Abstract
The food and beverage industry worldwide is trying to switch to using environment-friendly and bio-degradable materials in food packaging to avoid environmental concerns of using petroleum-derived plastic (synthetic polymers) materials. In this study, chitosan (CH) hydrogel films were fabricated by using its derivative chitooligosaccharides (COS) as an additive, polyvinyl alcohol as a plasticiser, and bioactive gallic acid as a cross-linker. The physical, mechanical, structural, barrier (e.g., moisture, water vapour permeability (WVP), and UV-barrier property), thermal properties, and biodegradation patterns of fabricated films were investigated. The use of bio–composite in CH films exhibited a synergistic effect. A film with a homogenous/smooth surface and excellent mechanical and thermal properties was obtained. Additionally, incorporating COS and gallic acid reduced the moisture content, WVP, and transparency. Moreover, the films exhibited good colour, strong UV-barrier properties, and good biodegradable capacity in soil. The results suggest that eco-friendly CH hydrogel films have promising potential to be used in food packaging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of plastic materials in food packaging sectors is on the rise, leading to an abundance of waste that clogs drainage systems and harms the aquatic environment, posing a threat to both plants and animals. Therefore, environmental concerns are ascending (Tardy et al. 2023; Zhao et al. 2023; Nguyen and Lee 2023). To combat this issue, many in the food and beverage industries are considering the use of sustainable, non-toxic, biodegradable biomaterials in food packaging as alternatives to traditional plastic. While this shift could help reduce waste and alleviate environmental concerns, it is not without its challenges. Research has shown that sustainable bio-based materials often have poorer mechanical, barrier, and thermal properties (Lamarra et al. 2020). Nevertheless, food industries and research organisations globally are exploring various combinations of biomaterials to create sustainable packaging materials that can overcome these shortcomings.
Recently, researchers have turned to polysaccharides such as chitosan, starch, cellulose, and carrageenan to convert and create eco-friendly and sustainable packaging materials to ensure food safety and safeguarding the environment (Wang et al. 2023; Yu et al. 2023; Antony et al. 2023). In addition, cellulose is widely considered to be most abundant; chitin, starch, glycogen, and dextran are among the top five. Of these polysaccharides, chitosan (CH, mainly derived from chitin through the deacetylation process) is gaining popularity due to its abundance, biodegradability, non-toxicity, and ability to form both films and gels (Bhowmik et al. 2023). However, neat chitosan has some limitations when used in packaging films, such as insufficient barrier, mechanical, and thermal properties (Haghighi et al. 2020). One effective way to overcome these drawbacks is through chemical modification by blending chitosan with biodegradable thermoplastic materials like PVA (polyvinyl alcohol) (Liu et al. 2023a, b). PVA is derived from polyvinyl acetate through hydrolysis, chemically stable, biocompatible, low toxic, and soluble in water (depending on particle size, molecular weight, and crystallinity) (Qureshi et al. 2021). The blending of PVA with CH acts as a plasticiser that improves the mechanical/flexibility properties; however, it has low functional properties, such as antioxidant and antimicrobial activity (Priyadarshi et al. 2018a, b).
Hence, the addition of bioactive molecules in the CH matrix has been considered for achieving desirable physical, mechanical, and chemical stability of film (Jiang et al. 2021). For instance, chitooligosaccharides (COS) is derived from chitosan by hydrolysis of unstable glycosidic bonds with low molecular weight and strong water solubility (Rajabi et al. 2022). COS has versatile functionalities, such as antimicrobial activity, antioxidant properties, probiotic action, gel-forming capacity, and immunostimulatory; thereby, it has potential in the application of functional food, perishable food preservation, and as a packaging material (Tabassum et al. 2021). Some research findings reported that using COS in the development of film improved antioxidant activity and mechanical strength, demonstrating its suitability for use as a functional packaging material (Yuan et al. 2023). Gallic acid (known as tri-hydroxybenzoic acid) is one of the representative natural phenolic substances derived from plants (vegetables and fruits) and has some unique properties such as antibacterial, anticancer, anti-inflammatory, chelating metal, anti-allergic, and antimutagenic (Zhao et al. 2022). As a result, the use of gallic acid in health science research is well-documented, and its usage in food packaging, particularly in coating and film production, is gaining attention (Thanyacharoen et al. 2018). Some literature suggests that using gallic acid in packaging film improves the antioxidant and antimicrobial activity and acts as a crosslinking agent that enhances the mechanical and barrier properties of the film (Zarandona et al. 2020).
Hydrogels are three-dimensional (3D) hydrophilic polymer network structures that have an excellent ability to retain water without dissolving (Rajabi et al. 2021). Due to their water absorption capacity, hydrogels have good potential in several applications, such as water treatment, drug release, scaffolds, and agriculture (Panpinit et al. 2020). The excessive moisture in food and/or accumulation of moisture from fresh foods such as meat, fish, poultry, vegetables, and fruits inside a package, accelerates the proliferation of microbes that render quality and nutritional loss of foods (Liu et al. 2023a, b). In this case, using hydrogel effectively in the food packaging sector may regulate the humidity inside the packaging, extending product shelf-life (Liu et al. 2022).
Owing to the good antimicrobial and antioxidant properties of COS and gallic acid, the biodegradable nature of PVA, and the gel/film forming capacity of chitosan, these biomaterials can be considered to develop sustainable food packaging materials (Gulzar et al. 2022; Zhang et al. 2019). Some studies have reported using chitosan and gallic acid in food packaging (Zhao et al. 2022). However, the potential utilisation of COS and gallic acid into CH in developing hydrogel film has yet to be explored. Therefore, the main objective of the study is to develop a hydrogel film containing biodegradable biomaterials and characterisation of the films’ physicochemical properties, which provides baseline information for potential application in food packaging sectors.
