore the practical applications of these adsorption structures, additional experiments and simulations could be conducted (Smolin and Zabneva 2023; Canbaz 2023; Chen et al. 2023).
Antioxidant activity of MNPs and CMNPs
The DPPH method is a comprehensive test to determine the free radical scavenging ability of plant NPs. Due to its simplicity, rapidity, and independence from sample polarity, this technique is ideal for measuring free radical scavenging ability (Salarbashi et al. 2014). The DPPH free radical scavenging method was used to measure the antioxidant activity of MNPs and CMNPs, which can be seen in Fig. 10 A–B. The green synthesis of magnetic nanoparticles significantly improves the antioxidant properties of MNPs and CMNPs, with IC50 values of 176.1 ± 4.0 and 70.92 ± 3.0 (µg/mL), respectively, compared to the standard drug Trolox IC50 = 44.43 ± 2.0 (µg/mL)—p ≤ 0.0001). Additionally, MNPs capped with CMC have higher antioxidant capacity than uncapped ones. Figure 10 C-D illustrates the radical scavenging ability of MNPs and CMNPs by inhibiting the ABTS radical. Both showed activity that varied with dose, with IC50 values of 141.3 ± 3.0 and 61.67 ± 2.5 (µg/mL), respectively, compared to Trolox, which had an IC50 of 23.10 ± 1.0 (µg/mL) (p ≤ 0.0001). The antioxidant activity of MNPs and CMNPs' is related to a number of factors. In this case, plant reducing agent molecules and CMC molecules are present on their reactive surfaces. Transition metal-based NPs are also present on the surfaces of MNPs and CMNPs, and the particles interact with the free radicals DPPH and ABTS (Moteriya and Chanda 2017). We repeated the measurements three times and calculated the standard deviations.
Anticancer activity of MNPs and CMNPs of C. camphora
After biosynthesis of MNPs and capping with carboxymethyl cellulose, the anticancer efficacy of MNPs and CMNPs against two cancer cell lines was investigated. For this reason, several in vitro tests were performed, including an MTT test and an apoptotic morphological examination. We evaluated the cytotoxicity of MNPs and CMNPs toward WI-38 using the in vitro MTT assay. Figure 11 shows that both MNPs and CMNPs exhibited good cell viability at all doses tested. Since they were non-toxic, we conducted further research on them. Two different HEPG-2 and HTC-11) were selected for the in vitro evaluation of the anticancer potential of both MNPs and CMNPs. The results showed that although both MNPs and CMNPs have low cytotoxicity against normal cells, their specific cytotoxicity against cancer cells is much higher (Abd El-Aziz and Farahat 2023). Another result is that both MNPs and CMNPs exhibit dose-dependent cytotoxicity. As the dose increased, fewer cancer cells remained alive. The CMNPs were found to be most effective and selective against human colon cancer cells: HCT-116 (IC50 = 20 (µg/mL)) followed by HEPG-2 (IC50 = 33 (µg/mL)).
MTT cytotoxicity study of MNPs, CMNPs, and doxorubicin (DOX) on normal human lung fibroblasts (WI-38) and two cancer cell lines: A Cell viability percentage of WI-38; B for HEPG-2 cells; and C for HCT-116 after treatment with different concentrations (6.5 to 100 (µg/mL)) of MNPs, CMNPs, and Dox, respectively. Determination of the inhibitory concentration responsible for the death of 50 (%) (IC50) D for WI-38 cells, E for HEPG-2 cells, and (F) for HCT-116 MNPs, CMNPs, and DOX
Morphological changes in HCT 116 colon cancer and HepG2 liver cancer cells
Apoptosis induction is one of the best methods for treating cancer. It has become increasingly important in the development of cancer drugs. AO penetrates the intact membranes of both normal and early apoptotic cells and binds to DNA. In normal cells, the DNA exhibits uniform green fluorescence, while in early apoptotic cells it was colored green and had bright green patches in the AO nuclei, while untreated control cells fluoresced green due to nuclear fragmentation and chromatin condensation, as seen in Fig. 12. Conversely, the color of late apoptotic cells attaching to EB changes from orange to red (Tambe et al 2018). This is due to its binding to DNA fragments or apoptotic moieties units within late apoptotic cells, which resemble viable cells in nuclear morphology. We stained HCT 116 and HepG2 cells with AO-EB and incubated them with MNPs and CMNPs for 24 h. The results shown in Fig. 12 show that HCT 116 and HepG2 cells treated with MNPs and CMNPs showed orange with red fluorescence and chromatin fragmentation. This indicates that the CMNPs significantly induce apoptosis in these cells.
