Abstract
In this study we investigate the use of in situ bioprocessing for the production and surface modification of bacterial cellulose (BC) with silicon additives. The surface properties and tensile strength of the BC were studied and compared with plain BC. The effect the modification exhibited on the survivability of the bacteria was assessed by optical density measurements and found that the addition of the modification marginally slowed growth in the case of Tetramethyl orthosilicate (TMOS) and did not affect the growth in the case of Tetraethyl orthosilicate (TEOS). Characterisation of the modified BC was carried out using FTIR, EDX and confirmed the presence of silicon in the material. The width of fibres in the microstructure of BC was measured using SEM. Two different silicon modifications were used to modify the BC, it was shown that the TMOS modification decreased the tensile strength but that the TEOS increased the tensile strength of the BC fibres compared to plain BC. In addition, we found that the washing conditions of 1% NaOH (w/v), industrial methylated spirit (IMS), and deionised water (DI) showed some impact on the properties of the samples, particularly the IMS produced a reduced contact angle in the modified samples. However, the contact angle increased in the case of TEOS modification with the NaOH wash. In conclusion this study shows a novel method of modifying BC materials in-situ using silicon additives for increased tensile strength and the potential for tuneable hydro interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacterial Cellulose (BC) is a biomaterial with many applications from medical materials for implants and wound dressing to construction (Lin et al. 2013a). BC is a biopolymer produced by several microorganisms from glucose molecules joined together by β-1,4 glycosidic links (Sakaguchi et al. 2010), and it is highly pure with a crystalline structure formed of 2 phases I and II. The I phase is highly organised in fine sheets and constitutes roughly 70% of the total structure and the II phase is more disorganised and amorphous and constitutes the remainder (Atalla and Vanderhart 1984; Gromovykh et al. 2017).
BC is produced through an enzyme-driven polymerisation process and can be created using waste products such as agricultural residues, food industry waste and wastewater (Kadier et al. 2021). It requires less energy, water and land compared to the production of plant cellulose fibre in theory as it is able to be produced in a lab or bioreactor and harvested there, however producing BC is of greater cost and lower yield than harvesting wood from trees or fibre from plants like cotton or flax (El-Saied et al. 2008; Vandamme et al. 1998). BC has unique properties of biocompatibility, high tensile strength (for a biopolymer), and ease of modification make it an attractive material for research and small-scale production (Budhiono et al. 1999; Kurosumi et al. 2009; Shoda and Sugano 2005).
Biodegradable materials are in high demand in the field of materials research and development with the ever growing threat of microplastics and the great pacific garbage patch which we are aiming to reduce (Lebreton et al. 2018; Prata et al. 2020). BC can be broken down by natural processes of biodegradation by certain organisms such as fungi into sugars and short chain polymers which can act as biomass for the production of more cellulose (Baldrian and Valášková 2008; Urbina et al. 2021). The ease of biodegradation into potentially useful materials or biomass for further processing makes BC a more sustainable alternative to synthetic materials. Being biodegradable is desirable as synthetic polymers produce damaging environmental impacts during their production and disposal from the toxic chemicals used to create them and from their disposal (Amarakoon et al. 2022).
To achieve its full potential in various applications, BC often requires post-production modification and functionalisation. Surface treatment has seen much interest as it is the simplest method of modifying BC. Surface modification can enhance the tensile strength and stiffness of BC, making it suitable for applications such as biocompatible scaffolds for medical implants. Surface modification can alter wettability of BC, making BC suitable for water oil separation (Leng et al. 2009; Pirzada et al. 2020; Zhang et al. 2020) and food packaging (Azeredo et al. 2019; Bandyopadhyay et al. 2018; Gregory et al. 2021; Kim et al. 2022; Padrao et al. 2016). Furthermore, surface modification can add new functionalities to BC, such as antimicrobial activity and antioxidant properties, making it suitable for reducing the immune response and promoting tissue regeneration (Stumpf et al. 2018).
Modifying the mechanical properties and hydrophobicity of BC is an attractive area of research with the aim to specialise the material for applications such as wound dressing and textiles. One method is to add more electronegative groups and stronger electronegative groups which will out compete water for hydrogen bonds in favour of hydrogen bonding between the fibres of the material preventing the absorbance of water and repelling water from the surface (Ma et al. 2020; Ye et al. 2020). Furthermore some of these electronegative groups drastically increase the surface energy of the material which correlates with an increase in hydrophobicity (Praeger et al. 2015). Another method to increase hydrophobicity is to add in longer chain hydrocarbons to reduce the availability of electronegative groups on the surface of the BC thus preventing water from binding to the surface of the material (Lee et al. 2011).
Several groups have silanated cellulose to produce novel effects such as Frank et al. (2018). who reacted methyl, propyl, or aminopropyl silanes with cellulose nanofibres, and polyhydroxyalkanoate (PHA). It was shown that compared to unmodified fibres all of the cellulose nano fibres modified showed a greater hydrophobicity and greater dispersion within the PHA polymer than the unmodified cellulose nano fibres. Improved hydrophobicity compared to the control was observed when the matrix was suspended in chloroform and remained stable over time. The increase in hydrophobicity was attributed to the formation of a hydrophobic silane layer on the surface of the matrix (Frank et al. 2018). The process of modifying a material with silicon is called silanisation and is performed using methods such as combining silica nanoparticles with cellulose or using cellulose as a scaffold in silica-based gel. The silanisation process increases the stability of the gel and the strength of it as well as the water holding capacity, making silicon an attractive molecule to modify BC with. (Cai et al. 2018; Frank et al. 2018; Nakatani et al. 2011; Pirzada et al. 2020; Rodríguez-Robledo et al. 2018).
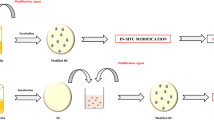
The methods to modify BC are in-situ and ex-situ treatments shown in Fig. 1 which illustrates the process of “conventional” in-situ modification used in the previous publications and the in-situ modification performed in this study. In-situ treatment is modification of a material during its production by the addition of new material or the modification of the production method. The current method took inspiration from the method described by Natalio et al. (2017) and Gao et al. (2019) whereby functional molecules are introduced to cellulose utilising glucose as a carrier molecule (Gao et al. 2019; Natalio et al. 2017). Whereas in traditional in-situ treatment is the modification takes place after the BC fibres are produced and secreted outside the cells (Lin et al. 2013a; Rouabhia et al. 2014; Stumpf et al. 2018).