Materials and methods
Materials
Chitosan (CH) (molecular weight (Mw): 503kDa and degree of deacetylation (DD): 91%) obtained from Weseta International, Shanghai, China. Polyvinyl alcohol (PVA) (Mw: 89,000−98,000 g/mol, CAS-No: 9002−89-5, and > 99% hydrolysed), acetic acid (CH3COOH, CAS-No: 64−19−7, and 99.7%), gallic acid (C7H6O5, Mw: 170.12 g/mol, CAS-No: 149−91−7, and 97.5−102.5%), and sodium hydroxide (NaOH, CAS-No: 1310−73−2, Mw: 40 g/mol, and > 98% assay) were supplied by Sigma-Aldrich, New Zealand. Hydrogen peroxide (H2O2, CAS-No: 7722−84−1, 30% (w/v) 100 volumes, and Fisher ChemicalTM) was purchased from Ajax Finechem Limited, New Zealand. The analytical grade ethanol (C2H5OH, 99.5%) was procured from the Department of Chemistry, University of Otago.
Chitooligosaccharide (COS) preparation
COS was fabricated, followed by Rajabi et al. (2022) method with some modifications. About 1.5 g CH powder was dissolved in 50 mL of 2% (v/v) acetic acid in a 250 mL Erlenmeyer flask. After that, 0.6% (v/v) (1 mL) of H2O2 (30%) was incorporated with CH solution. The mixture was kept at the microwave reactor (MCR-3, KEDA Instrument, Zhengzhou Co. Ltd.) to conduct the chemical reaction at 70 °C with 800 W microwave radiation power for 25 min. Afterwards, the solution was kept on ice and allowed to cool immediately to eliminate the possible chemical reaction or thermal effects. Then, 10 M of NaOH was added to the solution to adjust the pH 7 of the solution. After that, 250 mL of absolute ethanol was added to the mixture and kept at room temperature (23 °C) for 1 h to remove the H2O2 and salts. The precipitate was extracted by centrifugation (Megafuge 1.0, Kendro Laboratory Products, Germany) at 3000 rpm for 15 min three times; then, frozen (MDF-192, Ultra-low temperature freezer, SANYO Electric Biomedical Co. Ltd., Japan) at −80°C overnight and lyophilised (FreeZone 12, Labconco, Kansas City, USA).
Hydrogel film fabrication
Hydrogel films were prepared using the solvent casting technique to obtain a 2% CH solution, CH (2 g) was mixed in 100 mL of an acetic acid solution (2%, v/v) and stirred (Magnetic stirrer 34532, Cenco instrumenten b.v., Apeldoorn, Netherlands) vigorously for 3 h at room temperature (23°C). A 10% PVA solution was prepared by dissolving PVA powder (10 g) in 100 mL of distilled water and heated at 80°C for 4 h in a water bath (WCB-22, Daihan Scientific, South Korea). Different amounts of PVA (25%, 50%, 75%, and 100% based on CH) were added to the CH solution and stirred for 30 min., which resulted in CP1, CP2, CP3, and CP4, respectively. After performing the qualitative experiment, 0.2g (50%) of PVA was selected for film formulation (Table S1). Thereupon, different percentages (5% (0.02 g) and 10% (0.04 g)) of COS and gallic acid (based on chitosan) were added to the film solution. Finally, 20 mL film-forming solution was stirred at room temperature (23 °C) for 30 min, poured into 10 cm square Petri dishes, and kept at room temperature (23°C) for 72 h. The fabricated films were separated manually. The films developed with CH, PVA, COS, and gallic acid were designated CH, CP2, CP5, CP6, CP7, CP8, CP9, CP10, CP11, and CP12 (Table 1).
Physical and barrier properties of hydrogel film
Film thickness
The thickness of the developed films was measured using the digital vernier calliper (LCD 0-150MM, CALIPERS VERNIER DIG, RS PRO). Six random locations of each film were selected to calculate the average thickness. The unit of the film thickness is denoted as mm.
Moisture content
The film samples (12−15 mg and 1.5\(\times\)1.5 cm) were cut, weighed, and kept in a drying oven (THERMOTEC 2000, Lower Hutt, New Zealand) for 24 h at 100°C. The weight of dried films was measured. The moisture content of films was estimated based on initial and dried weight difference and calculated using Equation 1.
where Wiw and Wdw are the initial and dry weights of the film samples, respectively.
Water solubility
The water solubility of developed films was determined, followed by the Bhat et al. (2022) method with some modifications. The film samples (0.7 \(\times\) 0.7 cm) were kept at 100°C in a drying oven for 24 h. The dry weight of the film samples was recorded. After that, dry film samples were soaked in a tube (30 mL distilled water) at room temperature for 24h. Then, the wet film samples were kept in an oven under the exact situations to measure their final dry weight. The water solubility of the films was measured using Equation 2.
where Widw and Wfdw are the initial and final dry weights of the films, respectively.
Degree of swelling
The film samples (1 \(\times\) 1 cm) were kept at 70°C in a drying oven for 24 h (Bhat et al. 2022). The dry weight of the film samples was recorded. The dry film samples were transferred into the tubes containing 30 mL distilled water and kept at room temperature (23°C) for 24 h. After that, swollen film samples were collected from the tube and the excess water was removed using tissue paper. The swollen film samples’ weight was measured, and the degree of swelling was calculated according to the following Equation 3.
where Wsw and Wdw are the swollen and dry weights of the films, respectively.
Water vapour permeability (WVP)
The WVP of films was measured using ASTM E00996-00 with a slight modification. Briefly, 7 mL of distilled water is kept in a glass bottle with an internal diameter of 18 mm, mouth area of 13 mm, and 44 mm height. The mouth of each bottle was tightly covered with the films (20 mm diameter) and sealed with Teflon tape. The initial weight of the bottle was noted and placed in the oven (40°C). The bottle weight was taken every 24 h till five days. Then, WVP (g m−1s−1Pa−1) was measured using the following Equation 4.
where W, x, t, A, and Δp are the weight difference (g), film thickness (m), time (24 h), exposed area (m2), and pressure difference (Pa) between the inner and outer sides of the films, respectively.
Colour, transmittance, and opacity
The film surface colour was measured using the portable MiniScan EZ 45/0° spectrophotometer (4500L, Hunter Associates Laboratory, Inc., Reston, USA), followed by Giteru et al. (2019) method. Initially, the device was calibrated using the instrument standard (X: 81.07, Y: 85.97, and Z: 89.44). The colour parameters such as L* (light-darkness), a* (red-greenness), and b* (yellow-blueness) were considered during the measurement of film colour. The measurements were taken at three points of the films, and ΔE (the total colour difference) was calculated using the following Equation 5.
where L*, a*, and b* are the colour parameters of the standard (L*: 81.45, a*: 0.94, and b*: 13.34) and L0, a0, and b0 are the film samples’ colour properties.