In silico mode of action of CMNPs
Molecular docking simulations were performed to evaluate the binding interactions and affinity of doxorubicin, uncoated magnetic nanoparticles (MNPs), and carboxymethyl cellulose MNPs (CMNPs) toward the apoptotic protein targets Bcl-2-associated death promoter (BAD), caspase 3, and caspase 9. The absorbance values were calculated to predict the cellular uptake potential of MNPs and CMNPs.
Doxorubicin was found to have strong binding affinity for all three protein targets, with docking values ranging from −4.213 to −5.145 kcal/mol (Table 5). The main molecular interactions identified were hydrogen bonds and π-cation interactions with specific amino acid residues in the active site of the proteins (Table 5). This indicates that doxorubicin can effectively bind to the apoptotic proteins and exert its cytotoxic effect, confirming it as an anticancer therapeutic agent. Figure 13.
The absorbance values showed some difference between the three proteins for uncoated MNPs compared to MNPs coated with carboxymethyl cellulose (Figs. 13–15). For BAD binding, CMNPs showed an absorbance value of 0.0 (Fig. 14a, while MNPs showed a very high value of 1264.399 (Fig. 15a.
The differences in the absorptivity of MNPs can be attributed to the different surface properties of the protein targets. The CMNPs can preferentially bind to the more acidic BAD protein compared to caspases, leading to increased uptake of MNPs alone. For caspases, the carboxymethyl cellulose coating can induce favorable interactions that increase CMNP absorbency. In Figs. 14b and 14c, uncoated MNPs showed lower absorbency for caspase 3 and caspase 9 compared to CMNPs. Specifically, CMNPs had an absorbance value of 0.0, while MNPs had higher positive values of 735.050 and 1675.174 for caspases 3 and 9, respectively. Since higher positive absorbance values indicate lower uptake potential, this suggests that CMNPs coated with carboxymethyl cellulose have higher predicted uptake by caspases 3 and 9 compared to uncoated MNPs. In other words, coating the MNPs reduced the positive absorbance values closer to 0, indicating improved absorbance for caspase targets.
Conclusions
Based on the results of this study, the biosynthesized NPs derived from the hydroalcoholic extract of C. camphora have an SPR peak at 232, 240 nm for MNPs and CMNPs, respectively, an average mean diameter of 13.20, and 10.77 nm, respectively. for MNPs and CMNPs, respectively, a size of 10 nm for MNPs and 7 nm for CMNPs, and the NPs were spherical; MNPs and CMNPs are crystalline in nature. The biosynthesized MNPs and CMNPs were found to be effective as anticancer agents and antioxidants, and the CMNPs are better than MNPs. These results demonstrate that MNPs and CMNPs are effective in a variety of pharmaceutical, biotechnological, and biomedical applications. Low production cost, availability of raw materials, environmentally friendly production methods and cytotoxic studies showed that MNPs and CMNPs inhibited the growth of HEPG-2, HCT-116, and WI-38. The antioxidant properties of MNPs and CMNPs were demonstrated, with IC50 values of 176.1 ± 4.0 and 70.92 ± 3.0 (µg/mL) for DPPH and IC50 values of 141.3 ± 3.0 and 61.67 ± 2.5 (µg/mL) for ABTS, respectively.
Data availability
No datasets were generated or analysed during the current study.