In this study we modified BC fibres through an in-situ approach using silicon additives Tetraethyl orthosilicate (TEOS) and Tetramethyl orthosilicate (TMOS) as shown in Fig. 2 which were chosen as the silicon compounds to modify the BC due to their 4 electro negative oxygens as well as the methyl and ethyl groups attached to the oxygen. These molecules have available oxygen groups available for hydrogen bonding within the BC while the silicon acts as a electro positive anchor for the oxygen groups as well as providing a greater side chain length for the methyl and ethyl groups (Rao et al. 2003; Tang et al. 2003).
The surface hydrophobicity, tensile strength and thermal properties of the modified BC were studied and furthermore the effect that the silicon additives had on the survivability of the Komagataeibacter Xylinus was investigated using OD600nm measurements. Three washing methods were employed to ascertain if there was any drastic effect between the methods and if they affected the modification. These methods were the standard widely used NaOH wash, a wash with industrial methylated spirit (IMS), and a plain DI water wash.
Materials and methods
Materials preparation
Preparation of modified Si-glucose
The TEOS and TMOS glucose was prepared as follows, 20 g of glucose was dissolved in 100 mL of distilled water. The TEOS and TMOS were added in a molar ratio of 2:1 (glucose: Si-modification). The pH was adjusted to 4 with HCl and the remaining volume was made up with 100 ml of 100% methanol for a final dilution of 50% v/v methanol under constant agitation at room temperature. The solution was then heated under reflux at 100 ⁰C for 6 h.
Purification of modified Si-glucose
The vessel containing the solution of silicon and glucose was relocated to a rotary evaporator to remove the HCl and water. Once the solution showed no more change in volume the solution was poured into falcon tubes and distilled water was added in a ratio of 1:4 (solution: DI water) and the tube was filled to 25 ml (50% of the tubes volume). The solution was mixed and then transferred to the -80 ⁰C freezer overnight. Once frozen the solution was then lyophilized for 2 days using Edwards modulyo freezer and Edwards vacuum pump XDS10 (Edwards Ltd. UK).
Modified Si-glucose media preparation
Hestrin-Shcramm (HS) media was prepared using the standard composition. A solution of 5 g/L peptone, 5 g/L Yeast extract, 1.15 g/L citric acid, and 2.7 g/L disodium phosphate was prepared and autoclaved (Hestrin and Schramm 1954). The desired volume of water was prepared with 20 g/L of glucose and was autoclaved. For the Si modified samples instead of the 20 g/L glucose, 20 g/L of the TEOS and TMOS glucose was prepared and autoclaved.
Preparation of BC and modified BC
The starter culture was prepared by inoculating 15 mL of HS media with a thawed 2 mL of 50% (v/v) glycerol culture of K. xylinus DSM 2325. The modified or unmodified media was added to a 500 mL conical flask, filling the flask to 30% of its total volume (150 mL). The media was the inoculated with 200 μL of a starter culture of K. xylinus DSM 2325 and the culture was incubated at 30 ⁰C for 7 days then the pellicle was collected and washed. Three washing methods were employed: (1) The pellicle was immersed in 1% NaOH (w/v) and heated at between 80–100 ⁰C for 2 h, the pellicle was then neutralised in 1.5% (v/v) acetic acid; (2) The pellicle was washed in 70% IMS for 4 h at room temperature; and (3) The pellicle was immersed in DI water for 4 h.
After the initial washing step each pellicle was immersed in 300 mL of DI water and centrifuged at 6000 rpm for 10 min to ensure the full removal of any residual media or modification components that had not been tightly bound to the BC. The centrifugation step was repeated 3 times replacing the DI water each time. The fully washed pellicle was then dried in an oven at 60 ⁰C overnight.
Characterisation
Growth assay using optical density measurements (OD600nm)
The cultures were set up in 250 mL conical flasks using a modified method for BC production in HS medium containing either glucose or modified glucose (Gilmour et al. 2023). All cultures contained cellulase from Trichoderma reesei (Sigma Aldritch, UK) at the concentration of 1% (v/v). The addition of cellulase prevents the growth of the pellicle which allows for obtaining the absorbance at the wavelength of 600 nm. The medium was then inoculated with 200 µL of K. xylinus starter culture and the culture was grown in a static incubator for 14 days at 30 ⁰C. The absorbance of the culture at 600 nm was recorded with the WPA biowave CO8000 cell density meter (Biochrom Ltd, UK) every day during this period. Each sample was produced and analysed in triplicate.
FTIR
The pellicles grown using the standard growth protocol were analysed by placing a small section of the pellicle with the bottom facing the analyser on the analyser and the transmittance was recorded. The modified Si-glucose from the homogenisation step was tested by taking a small spatula of dried material, enough to cover the sensor, and placing it on the Fourier transform infrared spectroscopy (FTIR) analyser. The transmittance was then recorded taking reading over 2 min. The alpha FTIR (Bruker corporation, USA) was used to record the data points for the FTIR analysis. The software used to visualise and record the data points was OPUS (Bruker corporation, USA).
Microstructure analysis
BC was grown using the protocol for the preparation of BC and modified BC, small 1 cm2 sections were cut from the BC pellicles and used for the tests. The morphology of modified BC was characterised by Scanning Electron Microscopy (SEM) (Tescan MIRA, UK). The Energy Dispersive X-ray (EDX) analysis was carried out using the X-MaxN analyser and analysed by the AZtec software (Oxford instruments, UK). To analyse the pellicles each sample was coated in 5 nm of platinum using the quorum Q150R ES (QUARUM Technologies Ltd, England). An energy of 5 kV was used for the electron generator, the electron detector was set to “in beam” analysing the secondary electrons. The EDX was carried out using an X-ray detector and electron beam of 5 kV. The elemental analysis was carried out using the AZtec program.
The width of the BC fibres was measured using the TESCAN software (Oxford instruments, UK). A series of 10 random sites of 10 fibres selected for each average were chosen from an area with a view field of 5 µm.