The light transmittance and opacity of films were measured using the UV/Visible spectrophotometer (Ultrospec 3300 pro, Biochrom Ltd., Cambridge, England), followed by Khanzada et al. (2023) method. The film samples (1\(\times\)4 cm) were inserted into the cuvette, absorbance was taken at a different wavelength (200–800 nm), and the cuvette without film was used as a reference. According to the following Equations 6 and 7, transmittance and opacity were calculated at 600 nm.
where A600 is film absorbance at 600 nm, and x is the film thickness.
Mechanical properties
The tensile strength (TS) and elongation at break (EAB) of films were measured using the TA.HD plus texture analyser (Stable Micro Systems Limited, Godalming, United Kingdom) according to the ASTM D822_02/TG standard method. The film samples (60\(\times\)10 mm) were kept between the stationary lower grip and movable upper grip area. The films’ measured thickness was used to calculate TS and EAB. The pre-test speed, test speed, post-test speed, distance, trigger force, break sensitivity, contact force, return distance, and return speed were 4.8 mm/min, 30 mm/min, 480 mm/min, 50 mm, 0.049 N, 0.098 N, 50 g, 30 mm, and 20 mm/sec, respectively. Five specimens for each film sample were considered to represent the average value of TS and EAB.
Structural properties
Scanning electron microscopy (SEM) analysis
The surface and cross-sectional morphology of films were examined using the Hitachi TM3030 tabletop scanning electron microscope (Hitachi High-Technologies Corporation, Minato-Ku Tokyo, Japan). Before scanning, the film samples were coated with 10 nm gold/palladium using Q 150V ES plus (Quorum Technologies, East Sussex, United Kingdom). The scanning of films was done at 5−15 kV voltage and 1000−2000× magnifications.
Fourier-transform infrared spectroscopy (FTIR) analysis
The different functional groups of developed films were characterised using Varian 610-IR FTIR from Agilent Technologies, Santa Clara, USA. The OPUS software (8.1 version), 400−4000 cm−1 wavelength, and 4 cm-1 resolution were applied during the FTIR analysis. Before film samples analysis, clean ATR (Attenuated total reflectance) spectra were considered background for each film measurement.
Thermal properties
Differential scanning calorimetry (DSC) analysis
The film’s thermal properties were determined using DSC Q2000-V24.11 build 124 from TA Instruments, New Castle, USA. The film samples (5 mg) were inserted into the aluminium pan (Product of USA), and a blank pan was considered a reference. All film samples were heated at 10−300 °C and 10 °C/min heating rate under 50 mL/min of dynamic nitrogen conditions.
Thermogravimetric analysis (TGA)
The thermal stability of prepared films was examined using a TGA550 analyser (TA Instrument, USA) and TRIOS software (4.3 version). All film samples (5−10 mg) were kept in a platinum pan and heated at 25−600 °C with a 10 °C/min heating rate in a 50 mL/min flow rate of nitrogen atmosphere.
Biodegradation study of developed hydrogel film
The soil burial approach was used to test the biodegradability patterns of developed films, followed by Riaz et al. (2020) with slight modifications. The soil (mixture of natural materials called lawn soil) was purchased from the Warehouse in South Dunedin, New Zealand and placed into a plastic pot (34 × 14 × 13 cm). The film samples (1.5 × 1.5 cm) were weighed and buried 5 cm depth in the soil. The pot was kept in the laboratory at 21−23 °C for 15 weeks. Water was also sprinkled once a day during the experiment periods to keep the soil wet. To evaluate film degradation on a weekly basis, the film sample was meticulously collected, gently washed to remove soil, and surplus water was removed using tissue paper. The weight difference represented films that deteriorated over time, and the degradation percentage was computed using equation 8.
where Wi is the initial weight of film samples, and Wf is the weight of buried film samples over time.
Statistical analysis
GraphPad Prism version 9.4.1 (GraphPad Software, Boston, USA) was applied for statistical analysis. The obtained data are presented as mean±standard deviation (SD). Ordinary one-way analysis of variance (ANOVA) and Tukey’s test were considered to analyse significant differences (p < 0.05) in the fabricated films.
Results and discussion
Physical and barrier properties of hydrogel film
Thickness, moisture content, and water solubility
The results of thickness, moisture content, and water solubility of hydrogel films are presented in Table 2. Film thickness plays a crucial role in measurement the physical and mechanical characteristics of films. The fabricated hydrogel film’s thickness range is 0.04−0.06 mm. The incorporation of PVA, COS, and gallic acid into chitosan film resulted in consistent film thickness, with no significant difference in average thickness (p>0.05). Similar trends have been observed in chitosan film with different additives (Chakravartula et al. 2020; Khanzada et al. 2023). The thickness of films depends on the nature of the film-forming polymer and the type of additive (Moalla et al. 2021). In the current study, the organised structure of the chitosan film matrix was not greatly interrupted by the additives; therefore, there was no significant difference in the thickness of developed films.
Moisture content is another essential parameter of films associated with the free film space the water molecules occupy. The range of moisture content of the prepared films is 14.31−24.28%. The CH film exhibited the highest percentage of moisture value (24.28%), followed by CP2 (18.05%), CP12 (17.57%), CP6 (16.95%), CP11 (16.83%), CP5 (15.55%), CP8 (15.09%), CP7 (14.92%), CP10 (14.33%), and CP9 (14.31%). The inclusion of PVA, COS, and gallic acid has been shown to significantly reduce moisture content. These findings suggest that the amino (NH2) and hydroxyl (OH) groups CH may interact with PVA, COS, and gallic acid through covalent and/or hydrogen bonds, thereby rendering functional groups of CH to inaccessible to water. Similar results have been observed in biopolymer films containing CH and other biomaterials such as COS, PVA, caffeic acid, gallic acid, and essential oils (Lamarra et al. 2020; Roshandel-Hesari et al. 2022; Yuan et al. 2023).