References
Abd El-Aziz SM, Faraag AHI, Ibrahim AM, Albrakati A, Bakkar MR., (2023). Tyrosinase enzyme purification and immobilization from Pseudomonas sp. EG22 using cellulose coated magnetic nanoparticles: characterization and application in melanin production. World J Microbiol Biotechnol. 40 (1): 10
Abd El-Aziz SM, Farahat EA (2023) The Activity of Vossia cuspidata Polysaccharides-Derived Monometallic CuO, Ag, Au, and Trimetallic CuO-Ag-Au Nanoparticles Against Cancer, Inflammation, and Wound Healing. J Inorg Organomet Polym 33(3):853–865. https://doi.org/10.1007/s10904-023-02542-x
Abdel-Aziz MS, Shaheen MS, El-Nekeety AA, Abdel-Wahhab MA (2014) Antioxidant and antibacterial activity of silver nanoparticles biosynthesized using chenopodium murale leaf extract. J Saudi Chem Soc 18(4):356–363. https://doi.org/10.1016/j.jscs.2013.09.011
AbouTaleb MF, Abd El-Mohdy HL, Abd El-Rehim HA (2009) Radiation preparation of PVA/ CMC copolymers and their application in removal of dyes. J Hazard Mater 168:68–75. https://doi.org/10.1016/j.jhazmat.2009.02.001
Aguiler G, Berry CC, West RM, Gonzalez-Monterrubio E, Angulo-Molina A, Arias-Carrión Ó, Méndez-Rojas MÁ (2019) Carboxymethyl cellulose coated magnetic nanoparticles transport across a human lung microvascular endothelial cell model of the blood–brain barrier. Nanoscale Adv 1(2):671–685. https://doi.org/10.1039/C8NA00010G
Aguilera G, Berry CC, West RM, Gonzalez-Monterrubio E, Angulo-Molina A, Arias-Carrión Ó, Méndez-Rojas MÁ (2018) Carboxymethyl cellulose coated magnetic nanoparticles transport across a human lung microvascular endothelial cell model of the blood-brain barrier. Nanoscale Adv. 1(2):671–685. https://doi.org/10.1039/c8na00010g
Alam KM, Kumar P, Gusarov S, Kobryn AE, Kalra AP, Zeng S, Shankar K (2020) Synthesis and characterization of zinc phthalocyanine-cellulose nanocrystal (CNC) conjugates: toward highly functional CNCs. ACS Appl Mater Interfaces 12(39):43992–44006. https://doi.org/10.1021/acsami.0c07179
Arnao MB, Cano A, Acosta M (2001) The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem 73(2):239–244. https://doi.org/10.1016/S0308-8146(00)00324-1
Aslam M, Fozia F, Gul A et al (2021) Phyto-extract-mediated synthesis of silver nanoparticles using aqueous extract of sanvitalia procumbens, and characterization, optimization and photocatalytic degradation of azo dyes orange g and direct blue-15. Molecules 26(20):6144. https://doi.org/10.3390/molecules26206144
Attard E (2013) A rapid microtitre plate folin-Ciocalteu method for the assessment of polyphenols. Cent Eur J Biol 8(1):48–53. https://doi.org/10.2478/s11535-012-0107-3
Benkert P, Biasini M, Schwede T (2011) Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 27(3):343–350. https://doi.org/10.1093/bioinformatics/btq662
Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239(1):70–76. https://doi.org/10.1006/abio.1996.0292
Berradi A, Aziz F, Achaby ME, Ouazzani N, Mandi LA (2023) Comprehensive review of polysaccharide-based hydrogels as promising biomaterials. Polymers 15(13):2908. https://doi.org/10.3390/polym15132908
Bertoni M, Kiefer F, Biasini M et al (2017) Modeling protein quaternary structure of homo- and hetero-oligomers beyond binary interactions by homology. Sci Rep. https://doi.org/10.1038/s41598-017-09654-8
Bienert S, Waterhouse A, De Beer TAP et al (2017) The SWISS-MODEL Repository-new features and functionality. Nucleic Acids Res. https://doi.org/10.1093/nar/gkw1132
Bordbar AK, Rastegari AA, Amiri R, Ranjbakhsh E, Abbasi M, Khosropour AR (2014) Characterization of Modified magnetite nanoparticles for albumin immobilization. Biotechnol Res Int 2014:1–6. https://doi.org/10.1155/2014/705068