Water contact angle tests
Water contact angle (WCA) tests were performed using the Kruss Droplet Size Analyser 30 (DSA) (KRÜSS Scientific, Germany). The samples were prepared by cutting pellicles grown using the standard protocol into 1 cm × 3 cm strips and they were placed on to microscope glass slides. The strips were then mounted on the DSA where a DI water droplet of 2 μL was deposited onto the surface. A video was recorded from the moment of impact until 1 min had passed, the static contact angle was measured at the time of impact and 1 min after contact with the pellicle. The software used was DSA4 (KRÜSS Scientific, Germany) and the droplets contact angles were measured using ImageJ (NIH, USA).
Tensile strength measurements
Pellicles were grown using the standard protocol and each pellicle was cut into 3 cm × 5 cm strips. These strips were placed into the Instron and extended at 20 mm / min. The experiment was repeated 3 times for each washing condition, and each modification. The tensile testing was performed by the Instron 6800 series (Instron corporation, USA).
Statistical analysis
The p values, the standard error mean of each data set, and the R2 squared values were calculated using a T-test with the Welche’s correction for each data point for the box and whisker plots. The properties of the pellicles produced from the modified media were tested against the HS control media (plain). For cytotoxicity assay, the Pearson correlation coefficients analysis was applied. The software used was GraphPad Prism version 9 for macOS, GraphPad Software, San Diego, (Graph Pad Software, California USA).
Results and discussion
BC was modified by culturing the K. xylinus in HS medium with modified glucose and the Si-glucose was created by heating both the silicate and the glucose under reflux and then purifying the mixture by rotary evaporation and lyophilising. We analysed the affect the silicate had on the growth of the K. xylinus by measuring the optical density of the growth media containing cellulase over the course of two weeks. We then characterised the material as described below using FTIR, EDX analysis, mechanical testing, SEM, and droplet size analysis.
Cell growth assay
The cell growth assay (Fig. 3) was performed to check the affect that the modification had on the growth rate of K. xylinus. A drastically slower growth rate or even cytotoxic affect would be undesirable for the production of a new material, therefore the assay was used to determine if the method of modification would be a suitable alternative to standard growth conditions.
From the experiment it appears that the TMOS modification has a statistically significant negative impact on the growth of K. xylinus (P < 0.0001) as shown by the reduced OD600nm compared to the TEOS and plain media until day 7. After 7 days the growth rate follows the same pattern as the plain media growth indicating that there was a factor affecting the growth rate before day7. The TEOS modification shows almost no difference in the rate of growth from the plain media indicating that the modification has little or no impact on the growth of K. xylinus. After the third day the growth rate of the cells in TMOS dropped and the OD600nm reduced to the level below that of both the TEOS and plain media. The TMOS growth rate remained static at below 0.1 OD600nm until day 7 where the growth rate began to mirror the growth rate of the TEOS and the plain media. The plain media showed a consistent growth rate after day 3 with an exponential increase in growth rate over the 14 days. The TEOS media maintained a similar growth rate to the plain media up till day 11 where the growth rate of the TEOS media began to outpace the growth rate of the plain media suggesting an increase in growth rate over plain media (P < 0.0001).
The difference in growth rate is thought to be due to the side groups on each of the TEOS and the TMOS. In aqueous environments the methyl and ethyl groups experience some dissociation from the silicate molecule producing methanol and ethanol respectively due to the presence of acetic acid produced by K. xylinus during fermentation (Cihlář 1993; Tanaka et al. 2000). Methanol is highly cytotoxic, far more so than ethanol and as such would have a greater impact on the growth of the K. xylinus (Patterson and Ricke 2015; Ryngajllo et al. 2019). Ethanol is much more readily metabolised by K. xylinus and even has potential to increase cellulose yields, as such the small quantities that are imparted to the media from the modified feedstock may well be metabolised before they cause any reduction in cell growth (Tanaka et al. 2000).
Characterising the composition of BC
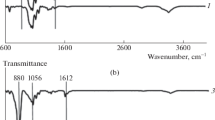
The FTIR spectra (Fig. 4) shows that there are distinct peaks that show the presence of the silicate modification in the homogenised glucose spectra. The presence of the -OH bonds shown by the peak at 3400 cm−1 – 3100 cm−1 in Fig. 4a, b, and c is expected in the glucose samples due to the -OH bonds present in glucose. However the shape of the wide -OH peak changes dramatically in the TMOS glu and the TEOS glu spectra and Fig. 4b and c respectively. We use the presence of this feature as evidence that the homogenisation process has caused some of the EtOH and MeOH groups had been lost from the Si-modifications. The peak indicated by the line at 3300 cm−1 Fig. 4b and c corresponds to Si–OH bonds as the peak would not be present otherwise and would be present in the TMOS and TEOS spectra (Phillips 2013; Smith 1960).
The FTIR spectra of (a) TEOS and glucose (b) TMOS and glucose and glucose (c) TEOS with TMOS and plain glucose (i) Si-OCH3 – 2840 cm−1 (ii) Si-OCH3 – 1190 cm−1 1100 – 1080 cm−1 (iii) Si-OCH2CH3 – 1170–1160 cm−1 1100 cm−1 1075 cm−1 970 -940 cm−1 (iV) Si–OH 3690 cm−1 (free OH), 3400 cm−1 – 3200 cm−1 (hydrogen bonded OH) 950 cm−1 – 810 cm.−1 (Smith AL 1960)
The peaks (i), (ii), and (iii) are conserved between the plain silicate spectra and the Si-glucose spectra in Fig. 4 suggesting that these peaks correspond to bonds present in the silicates. FTIR spectra to confirmed that glucose was still present in the homogenised Si-glucose mixture and the presence of the Si-modifications in the BC. The water was fully removed via lyophilisation prior to analysis with the intent of removing any noise generated by hydrogen bonding between the water and the -OH groups present on glucose and the Si-modifications (Griffith 1984).
The surface composition of the modified and plain BC obtained with EDX analysis as shown in Fig. 5. The TMOS and TEOS samples both showed the presence of silicon in small quantities and the plain BC control showed the complete absence of silicon. The percentage of atomic silicon was consistently higher in the TEOS samples than in the TMOS samples. However, the modification seems unevenly distributed on the surface in some cases. The surface microstructure of modified and plain BC was analysed using SEM (Fig. 6). The silicon modified BC shows a collection of aggregates on the surface of the material, they also show areas of thicker aggregate labelled in the red circles in Fig. 4a and b. The aggregate was especially clear in the NaOH TEOS sample in Fig. 4a. EDX analysis indicated that the silicon appears to be spread throughout the material in small amounts and in other areas concentrated on the surface.