The increased water solubility (41.56%) seen in CH film compared to other produced films is attributed to chitosan’s solubility and hydrophilicity. When gallic acid, COS, and PVA were mixed with CH, they formed a strong molecular contact with the CH molecules, preventing water molecules from permeating and therefore reducing the water solubility rate. Noticeably, higher concentrations of COS and gallic acid in CP10 film demonstrated lower water solubility (22.14%), as presented in Table 2. Zhao et al. (2022) reported that the water solubility of chitosan, silver nanoparticles, PVA and gallic acid composite film (11.12–26.5%) was reduced compared to chitosan film (41.58%). They proposed that molecular solid interactions between the additives and the polymer reduced the attraction of water molecules on the composite film. The current research findings are consistent with these findings. The lower water solubility of films is usually recommended for the film’s suitability in food packaging in humid conditions (Xu et al. 2021).
Degree of swelling and water vapour permeability
According to Table 2 and Fig. S1, the CH film exhibited the highest of swelling rate 3850.05%, due to its hydrophilic nature and strong capacity of hydrogen bonding with water molecules. The CH hydrogel film showed an impressive swelling value of up to 4900%, surpassing the value reported by (El-Mekawy et al. 2021). However, when blending with PVA, COS, and gallic acid, the swelling percentage decreased significantly to a range of 493.27–2196.64%. This downward trend was also observed in CH films containing COS, caffeic acid, ascorbic acid, copper sulphate hexahydrate, and Xanthan gum (Panneerselvam et al. 2022; Yuan et al. 2023). Also, a chitosan hydrogel film containing PVA, acrylamide, and 2-hydroxyethyl methacrylate showed a swelling value of 975%, as reported by Panpinit et al. (2020). Moreover, the swelling behaviour depends on various factors, including ionic strength, biomaterials type, crosslinking density, environment temperature, and pH of the aqueous media (Hegaard and Thormann 2023).
The WVP of developed films is demonstrated in Table 2. WVP of CH films blended with PVA, COS, and gallic acid (7.52−9.87×10−9 g m−1 s−1 Pa−1) was significantly (p<0.05) lower than WVP of CH film (14.63×10−9 g m−1 s−1 Pa−1), suggesting that the inclusion of PVA, COS, and gallic acid in the CH augmented the confrontation the movement of water molecules. This might have occurred due to the formation of hydrogen bonds and cross-linking properties with the active components of CH films blended with PVA, COS, and gallic acid that decreased the availability of O–H groups, and reduced water vapour diffusion rate (Bhat et al. 2022). The study findings align with Priyadarshi et al. (2018a, b) who reported that adding plasticiser (glycerol) and citric acid into CH film reduced WVP. The lower WVP of blended films compared to CH film could be attributed to acting as an excellent barrier to protect food from the external environment (Gulzar et al. 2022).
Colour, transparency, and opacity
The colour properties (L*, a*, and b*) and total colour difference (ΔE) of fabricated hydrogel films containing CH, PVA, COS, and gallic acid are shown in Table 3. There is no significant difference (p>0.05) detected in CH, CP2, CP5, and CP6 films that showed the highest L* value (73.22−75.14), whereas the addition of gallic acid in CP7−CP12 films exhibited the lowest L* value (62.86−64.94), which indicates CH−CP6 films are lighter than composite films containing gallic acid. Similar findings were stated by Zarandona et al. (2020) in CH film containing gallic acid. Additionally, the gallic acid free CH films (CH−CP6) showed the lowest a* (0.5−0.65), b* (16.41−21.51), and ΔE (8.51−10.39) values whereas the gallic acid-blended films (CP7−CP12) significantly increased the a* (6.49−7.53), b* (19.73−26.49), and ΔE (19.12−22.51) values. The results are aligned with Rui et al. (2017) who reported that the addition of gallic acid in CH film containing gelatine and glycerol increased the a* (0.53−9.74), b* (6.1−26.24), and ΔE (10.95−44.15). The increase of a*, b*, and ΔE suggested that the developed films became redder, yellower, and darker (Ebrahimzadeh et al. 2021).
The light transmittance and opacity of films are significant factors during the selection of packaging materials that affect the appearance of packaging products to the consumers’ level. As presented in Fig. 1, films containing gallic acid showed lower light transmittance (9.71−12.85%) and higher opacity (20.77−26.35) compared to films without gallic acid, including CH film (63.67% light transmittance and 4.1 opacity). This finding is in line with Zheng et al. (2023) who reported that the addition of gallic acid into CH film containing ε-polylysine and collagen showed lower light transmittance compared to CH film and suggested that gallic acid moieties have a strong UV absorption ability. A similar trend was also reported when CH film was fabricated with hydroxybenzoic acid (Liu et al. 2017). Zhang et al. (2019) reported that grafted gallic acid with chitosan exhibited higher opacity compared to CH film, resulting in lower transparency of films. Higher light transmittance could accelerate photooxidation activity, resulting in a product off-flavour (Cai and Wang 2021). In this case, gallic acid-containing fabricated films may protect food from UV damage by increasing film opacity and decreasing light transmittance. In addition, gallic acid-loaded CH films (CP7−CP12) showed the lowest light transmittance (0.91−1.19%) value at T280 compared to gallic acid-free CH films (24.52−35.03%), reconfirming the UV-blocking ability of the fabricated films (Fig. S2).
Mechanical properties
Fig. 2 presents the tensile strength and EAB of developed films. As can be seen, the PVA, COS, and gallic acid-treated CH films had higher tensile strength (45.49−80.85 MPa) and EAB (20.01−36.22%) than the untreated CH film (26.26 MPa and 18.04% EAB). Predominantly, films with COS (10%) and gallic acid (10%) exhibited significantly (p<0.05) high tensile strength when compared with other films except CP8 and CP9. However, the EAB of composite films decreased gradually with the addition of COS and gallic acid compared to the CP2 film. The findings are consistent with Zarandona et al. (2020) who reported that CH film containing gallic acid increased tensile strength and reduced EAB, implying that aromatic compounds in gallic acid structure improved the rigidity of CH film. In contrast, the potential interaction of gallic acid and chitosan, especially carboxylic and amino groups, may hinder the flexibility of CH films (An et al. 2019).