Canbaz GT (2023) Fe3O4@Granite: a novel magnetic adsorbent for dye adsorption. Processes 11(9):2681. https://doi.org/10.3390/pr11092681
Cardoso-Ugarte GA, López-Malo A, Sosa-Morales ME (2016)/ Cinnamon (Cinnamomum zeylanicum) Essential Oils. Essential Oils in Food Preservation, Flavor and Safety. https://doi.org/10.1016/B978-0-12-416641-7.00038-9
Chai MN, Isa MIN (2013) The oleic acid composition effect on the carboxymethyl cellulose based biopolymer electrolyte. JCPT 3:1–4. https://doi.org/10.4236/jcpt.2013.31001
Chen L, Yin S, Chai T, Wang J, Chen G, Zhou X, Yang F (2023) Ultra-high adsorption capacity of core–shell-derived magnetic zeolite imidazolate framework-67 as adsorbent for selective extraction of theophylline. Molecules 28(14):5573. https://doi.org/10.3390/molecules28145573
Chomchoey N, Bhongsuwan D, Tripob B (2010) Magnetic properties of magnetite nanoparticles synthesized by oxidative alkaline hydrolysis of iron powder. Kasetsart J Nat Sci 44(5):963–971
Compeán-Jasso ME, Ruiz F, Martínez JR, Herrera-Gómez A (2008) Magnetic properties of magnetite nanoparticles synthesized by forced hydrolysis. Mater Lett 62(27):4248–4250. https://doi.org/10.1016/j.matlet.2008.06.053
Dhar P, Saha P, Hasan Md, Amin K, Haque Md (2021) Green synthesis of magnetite nanoparticles using Lathyrus sativus peel extract and evaluation of their catalytic activity. CLCE 3:100117. https://doi.org/10.1016/j.clet.2021.100117
Domingues SZ, Timmers LFSM, Granada CE (2022) Cellulase production by bacteria is a strain-specific characteristic with a high biotechnological potential. a review of cellulosome of highly studied strains. Cellulose 29:8065–8083. https://doi.org/10.1007/s10570-022-04790-5
Du Toit EA, Rautenbach M (2000) A sensitive standardised micro-gel well diffusion assay for the determination of antimicrobial activity. J Microbiol Methods. https://doi.org/10.1016/S0167-7012(00)00184-6
Durán N, Durán M, de Jesus MB et al (2016) Silver nanoparticles: a new view on mechanistic aspects on antimicrobial activity. Nanomedicine Nanotechnology, Biol Med. 12(3):789–799. https://doi.org/10.1016/j.nano.2015.11.016
Elbagory AM, Marima RM, Dlamini Z (2021) Role and merits of green based nanocarriers in cancer treatment. Cancers 13(22):5686. https://doi.org/10.3390/cancers13225686
Eldeeb BA, El-Raheem WMA, Elbeltagi S (2023) Green synthesis of biocompatible Fe3O4 magnetic nanoparticles using Citrus Sinensis peels extract for their biological activities and magnetic-hyperthermia applications. Sci Rep 13:19000. https://doi.org/10.1038/s41598-023-46287-6
El-Naggar ME, Hasanin M, Hashem AH (2022) Eco-friendly synthesis of superhydrophobic antimicrobial film based on cellulose acetate/polycaprolactone loaded with the green biosynthesized copper nanoparticles for food packaging application. J Environ Polym Degrad 30(5):1820–1832. https://doi.org/10.1007/s10924-021-02318-9
El-Rafie HM, Abd El-Aziz SM, Zahran MK (2016) Bioactivities of gold and iron oxide nanoparticles biosynthesized from the edible plant Corchorus olitorius. Pharm Lett 8:156–164
El-Rafie HM, El-Aziz SA, Zahran MK (2023) In vitro cytotoxicity against breast cancer using biogenically synthesized gold and iron oxide nanoparticles derived from the hydroethanolic extract of Salvia officinalis L. Chem Pap 77:361–373. https://doi.org/10.1007/s11696-022-02464-x
El-Sayyad GS, Abdel Maksoud MIA, Fahim RA et al (2022) Gamma radiation induced synthesis of novel chitosan/gold/bioactive glass nanocomposite for promising antimicrobial, and antibiofilm activities. J Clust Sci. https://doi.org/10.1007/s10876-022-02357-9
Fan J, Tan Y, Jie L, Wu X, Yu R, Zhang M (2013) Biological activity and magnetic resonance imaging of superparamagnetic iron oxide nanoparticles-labeled adipose-derived stem cells. Stem Cell Res Ther 4:1–10. https://doi.org/10.1186/scrt191