The silicon content in some areas rose as high as 6.52% in the IMS TEOS sample, the range of the atomic percentage of silicon went up to as high as 2.97% on the surface in the case of TMOS IMS. The average surface atomic percentage of silicon were all below 1% being 0.78%, 0.89%, and 0.32% respectively for NaOH TMOS, IMS TMOS, and DI TMOS. The EDX data suggests that the incorporation of silica, at least on the surface, was not as substantial as the TEOS modification. We believe the lower percentage of atomic silicon in the TMOS samples is related to the methyl side group dissociating from the TMOS in small amounts and causing damage to the K. xylinus cells. The damaged caused by the dissociated methanol would result in a reduction of BC production as well as a reduced growth rate of K. xylinus reducing the growth rate around areas of high TMOS concentration and thus leading to a lower amount of silicon being incorporated into the BC (Patterson and Ricke 2015). We did not observe any reduced growth with the TEOS as K. xylinus regularly metabolises ethanol and will be relatively unaffected with low concentrations (Ryngajllo et al. 2019). The reduced growth rate can be observed in Fig. 2 which shows that the TMOS cultures lagged behind the plain media and the TEOS media in growth rate.
Physical and mechanical properties
The physical properties were analysed using droplet size analysis, and tensile strength testing. From these techniques we elucidated the water contact angle at the moment of contact and after 1 min of rest on the surface, as well as the tensile strength of the material.
Contact angle measurement for hydrophobicity tests
The WCA test (Fig. 7) provided insight about the hydrophobic and hydrophilic properties of the modified and plain BC. The contact angles were measured at the initial contact (ICA) and after 1 min (1mCA), similar to the surface composition analysis the different washing methods have some impact on the WCA. The ICAs are approximately 60° with the NaOH TEOS sample rising above to an average of 78°. The TEOS NaOH and TEOS IMS samples exhibited a significant change from the control (P < 0.05) showing that the addition of silicate had changed the hydrophobic properties. The NaOH TEOS modification increased the ICA by 20° but as the contact angle was still under 90° the modification did not impart hydrophobicity. The ICA of the IMS TEOS decreased by 3° from 60° in the plain IMS but the result was not significant (p > 0.05). The DI TEOS ICA decreased by 18° (p < 0.0001) when compared to the DI plain ICA showing a significant decrease of the ICA.
The ICA varied greatly in the TEOS samples depending on the washing procedure used indicating that the washing procedure and the modification in concert will affect the resulting hydrophobicity of each sample. Furthermore, the way that each washing process affects the sample is different between the TEOS samples and the plain samples, which tells us that the washing step produces different effects that are reliant on the type of modification or lack thereof.
Hydrophobicity is defined as a WCA of over 90° (Law 2014), none of the WCAs were above 90° and as such none of these materials can be considered hydrophobic. The increase in WCA for the NaOH TEOS was thought to be due to the ethyl groups present on the TEOS being affected by the NaOH washing step and potentially forming a marginally more hydrophobic layer on the surface of the BC (Choma et al. 2012). As the other samples tested with the NaOH wash did not show the same trend, we assume that the marginal increase in hydrophobicity was due to the TEOS present in the sample and its interaction with the NaOH washing step.
The 1mCA decreased when compared to all the ICAs for all samples and showed some distinct variation depending on the washing technique. The IMS wash decreased the 1mCA in the TEOS and the TMOS samples when compared to the plain BC control IMS wash. The TMOS showed similar 1mMCAs of 47°—26° to the plain BC control with a range of 31°—21°. The DI and IMS TMOS 1mCAs were significantly higher (P < 0.05) however the contact angle is still in line with other literature providing measured CAs of BC which range from 44⁰—36⁰ (Kim et al. 2022; Shao et al. 2017).
The TEOS samples had a significantly lower 1mCA (P < 0.05) in all cases, with the IMS wash for both TMOS and TEOS showing the greatest reduction in CA. The data suggests that the IMS wash affects the silicates providing a decrease in hydrophobicity.
The 1mCA decreased the most for the TEOS modification by 38°, 47°, and 32° for NaOH, IMS, and DI washes respectively. The results show that the TEOS modification absorbs water faster over the plain BC control as the contact angle was reduced more in the same time for the TEOS samples. Silicates are known for having a high water holding capacity (Schaller 2021), and if the ethyl and methyl groups have been lost from the silicates it would explain the decrease as there would no longer be any hydrophobic ethyl and methyl groups present. The reduced contact angle is thought to be due to the IMS washing dissolving the methyl and ethyl groups on the TEOS an TMOS, thus causing the remaining silicate to be comprised of various compounds based on SiO4(R1-4) where R is either ethyl or methyl groups. The unsaturated form of SiO4(R1-4) has a substantially larger water holding capacity than the fully saturated methyl and ethyl form (Ray et al. 2022; Wang et al. 2011).
The NaOH TMOS and DI samples 1mCA values were approximately 10° higher than the plain BC control equivalent samples. The increase in hydrophobicity was thought to be due to the methyl groups providing some hydrophobicity as well as the silicon acting as a filler for the pores preventing the absorbance of water into the material (Cheng et al. 2012; García et al. 2007; Meijer et al. 1998).
Impact of the modification on the fibre width of BC
The impact of modified glucose on the fibre width was also investigated in Fig. 8 which shows the average width of cellulose fibres present in both modified and unmodified cellulose samples. The TEOS samples have abroad range of results from 29 to 51 nm for the DI was and the NaOH wash respectively, the IMS TEOS and NaOH TEOS also have a wider range of results than other samples indicating a more varied width of fibre. The TMOS samples appear to have similar fibre widths averaging 26 nm (± 5.23 nm) for all 3. The TMOS results were similar to the plain BC control results (P > 0.05) with an average fibre width of 28.3 nm for all the different washing techniques. The only significantly different result was from the NaOH TEOS sample (P < 0.0001) with an average fibre width of 51 nm (± 10.7 nm) which was not conserved in the NaOH samples for the other samples. So we deduce that the increase in fibre width was due to the presence of the silicon modification acting as a fibre crosslinker, then the NaOH dissolving the silicon thus making the fibres less dense (Elshamy and Pantano 1977). The observed increase in fiber width did not correlate with the observed increase in tensile strength (see Fig. 9). We hypothesize that this discrepancy arises from the fact that although the fibers are thicker, they become less dense after undergoing the washing and drying process. The crosslinking action did not appear to occur with the TMOS modification as the TMOS modification consistently produced weaker fibres and exhibited less changes to the physical properties than the TEOS modification throughout the other experiments and tests.