The enhanced tensile strength is attributable to the denser interior structures of produced films caused by molecular interaction between the CH and gallic acid. The increased tensile strength of developed films is in line with Zhang et al. (2019) who showed that the presence of gallic acid increased film rigidity; however, no significant difference was identified in EAB. The difference in EAB percentage between their study and the current study might be attributed to several factors, including variations of synthetic approach and film development technique. The addition of PVA into CH film (CP2) increased the EAB; however, the value of EAB decreased when the inclusion of COS and gallic acid. The results are aligned with Zhao et al. (2022), who observed strong interaction between the PVA and CH film due to the formation of physical crosslinking in PVA and CH, leading to higher EAB. Also, the uniform distribution of different additives in the film matrix improved good stress distribution and reduced the possibility of stress concentration, which may be related to greater tensile strength and decreased EAB.
Gulzar et al. (2022) reported that the addition of COS and tannic acid in the development of polylactic acid film containing CH increased tensile strength and EAB. This phenomenon could have happened due to the strong molecular interaction of COS with the film matrix through hydroxyl or amino groups. Additionally, the conjugation of COS and caffeic acid is attributed to increasing tensile strength and EAB (Yuan et al. 2023). The current research findings are consistent with these findings; hence, COS and gallic acid-containing films demonstrated good mechanical characteristics. In general, good tensile strength and sufficient EAB value are important for the development of packaging materials to protect the food (Bhowmik et al. 2022).
SEM analysis
The surface morphology and cross-section analysis of developed films containing different concentrations of CH, PVA, COS, and gallic acid are illustrated in Fig. 3. It can be observed from the SEM analysis that the surface microstructure of developed films exhibited relatively homogenous and smooth. Similar results were observed by Zhao et al. (2022) who added CH, gallic acid, and silver nanoparticles in the development of film and exhibited good compatibility in the film matrix leads to flatter and smoother surface microstructure. However, some particles are spotted on the surface of CP10 and CP12 films leading to the creation of pores in the cross-section parts. Some dispersed particles are also observed by Chen et al. (2022) who included CH, gelatine, and nisin-corn starch in the production of films and stated that increasing nisin concentration in the film matrix which led to particle formation. The cross-section area of CH film exhibited some roughness in the current study; however, the roughness was decreased by the addition of PVA and returned when gallic acid was added. The smooth film cross-section may have happened due to the good interfacial adhesion of CH and PVA, and roughness of the cross-section might have occurred either after exfoliating or intercalating film matrix compounds (Hu et al. 2022; Zarandona et al. 2020). However, the incorporation of PVA, COS, and gallic acid in CH films reduced the roughness of the cross-section area. The study findings are consistent with Fernandez-de Castro et al. (2016) and Zhao et al. (2022).
SEM images of surface (15kV X1000 100 μm) and cross-section (5kV X2000 30 μm) of CH hydrogel films containing different concentrations of PVA, COS and gallic acid. CH (chitosan), CP2 (chitosan and PVA), CP5 and CP6 (chitosan, PVA, and COS), CP7 and CP8 (chitosan, PVA and gallic acid), and CP9−CP12 (chitosan, PVA, COS, and gallic acid)
FTIR analysis
The ATR-FTIR spectra of CH, PVA, COS, and gallic acid powder and their composite films are presented in Fig. S3 and Fig. 4. The CH, PVA, COS, and gallic acid powder showed bands at 693−3356, 603−3295, 693−3428, and 632−3492 cm−1 respectively. The band at 3354−3356cm−1 in CH and COS powders may be ascribed to the N–H/O–H stretching vibrations of the amino/hydroxyl groups, and the band at 2860−2869 cm−1 is related to the symmetric stretching of C–H. The CH and COS powder FTIR spectra are similar to those reported by other studies (Acosta-Ferreira et al. 2020; Rajabi et al. 2022). Additionally, PVA powder exhibited a band at 3295 cm−1 due to O–H stretching, which aligns with other findings (Islam et al. 2022). The gallic acid powder demonstrated bands at 3272-3492, 1663, and 1606-1645 cm−1, which is related to the O–H stretching, C=O stretching, and H-O–H bending, respectively (Tsioptsias and Tsivintzelis 2022). Also, different bands were detected in the developed CH (645−3273 cm−1), CP2 (647−3208 cm−1), CP5 (647−3355 cm−1), CP6 (648−3198 cm−1), CP7 (648−3428 cm−1), CP8 (647−3198 cm−1), CP9 (648−3213 cm−1), CP10 (647−3213 cm−1), CP11 (646−3221 cm−1), and CP12 (647−3304 cm−1) films. CH film exhibited a band at 3273 cm−1 assigned to N–H/O–H stretching, followed by 2929 cm−1 (C–H tension), 1635 cm−1 (C=O stretching, Amide I), 1540 cm−1 (N–H bending, Amide II), 1403 cm−1 (C–H bending), 1339 cm−1 (C-N stretching, Amide III), 1255 cm−1 (C–O–C/-C–O–H stretching), 1151 cm−1 (C–O stretching), 1065 cm−1 (C–O stretching), 1018 cm-1 (C–O stretching), 926 cm−1 (O–H/C–H bending), 897 cm−1 (C–H bending), and 645 cm−1 (C–H bending) (Bi et al. 2021; Giteru et al. 2021; Panpinit et al. 2020).
The addition of PVA into CH film, CP2 showed some changes, such as a pattern of absorption and shifting of band (3208 and 2918 cm−1), which might be happened due to the interaction of CH (-OH and -NH2) and PVA by generating hydrogen bonds (Ali and Ahmed 2021). The inclusion of COS into CH and PVA films, CP5 and CP6 demonstrated band changes (3355 and 3198 cm−1) that could be occurred due to the increase of the -OH group, which enhances the formation of hydrogen bond either between COS and PVA or within PVA chains (Qureshi et al. 2021). Similarly, adding gallic acid into CH and PVA films, CP7 and CP8 displayed shifting of spectra at 3428 and 3198 cm−1, respectively, due to the formation of hydrogen bonding between CH, PVA, and gallic acid and band at 1541 cm−1 due to the C=C stretching of gallic acid (Zhao et al. 2022). Additionally, the blending of CH with COS, PVA, and gallic acid, CP9−CP12 films exposed shifting of the band at 3213−3304 cm−1 (O–H stretching), 1636 cm−1 (Amide I, N–H bending) 1540−1557 cm−1 (Amide II, N–H bending) and 1339−1395 cm−1 (Amide III, C=O stretching), implying the potential interaction between CH, gallic acid, PVA, and COS that enhances the stability of composite films (Lamarra et al. 2020; Qureshi et al. 2021).