Gopalakrishnan R, Raghu K (2014) Biosynthesis and characterization of gold and silver nanoparticles using milk thistle (silybum marianum) seed extract. J Nanosci. https://doi.org/10.1155/2014/905404
Guex N, Peitsch MC, Schwede T (2009) Automated comparative protein structure modeling with SWISS-MODEL and swiss-pdbviewer: a historical perspective. Electrophoresis 30(S1):S162–S173
Han T, Wei Y, Jin X, Jiu H, Zhang L, Sun Y, Zhao R (2019) Hydrothermal self-assembly ofα-Fe2O3 nanorings@ graphene aerogel composites for enhanced Li storage performance. J Mater Sci 54:7119–7130. https://doi.org/10.1007/s10853-019-03371-5
Hazra RS, Kale N, Aland G et al (2020) Cellulose mediated transferrin nanocages for enumeration of circulating tumor cells for head and neck cancer. Sci Rep 10:10010. https://doi.org/10.1038/s41598-020-66625-2
Hazra RS, Kale N, Boyle C, Molina KB, D’Souza A, Aland G et al (2024) Magnetically activated, nanostructured cellulose for efficient capture of circulating tumor cells from the blood sample of head and neck cancer patients. Carbohydr Polym 323:121418. https://doi.org/10.1016/j.carbpol.2023.121418
He B, Ai J, Ren J et al (2022) Alkyl ketene dimer grafted cationic cellulose stabilizing nano magnesium oxide for efficient protection of acidic paper. Cellulose. https://doi.org/10.1007/s10570-022-04812-2
Huang J, Li Q, Sun D et al (2007) Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. J Nanotechnol 18:105104. https://doi.org/10.1088/0957-4484/18/10/105104
Jabareen L, Maruthapandi M, Saravanan A et al (2021) Effective degradation of cellulose by Microwave irradiation in alkaline solution. Cellulose 28:11275–11285. https://doi.org/10.1007/s10570-021-04274-y
Jamuna Bai A (2011) Nanoparticles and their potential application as antimicrobials. Science against microbial pathogen.
Jia H et al (2016) Immobilization of transaminase by magnetic PVA-Fe3O4 nanoparticles. Biotechnol Rep. https://doi.org/10.1016/j.btre.2016.03.004
Kalishwaralal K, Deepak V, Ram Kumar Pandian S et al (2010) Biosynthesis of silver and gold nanoparticles using Brevibacterium casei. Colloids Surf B 77:257–262. https://doi.org/10.1016/j.colsurfb.2010.02.007
Kiranmai M, Kumar CM, Mohammed I (2011) Comparison of total flavanoid content of Azadirachta indica root bark extracts prepared by different methods of extraction. Biol Chem Sci 2(3):254–261
Kraeling MEK, Topping VD, Keltner ZM et al (2018) In vitro percutaneous penetration of silver nanoparticles in pig and human skin. Regul Toxicol Pharmacol 95:314–322
Kumar S, Shukla A, Baul PP et al (2018) Biodegradable hybrid nanocomposites of chitosan/gelatin and silver nanoparticles for active food packaging applications. Food Packag Shelf Life 16:178–184. https://doi.org/10.3390/foods9091143
Lara HH, Garza-Treviño EN, Ixtepan-Turrent L, Singh DK (2011) Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J Nanobiotechnology 9:1–8. https://doi.org/10.1186/1477-3155-9-30
Lee HJ, Hyun EA, Yoon WJ et al (2006) In vitro anti-inflammatory and anti-oxidative effects of Cinnamomum camphora extracts. J Ethnopharmacol. https://doi.org/10.1016/j.jep.2005.08.009
Lee SH, Kim DS, Park SH, Park H (2022) Phytochemistry and applications of Cinnamomum camphora essential oils. Molecules 27(9):2695. https://doi.org/10.3390/molecules27092695
Marasini BP, Baral P, Aryal P et al (2015) Evaluation of antibacterial activity of some traditionally used medicinal plants against human pathogenic bacteria. Biomed Res Int. https://doi.org/10.1155/2015/265425
Mariselvam R, Ranjitsingh AJA, Usha Raja Nanthini A et al (2014) Green synthesis of silver nanoparticles from the extract of the inflorescence of Cocos nucifera (Family: Arecaceae) for enhanced antibacterial activity. Spectrochim Acta - Part A Mol Biomol Spectrosc. https://doi.org/10.1016/j.saa.2014.03.066