Tensile strength measurements
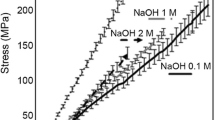
The tensile strength testing of the modified and unmodified plain BC control (Fig. 9) showed that the TMOS modification had a significant effect on the BCs tensile properties in all cases (P < 0.05). For all TMOS modified samples the average tensile strength decreased compared to the plain BC control where the reduction in tensile strength was most noticeable in the TMOS IMS wash resulting in a decrease of 13.3 MPa compared to the plain BC control IMS, however the TMOS IMS still showed the greatest tensile strength of all the TMOS samples. The reduction in tensile strength in the case of TMOS modification is further evidence supporting the claim that the TMOS inhibits the growth of the pellicle as discussed in Section 3.2 and shown in Fig. 9.
The TEOS modification increased the tensile strength compared to the control plain BC control (P < 0.05) except in the case of the IMS wash which did not show significant change in tensile strength (P = 0.0985), however did show a slight increase of the average tensile strength by 6.1 MPa compared to the IMS plain BC control. The average tensile strength for the sample was substantially higher in the TEOS DI at 14.27 MPa than the plain BC control DI at 3.9 MPa. The increase in tensile strength is theorized to be caused by the silicate aggregates crosslinking the BC fibres by acting as a cement.
It was found that the TEOS IMS modified BC showed the greatest tensile strength out of all the samples tested with a value at 22.1 MPa. The IMS washing method produced the greatest tensile strength out of every sample tested and we suspect that this is because the IMS causes the nano pores in the BC fibres to shrink after replacing the water within the BC. The removal of water would cause more hydrogen bonding between cellulose chains preferentially binding the other cellulose chains in the BC network, rather than the ethanol and methanol in the IMS. Therefore, the fibres would be more tightly bound together by the increased abundance of hydrogen bonding between the cellulose chains (Mao et al. 2010; Stanisławska et al. 2020). Although the cellulose pellicles were centrifuged 3 times to remove the excess unbound modification reagent, media and washing solutions. The centrifugation step and immediate drying afterwards prevented the rehydration of the BC with water and thus would preserve the properties gained in each washing step. Therefore, IMS washing shows the potential to be a useful method of washing to increase BCs inherent tensile strength. Greater tensile strength would be useful for applications requiring BC fibres to resist mechanical stress, such as being added to concrete as an additive plasticiser.
In both the TEOS and the TMOS samples, the NaOH washing produced the weakest pellicles. We believe the decrease in tensile strength is due to the NaOH interacting with the silicate and breaking down the aggregates which contributed to the tensile strength by acting as a crosslinker between the fibres (Elshamy and Pantano 1977). Therefore, NaOH breaks down the silicate within the BC, which in turn causes the disruption of the microstructure caused by the silicate modification to become apparent, as the silicon modification no longer contributes to the strength of the material.
The general consensus in the literature is that BC usually has a tensile strength of 15–20 MPa with either alkaline washing (NaOH), or water washing (DI) (Gea et al. 2010; Lin et al. 2013b). Comparing the tensile strength results of these experiments recorded results against those in the literature and adjusting for the difference in observed values, the plain BC controls tensile strength appears significantly greater when IMS was used as the washing method over NaOH or DI. The increase in tensile strength observed for the TEOS modified BC indicates that the modification has the potential to strengthen BC fibres especially when washed with IMS.
Further directions
There are potential routes for the optimising the method and changing the materials used to generate novel materials with useful properties. Further directions for the research would be to test varying concentrations of the silicate-based modifications and observe if the properties change further with greater amounts of the silicon present. The addition of the silicon modification appears to have little effect on the growth of the BC except in the case of the TMOS which consistently produced weaker and malformed pellicles compared to the TEOS modification or the plain BC control.
The FTIR results when analysed with the EDX results show that silicon is present within the BC in the modified samples. The method of analysis did not probe the internal composition of the BC so it is assumed that for this paper the atomic percentage of the elements does not differ drastically from the surface. Further testing with Tunneling electron microscopy would be able to elucidate the internal make up of the modified BC and would be a useful experiment to run in future. The greatest contact angle observed in the raw data was 86° for the NaOH TEOS and as such none of the samples could be considered hydrophobic. However, the increase in contact angle was significant and shows potential for further experimentation with silicates that contain more hydrophobic groups.
The TEOS modification shows the greatest promise for further modification as the pellicles produced were consistently stronger and impacted the growth of the K. xylinus the least out of both modifications. The method of homogenising the modification with glucose to use as the base for in-situ modification is a viable method of modifying the properties of BC and has the potential to be applied to different materials that could provide novel properties to the BC. The in-situ nature of the method of modification also presents an alternative to post production modification that has the potential to modify the BC throughout the structure rather than just on the surface as ex-situ modification does.
Conclusion
BC was modified by culturing K. xylinus in silicon modified media to create a composite material. Three washing methods (DI, IMS, and NaOH) were performed and compared for each modification. FTIR confirmed the presence of sugar in the homogenised silicate glucose feedstock. The SEM and EDX showed the presence of silicate in the micro and nano structure visually and with elemental analysis. DSA was performed on the BC and found that the IMS washed negatively affected the contact angle after 1 min when compared to the other washing methods for the Si-modifications. It was found that the addition of the silicon had no adverse effect on the growth rate of the K. xylinus for the TEOS modification and only mild inhibition for the TMOS modification. The IMS TEOS modification exhibited the greatest increase in tensile strength when compared to the plain and TMOS modifications, with all the TMOS samples showing a marked decrease in mechanical strength compared to the plain BC control. The increase in tensile strength was thought to be due to the BC fibres binding around the silicon aggregates and forming a crosslinked composite material which was confirmed in the SEM images. The washing conditions showed different effects on the properties of BC and as such can be used to tailor properties to suit the end use of the material. The results showcase a novel method to modify the properties of BC in a one pot method, with room for further modifications and optimisation of the methodology.