Thermal properties
DSC analysis
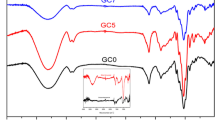
The thermal transition of developed films was monitored using DSC at 10–300°C and presented in Fig. 5A. The CH film demonstrated different peaks at 105°C, 188°C, 239°C, and 274°C. The peak appeared at 105°C, attributed to the evaporation of solvents such as acetic acid and water, and peaks exhibited at 188°C, and 239°C could have happened due to the melting of the chitosan structure, indicating melting temperature (endothermic). The final peak at 274°C showed the decomposition of chitosan, representing exothermic temperature. The findings are consistent with Khanzada et al. (2023) who detected peaks at 109°C, 210°C, 250°C, and 293°C in chitosan film during the DSC analysis, representing water evaporation, melting temperature, and decomposition of chitosan, respectively.
The addition of PVA, COS, and gallic acid into CH relatively improves the thermal stability of films, thereby delaying the moisture evaporation peak (109−115°C), melting temperature (192−199°C and 244−254°C) and decomposition peak (277−285°C) observed, especially in CP8, CP10, and CP12 films. It indicates that the inclusion of COS, PVA, and gallic acid strongly interacts with the chitosan matrix leading to more thermostable films. The findings are consistent with Kaczmarek-Szczepańska et al. (2022) and Qureshi et al. (2021), who reported that the addition of gallic acid and COS improved the thermal stability of CH film through effective interaction in molecular level.
TGA analysis
Fig. 5B illustrates the TGA analysis of prepared films and their three stages of weight loss. The initial weight loss observed at 25−150°C, followed by the second stage between 151−300°C, and the third stage between 301−600°C. The films experienced a slight weight loss of 14.98−18.57% during the first stage due to the evaporation of free and bound moisture caused by the breakdown of intra- and intermolecular hydrogen bonds (Khanzada et al. 2023). In the second stage, a weight loss of 46.49−50.34% was observed that related to the degradation of the PVA (Zhang et al. 2019). Additionally, 62.64−66.14% mass loss was monitored at the third stage due to the thermal decomposition of CH, COS, and gallic acid (Zhang et al. 2022). Further low molecular weight gas generated may be a breakdown of the char (Chen et al. 2021). Furthermore, CH film experienced a 50% mass loss at 301°C, while CP10 and CP12 films showed a 50% loss at 323°C and 318°C, respectively, indicating that the addition of PVA, COS, and gallic acid improved the thermal stability of the film through stronger interaction. Additionally, CP10 and CP12 films exhibited the highest residue of char at 37.36%. These research findings coincide with those of Chen et al. (2022) and Yuan et al. (2023) who stated that the thermal stability of CH composite films was higher compared to neat CH film.
The DTG curve illustrates the rate of maximal weight loss and displays two or three weight loss zones as a result of either strong or weak interactions between the CH, PVA, COS, and gallic acid (Fig. S4). The gallic acid-free CH films (CH−CP6) exhibited higher weight loss around 250−255°C, whereas gallic acid-loaded CH films (CP7−CP12) demonstrated maximum derivative weight loss at delayed temperature (260−265°C). This might have happened due to the strong interaction and evenly distributing of PVA, COS, and gallic acid inside the chitosan matrix. The current research findings are similar to those of Yuan et al. (2023) and Zhang et al. (2019), who elucidated that caffeic and gallic acid-loaded CH films exhibited maximum weight loss at delayed temperature (approximately 295−305°C) than neat chitosan film (around 275−285°C).
Biodegradation patterns
The biodegradation patterns of the fabricated hydrogel film were measured using the soil-burial technique, and their quantitative weight loss percentage is depicted in Fig. 6. It is observed that all developed films gained weight after seven days of the experimental period that might have happened due to the hydrogel nature of the film. However, the rate of weight loss increased gradually, and 50% of weight loss was detected in CH and CP2−CP6 films at 28 days, whereas CP7, CP8, CP11, and CP12 were taken at 49 days, and 56 days attained by CP9 and CP10 films. At the end of experiments, CP10 totally decomposed at 98 days, followed by CP9 and CP12 (84 days), CP7, CP8, and CP11 (77 days), CP2 and CP5 (70 days), and CP6 and CH taken at lowest days (63 days). The results revealed that neat chitosan film’s degradation rate was higher than COS and gallic acid-loaded films. The findings are consistent with Zhao et al. (2022), who observed 67% weight loss in neat chitosan film at 28 days, whereas only 30% weight loss was detected in gallic acid-loaded films. Additionally, neat chitosan film demonstrated 91% weight loss at 56 days, whereas the plant extract-loaded chitosan film showed 65%, as reported by Surendhiran et al. (2022), which is aligned with current study findings. This could have occurred due to the presence of COS and gallic acid in the film, retarding the microbial activity in the soil and delaying decomposition. In general, the polyphenolic compounds in the packaging film inhibit the growth of microbes present in the soil (Cerruti et al. 2011).
Conclusion
In this study, we successfully fabricated CH hydrogel films enriched with PVA, COS, and gallic acid. Based on property characterisations, it is observed that the inclusion of neat COS/gallic acid in CH films improved their physical, barrier, mechanical, structural, and thermal properties slightly compared to CH film. However, enriching CH film with COS-gallic acid greatly reduced the moisture, water solubility, degree of swelling, transparency and WVP, while also improving the colour, tensile strength, EAB, structural and thermal characteristics. We observed greater improvements in CH films with higher levels of gallic acid and COS, particularly CP10 film, which exhibited strong barrier, tensile strength, and thermal properties compared to CH films. Therefore, the findings of the current study revealed that the incorporation of COS and gallic acid in CH hydrogel films could have potential for use in food packaging. Additionally, the assessment of bioactivities such as antioxidant ability and antimicrobial activity and the application of fabricated hydrogel films in the food packaging sectors are of great interest to us for further study.
Data availability
No datasets were generated or analysed during the current study.