Masri A, Anwar A, Ahmed D et al (2018) Silver nanoparticle conjugation-enhanced antibacterial efficacy of clinically approved drugs cephradine and vildagliptin. Antibiotics. https://doi.org/10.3390/antibiotics7040100
Mehta BK, Chhajlani M, Shrivastava BD (2017) Green synthesis of silver nanoparticles and their characterization by XRD. In J Phys: Conf Ser 836(1):012050
Milovanovic M, Arsenijevic A, Milovanovic J, et al (2017) Nanoparticles in antiviral therapy. In Antimicrobial nanoarchitectonics 383–410. Elsevier
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1–2):55–63. https://doi.org/10.1016/0022-1759(83)90303-4
Moteriya P, Chanda S (2017) Synthesis and characterization of silver nanoparticles using caesalpinia pulcherrima flower extract and assessment of their in vitro antimicrobial, antioxidant, cytotoxic, and genotoxic activities. Artif Cells, Nanomedicine Biotechnol 45(8):1556–1567. https://doi.org/10.1080/21691401.2016.1261871
Musino D, Rivard C, Landrot G, Novales B, Rabilloud T, Capron I (2021) Hydroxyl groups on cellulose nanocrystal surfaces form nucleation points for silver nanoparticles of varying shapes and sizes. J Colloid Interface Sci 584:360–371. https://doi.org/10.1016/j.jcis.2020.09.082
Nayeem A (2006) A comparative study of available software for high-accuracy homology modeling: from sequence alignments to structural models. Protein Sci 15:808–824. https://doi.org/10.1110/ps.051892906
Ohno M, Abe T (1991) Rapid colorimetric assay for the quantification of leukemia inhibitory factor (LIF) and interleukin-6 (IL-6). J Immunol Methods. https://doi.org/10.1016/0022-1759(91)90327-C
Parekh J, Chanda SV (2007) In vitro antimicrobial activity and phytochemical analysis of some Indian medicinal plants. Turkish J Biol 31(1):53–58
Pfeffer CM, Singh ATK (2018) Apoptosis: a target for anticancer therapy. Int J Mol Sci 19(2):448. https://doi.org/10.3390/ijms19020448
Poole CP, Owens FJ (2003) Introduction to nanotechnology - poole, owens.pdf. Collect Manag 21:1–2
Prabhu K, Karar PK, Hemalatha S, Ponnudurai K (2011) A comparative preliminary phytochemical screening on the leaves, stems, and roots of three viburnum Linn. Species der Pharmacia Sinica 2:81–93
Pragadheesh VS, Saroj A, Yadav A et al (2013) Chemical characterization and antifungal activity of cinnamomum camphora essential oil. Ind Crops Prod. https://doi.org/10.1016/j.indcrop.2013.06.023
Qi X, Zhang HB, Xu J et al (2017) Highly efficient high pressure homogenization approach for scalable production of high-quality graphene sheets and sandwich-structured aFe2O3/graphene hybrids for high-performance lithium-ion batteries. ACS Appl Mater Interfaces 9:11025–11034. https://doi.org/10.1021/acsami.7b00808
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. https://doi.org/10.1016/j.biotechadv.2008.09.002
Remmert M, Biegert A, Hauser A, Söding J (2012) HHblits: lightning-fast iterative protein sequence searching by HMM-HMM alignment. Nat Methods. https://doi.org/10.1038/nmeth.1818
Rodríguez-Tudela JL, Barchiesi F, Bille J et al (2003) Method for the determination of minimum inhibitory concentration (MIC) by broth dilution of fermentative yeasts. Clin Microbiol Infect 9(8):1–8. https://doi.org/10.1046/j.1469-0691.2003.00789.x
Roy A, Bulut O, Some S et al (2019) Green synthesis of silver nanoparticles: bio$molecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv 9(5):2673–2702
Rozali MLH et al (2016) Effect of Adipic Acid Composition on Structural and Conductivity Solid Biopolymer Electrolytes Based on Carboxymethyl cellulose Studies. American-Eurasian J Sustain Agriculture. 9(2):39–45