Data availability
No datasets were generated or analysed during the current study.
References
Amarakoon M, Alenezi H, Homer-Vanniasinkam S, Edirisinghe M (2022) Environmental impact of polymer fiber manufacture. Macromol Mater Eng 307(11):2200356. https://doi.org/10.1002/mame.202200356
Atalla RH, Vanderhart DL (1984) Native cellulose - a composite of 2 distinct crystalline forms. Science 223(4633):283–285. https://doi.org/10.1126/science.223.4633.283
Azeredo HMC, Barud H, Farinas CS, Vasconcellos VM, Claro AM (2019) Bacterial cellulose as a raw material for food and food packaging applications. Front Sustain Food S 3. https://doi.org/10.3389/fsufs.2019.00007
Baldrian P, Valášková V (2008) Degradation of cellulose by basidiomycetous fungi. FEMS Microbiol Rev 32(3):501–521. https://doi.org/10.1111/j.1574-6976.2008.00106.x
Bandyopadhyay S, Saha N, Brodnjak UV, Saha P (2018) Bacterial cellulose based greener packaging material: a bioadhesive polymeric film. Mater Res Express 5(11) https://doi.org/10.1088/2053-1591/aadb01
Budhiono A, Rosidi B, Taher H, Iguchi M (1999) Kinetic aspects of bacterial cellulose formation in nata-de-coco culture system. Carbohydr Polym 40(2):137–143. https://doi.org/10.1016/S0144-8617(99)00050-8
Cai M, Shafi S, Zhao Y (2018) Preparation of compressible silica aerogel reinforced by bacterial cellulose using tetraethylorthosilicate and methyltrimethoxylsilane co-precursor. J Non·Cryst Solids 481:622-626. https://doi.org/10.1016/j.jnoncrysol.2017.12.015
Cheng Y, He C, Xiao C, Ding J, Zhuang X, Huang Y, Chen X (2012) Decisive role of hydrophobic side groups of polypeptides in thermosensitive gelation. Biomacromol 13(7):2053–2059. https://doi.org/10.1021/bm3004308
Choma J, Jamiola D, Augustynek K, Marszewski M, Gao M, Jaroniec M (2012) New opportunities in stober synthesis: preparation of microporous and mesoporous carbon spheres. J Mater Chem 22(25):12636–12642. https://doi.org/10.1039/c2jm31678a
Cihlář J (1993) Hydrolysis and polycondensation of ethyl silicates. 1. Effect of ph and catalyst on the hydrolysis and polycondensation of tetraethoxysilane (teos). Colloids Surf Physicochem Eng Asp 70(3):239–251. https://doi.org/10.1016/0927-7757(93)80298-S
El-Saied H, El-Diwany AI, Basta AH, Atwa NA, El-Ghwas DE (2008) Production and characterization of economical bacterial cellulose. BioResources 3(4):1196–1217. https://doi.org/10.15376/biores.3.4.1196-1217
Elshamy TM, Pantano CG (1977) Decomposition of silicate-glasses in alkaline-solutions. Nature 266(5604):704–706. https://doi.org/10.1038/266704a0
Frank BP, Durkin DP, Caudill ER, Zhu L, White DH, Curry ML, Pedersen JA, Fairbrother DH (2018) Impact of silanization on the structure, dispersion properties, and biodegradability of nanocellulose as a nanocomposite filler. ACS Appl Nano Mater 1(12):7025–7038. https://doi.org/10.1021/acsanm.8b01819
Gao M, Li J, Bao Z, Hu M, Nian R, Feng D, An D, Li X, Xian M, Zhang H (2019) A natural in situ fabrication method of functional bacterial cellulose using a microorganism. Nat Commun 10(1):437. https://doi.org/10.1038/s41467-018-07879-3
García N, Benito E, Guzmán J, Tiemblo P (2007) Use of p-toluenesulfonic acid for the controlled grafting of alkoxysilanes onto silanol containing surfaces: preparation of tunable hydrophilic, hydrophobic, and super-hydrophobic silica. J Am Chem Soc 129(16):5052–5060. https://doi.org/10.1021/ja067987a
Gea S, Bilotti E, Reynolds CT, Soykeabkeaw N, Peijs T (2010) Bacterial cellulose–poly (vinyl alcohol) nanocomposites prepared by an in-situ process. Mater Lett 64(8):901–904. https://doi.org/10.1016/j.matlet.2010.01.042
Gilmour KA, Aljannat M, Markwell C, James P, Scott J, Jiang Y, Torun H, Dade-Robertson M, Zhang M (2023) Biofilm inspired fabrication of functional bacterial cellulose through ex-situ and in-situ approaches. Carbohydr Polym 304:120482. https://doi.org/10.1016/j.carbpol.2022.120482
Gregory DA, Tripathi L, Fricker ATR, Asare E, Orlando I, Raghavendran V, Roy I (2021) Bacterial cellulose: a smart biomaterial with diverse applications. Mater Sci Eng, R R 145:100623. https://doi.org/10.1016/j.mser.2021.100623
Griffith GW (1984) Quantitation of silanol in silicones by ftir spectroscopy. Ind Eng Chem Prod Res Dev 23(4):590–593. https://doi.org/10.1021/i300016a015
Gromovykh TI, Sadykova VS, Lutcenko SV, Dmitrenok AS, Feldman NB, Danilchuk TN, Kashirin VV (2017) Bacterial cellulose synthesized by gluconacetobacter hansenii for medical applications. Appl Biochem Microbiol 53(1):60–67. https://doi.org/10.1134/S0003683817010094
Hestrin S, Schramm M (1954) Synthesis of cellulose by acetobacter xylinum. Ii. Preparation of freeze-dried cells capable of polymerizing glucose to cellulose. Biochem J 58(2):345–352. https://doi.org/10.1042/bj0580345
Kadier A, Ilyas RA, Huzaifah MRM, Harihastuti N, Sapuan SM, Harussani MM, Azlin MNM, Yuliasni R, Ibrahim R, Atikah MSN et al (2021) Use of industrial wastes as sustainable nutrient sources for bacterial cellulose (bc) production: Mechanism, advances, and future perspectives. Polymers 13(19):3365. https://doi.org/10.3390/polym13193365