References
Acosta-Ferreira S, Castillo OS, Madera-Santana JT et al (2020) Production and physicochemical characterization of chitosan for the harvesting of wild microalgae consortia. Biotech Rep 28:e00554
Ali A, Ahmed S (2021) Eco-friendly natural extract loaded antioxidative chitosan/polyvinyl alcohol based active films for food packaging. Heliyon 7(3):e06550
An X, Kang Y, Li G (2019) The interaction between chitosan and tannic acid calculated based on the density functional theory. Chem Phy 520:100–107
Antony T, Cherian RM, Varghese RT et al (2023) Sustainable green packaging based on nanocellulose composites-present and future. Cellulose 30(17):10559–10593
Bhat VG, Narasagoudr SS, Masti SP et al (2022) Development and evaluation of Moringa extract incorporated Chitosan/Guar gum/Poly (vinyl alcohol) active films for food packaging applications. Int J Bio Macro 200:50–60
Bhowmik S, Agyei D, Ali A (2022) Bioactive chitosan and essential oils in sustainable active food packaging: Recent trends, mechanisms, and applications. Food Pack Shel Lif 34:100962
Bhowmik S, Agyei D, Ali A (2023) Application of nanochitosan in the preservation of fish and oil. In: Next Generation Nanochitosan, Elsevier, pp. 447–474
Bi J, Tian C, Zhang GL et al (2021) Novel procyanidins-loaded chitosan-graft-polyvinyl alcohol film with sustained antibacterial activity for food packaging. Food Chem 365:130534
Cai L, Wang Y (2021) Physicochemical and antioxidant properties based on fish sarcoplasmic protein/chitosan composite films containing ginger essential oil nanoemulsion. Food Biopro Tech 14(1):151–163
Cerruti P, Santagata G, d’Ayala GG et al (2011) Effect of a natural polyphenolic extract on the properties of a biodegradable starch-based polymer. Poly Degraand Stab 96(5):839–846
Chakravartula SSN, Lourenço RV, Balestra F et al (2020) Influence of pitanga (Eugenia uniflora L.) leaf extract and/or natamycin on properties of cassava starch/chitosan active films. Food Pack Shel Li 24:100498
Chen C, Zong L, Wang J et al (2021) Microfibrillated cellulose reinforced starch/polyvinyl alcohol antimicrobial active films with controlled release behavior of cinnamaldehyde. Carbo Poly 272:118448
Chen J, Zhang J, Liu D et al (2022) Preparation, characterization, and application of edible antibacterial three-layer films based on gelatin–chitosan–corn starch–incorporated nisin. Food Pack Shel Li 34:100980
Ebrahimzadeh S, Bari MR, Hamishehkar H et al (2021) Essential oils-loaded electrospun chitosan-poly (vinyl alcohol) nonwovens laminated on chitosan film as bilayer bioactive edible films. LWT 144:111217
El-Mekawy RE, Elhady HA, Al-Shareef HF (2021) Highly stretchable, smooth, and biodegradable hydrogel films based on chitosan as safety food packaging. Poly Poly Comp 29(6):563–573
Fernandez-de Castro L, Mengíbar M, Sánchez Á et al (2016) Films of chitosan and chitosan-oligosaccharide neutralized and thermally treated: effects on its antibacterial and other activities. LWT 73:368–374
Giteru SG, Ali A, Oey I (2021) Understanding the relationship between rheological characteristics of pulsed electric fields treated chitosan-zein-poly (vinyl alcohol)-polyethylene glycol composite dispersions and the structure-function of their resulting thin-films. Food Hydro 113:106452
Giteru SG, Ali A, Oey I (2019) Solvent strength and biopolymer blending effects on physicochemical properties of zein-chitosan-polyvinyl alcohol composite films. Food Hydro 87:270–286
Gulzar S, Tagrida M, Nilsuwan K et al (2022) Electrospinning of gelatin/chitosan nanofibers incorporated with tannic acid and chitooligosaccharides on polylactic acid film: characteristics and bioactivities. Food Hydro 133:107916
Haghighi H, Licciardello F, Fava P et al (2020) Recent advances on chitosan-based films for sustainable food packaging applications. Food Pack Shel Li 26:100551
Hegaard F, Thormann E (2023) Influence of ionic strength and specific ion effects on polyelectrolyte multilayer films with pH-responsive behavior. Langmuir 39(14):5012–5020
Hu H, Yong H, Yao X et al (2022) Effect of starch aldehyde-catechin conjugates on the structural, physical and antioxidant properties of quaternary ammonium chitosan/polyvinyl alcohol films. Food Hydro 124:107279
Islam MT, Laing RM, Wilson CA et al (2022) Fabrication and characterization of 3-dimensional electrospun poly (vinyl alcohol)/keratin/chitosan nanofibrous scaffold. Carbo Poly 275:118682
Jiang Y, Yin H, Zhou X et al (2021) Antimicrobial, antioxidant and physical properties of chitosan film containing Akebia trifoliata (Thunb.) Koidz. peel extract/montmorillonite and its application. Food Chem 361:130111
Kaczmarek-Szczepańska B, Zasada L, Grabska-Zielińska S (2022) The physicochemical, antioxidant, and color properties of thin films based on Chitosan modified by different phenolic acids. Coatings 12(2):126
Khanzada B, Mirza B, Ullah A (2023) Chitosan based bio-nanocomposites packaging films with unique mechanical and barrier properties. Food Pack Shel Li 35:101016
Lamarra J, Rivero S, Pinotti A (2020) Nanocomposite bilayers based on poly (vinyl alcohol) and chitosan functionalized with gallic acid. Inter J Biol Macro 146:811–820
Liu D, Guo Y, Ma H (2023a) Production of value-added peptides from agro-industrial residues by solid-state fermentation with a new thermophilic protease-producing strain. Food Bios 53:102534
Liu F, Zhang X, Xiao X et al (2023b) Improved hydrophobicity, antibacterial and mechanical properties of polyvinyl alcohol/quaternary chitosan composite films for antibacterial packaging. Carbo Poly 312:120755
Liu J, Cg M, Liu S et al (2017) Preparation and characterization of protocatechuic acid grafted chitosan films with antioxidant activity. Food Hydro 63:457–466
Liu Y, Wang R, Wang D et al (2022) Development of a food packaging antibacterial hydrogel based on gelatin, chitosan, and 3-phenyllactic acid for the shelf-life extension of chilled chicken. Food Hydro 127:107546