Saberi Riseh R, Gholizadeh Vazvani M, Hassanisaadi M, Skori YA (2023) Micro-/nano-carboxymethyl cellulose as a promising biopolymer with prospects in the agriculture sector: a review. Polymers 15:440. https://doi.org/10.3390/polym15020440
Salarbashi D, Tajik S, Shojaee-Aliabadi S, Ghasemlou M, Moayyed H, Khaksar R, Noghabi MS (2014) Development of new active packaging film made from a soluble soybean polysaccharide incorporated Zataria multiflora Boiss and Mentha pulegium essential oils. Food Chem 146(614–622):10. https://doi.org/10.1016/j.foodchem.2013.09.014
Salem SS, Hashem AH, Sallam AM, Doghish AS, Al-Askar AA, Arishi AA, Shehabeldine AM (2022) Synthesis of silver nanocomposite based on carboxymethyl cellulose: antibacterial. Antifungal and Anticancer Activities. Polymers. 14(16):3352. https://doi.org/10.3390/polym14163352
Saratale RG, Karuppusamy I, Saratale GD et al (2018) A comprehensive review on green nanomaterials using biological systems: Recent perception and their future applications. Colloids Surf B 170:20–35
Schiavi LSDO, Gonçalves MA, Delgado-Silva ADO et al (2024) Properties of fiber cement reinforced with cellulose pulp modified by plasma treatment with sulfur hexafluoride (SF6). Cellulose 31(8):1–22. https://doi.org/10.1007/s10570-024-05885-x
Shinde PR, Patil PS, Bairagi VA (2012) Pharmacognostic, phytochemical properties and antibacterial activity of callistemon citrinus viminalis leaves and stems. Int J Pharm Pharm Sci 4(4):406–408
Singh R, Jawaid T (2012) Cinnamomum camphora (Kapur): review. Pharmacogn J 4(28):1–5
Smolin S, Zabneva OV (2023) Using surrogate equilibrium constants for assessing the energy of adsorption of synthetic organic substances from aqueous phase onto carbonized adsorbents. J Water Chem Technol 45(5):467–474. https://doi.org/10.3103/S1063455X23050107
Sotiriou GA, Meyer A, Knijnenburg JTN et al (2012) Quantifying the origin of released Ag+ ions from nanosilver. Langmuir. https://doi.org/10.1021/la303370d
Spiliopoulos P, Gestranius M, Zhang C et al (2022) Cellulose-inorganic hybrids of strongly reduced thermal conductivity. Cellulose 29:8151–8163. https://doi.org/10.1007/s10570-022-04768-3
Su JF, Huang Z, Yuan XY, Wanga XY, Li M (2010) Structure and properties of carboxymethyl cellulose/soy protein isolate blend edible films crosslinked by Maillard reactions. Carbohyd Polym 79:145–153
Takai ZI, Mustafa MK, Asman S, Sekak KA (2019) Preparation and characterization of magnetite (Fe3O4) nanoparticles by sol-gel method. Int J Nanoelectron Mater 12:37–46
Tambe P, Kumar P, Paknikar KM, Gajbhiye V (2018) Decapeptide functionalized targeted mesoporous silica nanoparticles with doxorubicin exhibit enhanced apoptotic effect in breast and prostate cancer cells. Int J Nanomedicine 13:7669–7680. https://doi.org/10.2147/ijn.s184634
Tang R, Yan X, Hu J et al (2024) Carboxymethyl chitosan/alginate/polyethylene glycol composite hydrogel for nucleic acid lysis solution storage at the point of care. Cellulose. https://doi.org/10.1007/s10570-024-05889-7
Thomsen R, Christensen MH (2006) MolDock: a new technique for high-accuracy molecular docking. J Med Chem 49:3315–3321. https://doi.org/10.1021/jm051197e
Tippayawat P, Phromviyo N, Boueroy P, Chompoosor A (2016) Green synthesis of silver nanoparticles in aloe vera plant extract prepared by a hydrothermal method and their synergistic antibacterial activity. PeerJ. https://doi.org/10.7717/peerj.2589
Waldron RD (1955) Infrared Spectra of Ferrites. Phys Rev 99:1727–1735. https://doi.org/10.1103/PhysRev.99.1727
Wang S, Chen X, Li Z et al (2022a) Click chemistry-induced selective adsorption of cationic and anionic dyes using functionalized cellulose methacrylate hydrogels. Cellulose 29(16):8843–8861. https://doi.org/10.1007/s10570-022-04822-0