Kim GH, Kang DH, Jung BN, Shim JK (2022) Fabrication and characterization of hydrophobic cellulose nanofibrils/silica nanocomposites with hexadecyltrimethoxysilane. Polymers 14(4). https://doi.org/10.3390/polym14040833
Kurosumi A, Sasaki C, Yamashita Y, Nakamura Y (2009) Utilization of various fruit juices as carbon source for production of bacterial cellulose by acetobacter xylinum nbrc 13693. Carbohydr Polym 76(2):333–335. https://doi.org/10.1016/j.carbpol.2008.11.009
Law KY (2014) Definitions for hydrophilicity, hydrophobicity, and superhydrophobicity: Getting the basics right. J Phys Chem Lett 5(4):686–688. https://doi.org/10.1021/jz402762h
Lebreton L, Slat B, Ferrari F, Sainte-Rose B, Aitken J, Marthouse R, Hajbane S, Cunsolo S, Schwarz A, Levivier A (2018) Evidence that the great pacific garbage patch is rapidly accumulating plastic. Sci Rep 8(1):1–15. https://doi.org/10.1038/s41598-018-22939-w
Lee K-Y, Quero F, Blaker J, Hill C, Eichhorn S, Bismarck A (2011) Surface only modification of bacterial cellulose nanofibres with organic acids. Cellu 18:595–605. https://doi.org/10.1007/s10570-011-9525-z
Leng BX, Shao ZZ, de With G, Ming WH (2009) Superoleophobic cotton textiles. Langmuir 25(4):2456–2460. https://doi.org/10.1021/la8031144
Lin S-P, Loira Calvar I, Catchmark JM, Liu J-R, Demirci A, Cheng K-C (2013a) Biosynthesis, production and applications of bacterial cellulose. Cellu 20(5):2191–2219. https://doi.org/10.1007/s10570-013-9994-3
Lin WC, Lien CC, Yeh HJ, Yu CM, Hsu SH (2013b) Bacterial cellulose and bacterial cellulose-chitosan membranes for wound dressing applications. Carbohydr Polym 94(1):603–611. https://doi.org/10.1016/j.carbpol.2013.01.076
Ma W, Wang N, Guo Y, Yang LQ, Lv MF, Tang X, Li ST (2020) Enhanced photoreduction co2 activity on g-c3n4: by synergistic effect of nitrogen defective-enriched and porous structure, and mechanism insights. Chem Eng J 388. https://doi.org/10.1016/j.cej.2020.124288
Mao Z, Cao Y, Jie X, Kang G, Zhou M, Yuan Q (2010) Dehydration of isopropanol–water mixtures using a novel cellulose membrane prepared from cellulose/n-methylmorpholine-n-oxide/h2o solution. Sep Purif Technol 72(1):28–33. https://doi.org/10.1016/j.seppur.2010.01.002
Meijer A, Otto S, Engberts JBFN (1998) Effects of the hydrophobicity of the reactants on diels−alder reactions in water. J Org Chem 63(24):8989–8994. https://doi.org/10.1021/jo981359x
Nakatani H, Iwakura K, Miyazaki K, Okazaki N, Terano M (2011) Effect of chemical structure of silane coupling agent on interface adhesion properties of syndiotactic polypropylene/cellulose composite. J Appl Polym Sci 119(3):1732–1741. https://doi.org/10.1002/app.32873
Natalio F, Fuchs R, Cohen SR, Leitus G, Fritz-Popovski G, Paris O, Kappl M, Butt HJ (2017) Biological fabrication of cellulose fibers with tailored properties. Science 357(6356):1118–1122. https://doi.org/10.1126/science.aan5830
Padrao J, Goncalves S, Silva JP, Sencadas V, Lanceros-Mendez S, Pinheiro AC, Vicente AA, Rodrigues LR, Dourado F (2016) Bacterial cellulose-lactoferrin as an antimicrobial edible packaging. Food Hydrocolloid 58:126–140. https://doi.org/10.1016/j.foodhyd.2016.02.019
Patterson JA, Ricke SC (2015) Effect of ethanol and methanol on growth of ruminal bacteria selenomonas ruminantium and butyrivibrio fibrisolvens. J Environ Sci Health B 50(1):62–67. https://doi.org/10.1080/03601234.2015.965639
Phillips JP (2013) Spectra-structure correlation. https://doi.org/10.1016/c2013-0-12517-2
Pirzada T, Ashrafi Z, Xie W, Khan SA (2020) Cellulose silica hybrid nanofiber aerogels: from sol–gel electrospun nanofibers to multifunctional aerogels. Adv Funct Mater 30(5):1907359. https://doi.org/10.1002/adfm.201907359
Praeger M, Hosier IL, Vaughan AS, Swingler SG (2015) The effects of surface hydroxyl groups in polyethylene-silica nanocomposites. 2015 IEEE electrical insulation conference (EIC), pp 201–204. https://doi.org/10.1109/ICACACT.2014.7223514
Prata JC, da Costa JP, Lopes I, Duarte AC, Rocha-Santos T (2020) Environmental exposure to microplastics: an overview on possible human health effects. Sci Total Environ 702:134455. https://doi.org/10.1016/j.scitotenv.2019.134455
Rao AV, Kalesh RR, Pajonk GM (2003) Hydrophobicity and physical properties of teos based silica aerogels using phenyltriethoxysilane as a synthesis component. JMatS 38(21):4407–4413. https://doi.org/10.1023/A:1026311905523
Ray SS, Soni R, Kim I-C, Park Y-I, Lee CY, Kwon Y-N (2022) Surface innovation for fabrication of superhydrophobic sand grains with improved water holding capacity for various environmental applications. Environ Technol Innov 28:102849. https://doi.org/10.1016/j.eti.2022.102849
Rodríguez-Robledo MC, González-Lozano MA, Ponce-Peña P, Quintana Owen P, Aguilar-González MA, Nieto-Castañeda G, Bazán-Mora EA-O, López-Martínez R, Ramírez-Galicia G, Poisot MA-O Cellulose-silica nanocomposites of high reinforcing content with fungi decay resistance by one-pot synthesis. Lid lid - 575. (1996–1944 (Print)). https://doi.org/10.3390/ma11040575
Rouabhia M, Asselin J, Tazi N, Messaddeq Y, Levinson D, Zhang Z (2014) Production of biocompatible and antimicrobial bacterial cellulose polymers functionalized by rgdc grafting groups and gentamicin. ACS Appl Mater Interf 6(3):1439–1446. https://doi.org/10.1021/am4027983