Moalla S, Ammar I, Fauconnier ML et al (2021) Development and characterization of chitosan films carrying Artemisia campestris antioxidants for potential use as active food packaging materials. Inter J Biolo Macro 183:254–266
Nguyen SV, Lee BK (2023) Multifunctional food packaging polymer composites based on polyvinyl alcohol/cellulose nanocrystals/apple peel extract. Cellulose 30(3):1697–1716
Panneerselvam N, Sundaramurthy D, Maruthapillai A (2022) Antibacterial/antioxidant activity of CuO impacted xanthan gum/chitosan@ ascorbic acid nanocomposite films. J Poly Envir 30(8):3239–3249
Panpinit S, Sa P, Keawin T et al (2020) Development of multicomponent interpenetrating polymer network (IPN) hydrogel films based on 2-hydroxyethyl methacrylate (HEMA), acrylamide (AM), polyvinyl alcohol (PVA) and chitosan (CS) with enhanced mechanical strengths, water swelling and antibacterial properties. Reac Func Poly 156:104739
Priyadarshi R, Kumar B, Deeba F et al (2018a) Chitosan films incorporated with Apricot (Prunus armeniaca) kernel essential oil as active food packaging material. Food Hydro 85:158–166
Priyadarshi R, Kumar B, Negi YS (2018b) Chitosan film incorporated with citric acid and glycerol as an active packaging material for extension of green chilli shelf life. Carbo Poly 195:329–338
Qureshi D, Sahoo A, Mohanty B et al (2021) Fabrication and characterization of poly (vinyl alcohol) and chitosan oligosaccharide-based blend films. Gels 7(2):55
Rajabi M, Cabral J, Saunderson S et al (2022) Green synthesis of chitooligosaccharide-PEGDA derivatives through aza-Michael reaction for biomedical applications. Carbo Poly 295:119884
Rajabi M, McConnell M, Cabral J et al (2021) Chitosan hydrogels in 3D printing for biomedical applications. Carbo Poly 260:117768
Riaz A, Lagnika C, Luo H et al (2020) Chitosan-based biodegradable active food packaging film containing Chinese chive (Allium tuberosum) root extract for food application. Inter J Biolo Macro 150:595–604
Roshandel-Hesari N, Mokaber-Esfahani M, Taleghani A et al (2022) Investigation of physicochemical properties, antimicrobial and antioxidant activity of edible films based on chitosan/casein containing Origanum vulgare L essential oil and its effect on quality maintenance of cherry tomato. Food Chem 396:133650
Rui L, Xie M, Hu B et al (2017) A comparative study on chitosan/gelatin composite films with conjugated or incorporated gallic acid. Carbo Poly 173:473–481
Surendhiran D, Roy VC, Park JS et al (2022) Fabrication of chitosan-based food packaging film impregnated with turmeric essential oil (TEO)-loaded magnetic-silica nanocomposites for surimi preservation. Inter J Biolo Macro 203:650–660
Tabassum N, Ahmed S, Ali MA (2021) Chitooligosaccharides and their structural-functional effect on hydrogels: A review. Carbo Poly 261:117882
Tardy BL, Richardson JJ, Greca LG et al (2023) Advancing bio-based materials for sustainable solutions to food packaging. Nat Sustain 6(4):360–367
Thanyacharoen T, Chuysinuan P, Techasakul S et al (2018) Development of a gallic acid-loaded chitosan and polyvinyl alcohol hydrogel composite: release characteristics and antioxidant activity. Inter J Biolo Macro 107:363–370
Tsioptsias C, Tsivintzelis I (2022) Insights on thermodynamic thermal properties and infrared spectroscopic band assignments of gallic acid. J Pharm Biomed Ana 221:115065
Wang Y, Liu K, Zhang M et al (2023) Sustainable polysaccharide-based materials for intelligent packaging. Carbo Poly 313:120851
Xu Y, Liu X, Jiang Q et al (2021) Development and properties of bacterial cellulose, curcumin, and chitosan composite biodegradable films for active packaging materials. Carbo Poly 260:117778
Yu Q, Yang L, Wang S et al (2023) Citric acid cross-linked regenerated bacterial cellulose as biodegradable film for food packaging. Cellulose 30(16):10273–10284
Yuan Y, Tan W, Lin C et al (2023) Development of antioxidant chitosan-based films incorporated with chitooligosaccharide-caffeic acid conjugates. Food Hydro 138:108431
Zarandona I, Puertas A, Dueñas M et al (2020) Assessment of active chitosan films incorporated with gallic acid. Food Hydro 101:105486
Zhang T, Yu Z, Ma Y et al (2022) Modulating physicochemical properties of collagen films by cross-linking with glutaraldehyde at varied pH values. Food Hydro 124:107270
Zhang X, Liu J, Qian C et al (2019) Effect of grafting method on the physical property and antioxidant potential of chitosan film functionalized with gallic acid. Food Hydro 89:1–10
Zhao X, Wang Y, Chen X et al (2023) Sustainable bioplastics derived from renewable natural resources for food packaging. Matter 6(1):97–127
Zhao Y, Yang L, Xu M et al (2022) Gallic acid functionalized chitosan immobilized nanosilver for modified chitosan/Poly (vinyl alcohol) composite film. Inter J Biolo Macro 222:2987–3000
Zheng T, Tang P, Yang C et al (2023) Development of active packaging films based on collagen/gallic acid-grafted chitosan incorporating with ε-polylysine for pork preservation. Food Hydro 140:108590
Acknowledgments
Shuva Bhowmik would like to acknowledge the University of Otago, New Zealand, for supporting research and studies through the Otago Doctoral Scholarship.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
SB: Conceptualization, Investigation, Methodology, Data curation, Visualization, Writing—Original draft. DA: Supervision, Writing—review and editing. AA: Supervision, Conception, Planning, Writing—review and editing.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no competing interests.
Ethical approval
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bhowmik, S., Agyei, D. & Ali, A. Biodegradable chitosan hydrogel film incorporated with polyvinyl alcohol, chitooligosaccharides, and gallic acid for potential application in food packaging. Cellulose (2024). https://doi.org/10.1007/s10570-024-06080-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10570-024-06080-8