Wang K, Hazra RS, Ma Q, Jiang L, Liu Z, Zhang Y et al (2022b) Multifunctional silk fibroin/PVA bio-nanocomposite films containing TEMPO-oxidized bacterial cellulose nanofibers and silver nanoparticles. Cellulose 29(3):1647–1666. https://doi.org/10.1007/s10570-021-04369-6
Waterhouse A, Bertoni M, Bienert S et al (2018) SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res 46(W1):W296–W303. https://doi.org/10.1093/nar/gky427
Xie S, Zhang M, Liu P et al (2017) Advanced negative electrode of Fe2O3/graphene oxide paper for high energy supercapacitors. Mater Res Bull 96:413–418. https://doi.org/10.1016/J.MATERRESBULL.2017.04.002
Xu L, Wang YY, Huang J et al (2020) Silver nanoparticles: Synthesis, medical applications and biosafety. Theranosticsy 10(20):8996. https://doi.org/10.7150/thno.45413
Yamashita T, Hayes P (2008) Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl Surf Sci 254(8):2441–2449. https://doi.org/10.1016/j.apsusc.2007.09.063
Yew YP, Shameli K, Miyake M et al (2016) Green synthesis of magnetite (Fe3O4) nanoparticles using seaweed (Kappaphycus alvarezii) extract. Nanoscale Res Lett 11:276. https://doi.org/10.1186/s11671-016-1498-2
Yusefi M, Shameli K, Lee-Kiun MS, Teow SY, Moeini H, Ali RR, Kia P, Jie CJ, Abdullah NH (2023) Chitosan coated magnetic cellulose nanowhisker as a drug delivery system for potential colorectal cancer treatment. Int J Biol Macromol 1(233):123388. https://doi.org/10.1016/j.ijbiomac.2023.126187
Zaidi WY, Oumellal JP, Bonnet J, Zhang F, Cuevas M, Latroche, et al (2011) Carboxymethyl cellulose and carboxymethyl cellulose formate as binders in MgH2-carbon composites negative electrode for lithium-IonBateries. J Power Sources 96(5):2854–2857
Zhang S, Wu W, Xiao X, Zhou J, Ren F, Jiang C (2011) Preparation and characterization of spindle-like Fe 3 O 4 mesoporous nanoparticles. Nanoscale Res Lett 6:1–9. https://doi.org/10.1186/1556-276x-6-89
Zhou Y, Tang R-C (2018) Facile and eco-friendly fabrication of AgNPs coated silk for antibacterial and antioxidant textiles using honeysuckle extract. J Photochem Photobiol B Biol 178:463–471. https://doi.org/10.1016/j.jphotobiol.2017.12.003
Acknowledgments
We express our gratitude to the Nanotechnology Center at Helwan University, Egypt, for their technical support. We express our gratitude to Dr. M.I.A. Abdel Maksoud (Radiation Physics Department, National Center for Radiation Research and Technology (NCRRT), Egyptian Atomic Energy Authority (EAEA), Cairo, Egypt) for his support.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
Salim Mohamed Abd El-Aziz collection of plant materials, conceptualization, research design, experiment development, data analysis, paper writing, editing, supervising the whole work, and implementation. Ahmed H. I. Faraag contributed to the conceptualization of the study and the analysis of the data. Enas A. Abdul-Baki participated in the design and implementation of the experiments and paper writing. Manar Seleem Fouda contributed to the manuscript’s writing, editing, experiment design, and implementation. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Ethical approval
The current experimental design was approved by the research ethical committee of Science Faculty, Helwan University (approval no. REC-Sci-HU/C19-7–2023).
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abd El-Aziz, S.M., Faraag, A.H.I., Abdul-Baki, E.A. et al. Enhancement of Cinnamomum camphora magnetic nanoparticle bioactivities via carboxymethyl cellulose immobilization for potential therapeutic applications in cancer treatment. Cellulose (2024). https://doi.org/10.1007/s10570-024-06104-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10570-024-06104-3