Ryngajllo M, Jacek P, Cielecka I, Kalinowska H, Bielecki S (2019) Effect of ethanol supplementation on the transcriptional landscape of bionanocellulose producer komagataeibacter xylinus e25. Appl Microbiol Biotechnol 103(16):6673–6688. https://doi.org/10.1007/s00253-019-09904-x
Sakaguchi M, Ohura T, Iwata T, Takahashi S, Akai S, Kan T, Murai H, Fujiwara M, Watanabe O, Narita M (2010) Diblock copolymer of bacterial cellulose and poly(methyl methacrylate) initiated by chain-end-type radicals produced by mechanical scission of glycosidic linkages of bacterial cellulose. Biomacromol 11(11):3059–3066. https://doi.org/10.1021/bm100879v
Schaller J (2021) Amorphous silica increases the water holding capacity of soils - from mechanistic understanding to field experiments. EGU21-663. https://doi.org/10.5194/egusphere-egu21-663
Shao W, Wu J, Liu H, Ye S, Jiang L, Liu X (2017) Novel bioactive surface functionalization of bacterial cellulose membrane. Carbohydr Polym 178:270–276. https://doi.org/10.1016/j.carbpol.2017.09.045
Shoda M, Sugano Y (2005) Recent advances in bacterial cellulose production. Biotechnol Bioproc e 10(1):1–8. https://doi.org/10.1007/Bf02931175
Smith AL (1960) Infrared spectra-structure correlations for organosilicon compounds. Spectrochim Acta 16(1):87–105. https://doi.org/10.1016/0371-1951(60)80074-4
Stanisławska A, Staroszczyk H, Szkodo M (2020) The effect of dehydration/rehydration of bacterial nanocellulose on its tensile strength and physicochemical properties. Carbohydr Polym 236:116023. https://doi.org/10.1016/j.carbpol.2020.116023
Stumpf TR, Yang X, Zhang J, Cao X (2018) In situ and ex situ modifications of bacterial cellulose for applications in tissue engineering. Mater Sci Eng C Mater Biol Appl 82(1873–0191 (Electronic)). https://doi.org/10.1016/j.msec.2016.11.121
Tanaka M, Murakami S, Shinke R, Aoki K (2000) Genetic characteristics of cellulose-forming acetic acid bacteria identified phenotypically as gluconacetobacter xylinus. Biosci, Biotechnol, Biochem 64(4):757–760. https://doi.org/10.1271/bbb.64.757
Tang HR, Covington AD, Hancock RA (2003) Structure-activity relationships in the hydrophobic interactions of polyphenols with cellulose and collagen. Biopolymers 70(0006–3525 (Print)):403–413. https://doi.org/10.1002/bip.10499
Urbina L, Corcuera MÁ, Gabilondo N, Eceiza A, Retegi A (2021) A review of bacterial cellulose: sustainable production from agricultural waste and applications in various fields. Cellu 28(13):8229–8253. https://doi.org/10.1007/s10570-021-04020-4
Vandamme EJ, De Baets S, Vanbaelen A, Joris K, De Wulf P (1998) Improved production of bacterial cellulose and its application potential. Polym Degrad Stabil 59(1–3):93–99. https://doi.org/10.1016/S0141-3910(97)00185-7
Wang T-H, Gole JL, White MG, Watkins C, Street SC, Fang Z, Dixon DA (2011) The surprising oxidation state of fumed silica and the nature of water binding to silicon oxides and hydroxides. Chem Phys Lett 501(4):159–165. https://doi.org/10.1016/j.cplett.2010.11.013
Ye H, Wang Z, Yu F, Zhang S, Kong K, Gong X, Hua J, Tian H (2020) Fluorinated conjugated poly (benzotriazole)/g-c3n4 heterojunctions for significantly enhancing photocatalytic h2 evolution. Appl Catal B Env 267:118577. https://doi.org/10.1016/j.apcatb.2019.118577
Zhang XX, Xiao FC, Feng QF, Zheng JX, Chen CX, Chen HX, Yang WB (2020) Preparation of sio2 nanoparticles with adjustable size for fabrication of sio2/pmhs ormosil superhydrophobic surface on cellulose-based substrates. Prog Org Coat 138. https://doi.org/10.1016/j.porgcoat.2019.105384
Acknowledgments
We thank Oliver Perry (Newcastle University) for use of the workshop. We thank Prof. Steven Stanforth (Northumbria University) for early aid with characterizing materials. We thank Samantha Davies and the technical team (Northumbria University) for providing support service and equipment.
Funding
This work was supported by Research England E3 scheme and the Engineering and Physical Sciences Research Council (EPSRC, UK) grants – EP/X02041X. YHJ and MX acknowledge the support from Leverhulme Trust grant—RPG-2022–177. MZ and YHJ also acknowledges the support from the Biotechnology and Biological Sciences Research Council (BBSRC, UK) grant—BB/X011402/1.
Author information
Authors and Affiliations
Contributions
All authors contributed to the experimental design. The material preparation and data analysis of the experiment was carried out by Peregrine C.G. Greenhope. Expertise with the Kruss water droplet analyser and the analysis of that data was given by Luke Haworth. Writing of the manuscript was carried out by Peregrine C.G. Greenhope. All authors commented on the manuscript during the writing process. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent to publish
All authors commented on the manuscript during the writing process. All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Greenhope, P.C.G., Loh, J., Gilmour, K.A. et al. Silicon-infused bacterial cellulose: in situ bioprocessing for tailored strength and surface characteristics. Cellulose (2024). https://doi.org/10.1007/s10570-024-06031-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10570-024-06031-3