Abstract
This study aims to examine the antimicrobial properties and washing resistance of cotton fabrics coated with propolis-doped hydrogel. More specifically, we compared the hygienic properties of AgNO3 (a common antimicrobial agent in textile materials), and the antimicrobial properties of propolis for the first time. We used PVA and NaCMC for hydrogel production because they are biocompatible and non-toxic. Later, we looked at how effective the propolis or AgNO3-doped hydrogel-coated cotton fabrics are against gram-positive and gram-negative bacteria and Candida albicans fungus, and compared their findings. Our results demonstrated that propolis could be a natural antibacterial alternative to AgNO3. The more active substance content there was, the more antibacterial and washing resistant it became. We used SEM images of the hydrogel coating and SEM–EDX images to how much silver the silver-doped layers contained. ATR–FTIR results also supported flavonoids and phenol in the structure of propolis itself. The changes in the basic comfort properties of the products were at acceptable levels.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the past, people used to use textiles solely for upholstery and clothing. Today, their application range has broadened significant, and can also embody a plethora of properties including strength, antibacterial, UV protection, hydrophilicity, flame retardant, hydrophobicity. Therefore, it is necessary to bring textile materials with some properties (such as the antibacterial properties of textile materials used in open wounds), depending on their usage areas.
Different industries use textiles via numerous functionalizing methods as finishing, microcapsules, grafting, and—ideally—nanocoating with hydrogel. Hydrogels, by their nature, have a three-dimensional structure (Latif et al. 2016; Ganji et al. 2007), thereby making them popular in the medical field, requiring a controlled release (Costa-Júnior et al. 2009; Davis and Anseth 2002). Polyvinyl alcohol is frequently preferred for hydrogel applications due to its properties such as ease of synthesis, low toxicity, easy control of product quality, and low cost(Xiao et al. 2022; Sankarganesh et al. 2022). The same goes for PVA/NaCMC hydrogels, because they are non-cytotoxic and highly biocompatible (Shin et al. 2018). Hydrogels release actively with external factors such as temperature, pH, electrical field, and radiation and can be used in functional products (Hosseinkhani et al. 2006). Hydrogel structures containing hydrophilic and hydrophobic groups release the active substance absorbed in the structure in a controlled way because of exposure to external factors (Yin et al. 2002; Tavakol et al. 2009).
Hydrogels are commonly added to textiles as they improve washing resistance and bring many functional properties (Vílchez et al. 2020). Medical professionals prefer Textile materials functionalized with hydrogel because they keep surgical areas moist after surgery, accelerate healing, and facilitate wound observation (Bothra 2014; Gonçalves et al. 2023; Salama and El-Sakhawy 2023). Those engaging in agriculture use hydrogels because they enhance water absorption capacity (El Salmawi 2007; Saleh et al. 2023). Textile materials featuring hydrogels loaded with different antibiotic and antibacterial agents are good at killing bacteria and preventing infections, and are biocompatible (cytotoxicity and hemolytic) (Costa-Júnior et al. 2009; Abdel-Mohsen et al. 2011; Wikanta et al. 2012; Yu et al. 2011; Islama et al. 2016; Zhang et al. 2015; Glampedaki et al. 2011). One study reported that the hydrogel-coated catheter reduced catheter-induced urinary infections (Demirci 2010). The invention of different active ingredients and hydrogel structures has made it possible to develop a wide range of dual-effect (moisturizing/healing) functional products (Wang et al. 2016).
The COVID-19 pandemic has driven people to search for antibacterial, antiviral, and antimicrobial clothing and other textile materials to protect themselves from viruses and microorganisms.
Environmental concerns are also pushing people to want natural and renewable textile fibres and chemicals—this is especially true for organic antimicrobial agents like peptides (Gabriel et al. 2008), guanidine (Cao et al. 2020), and halides (El Hage et al. 2014) in the textile industry. Many products enriched with plant and animal-based extract like chitosan and its derivatives also exist (Zou et al. 2018). Propolis extract is one such an organic antimicrobial (Oliveira et al. 2016) and commonly used by the pharmaceutical and cosmetic industries (Dantas et al. 2010). It can accelerate wound healing and proliferation, and yield bioactive materials for skin care (Jastrzębska-Stojko et al. 2013; Kurek-Górecka et al. 2020; Yaman Turan et al. 2021; Ramadan et al. 2019; Chirila et al. 2020; Rogina-Car et al. 2020).
In one study, the researchers applied ethanolic extracts of propolis to cotton fabric using the pad/dry/cure technique, and then assessed the resulting fabrics for their antibacterial properties. Glyoxal and Al2(SO4)3 catalysed pad-dry (3 min/80 °C) and cure (5 min/140 °C) processes achieved excellent antibacterial results, including different levels of UV protection based on the type of cross-linker (Sharaf et al. 2013). Other studies showed that the effects of nanowebs made from a homogeneous mixture of PVA+Propolis on gram-positive and gram-negative bacteria were excellent (Arıkan and Solak 2017). Yaman et al. microencapsulated propolis extracted with ethanol and assessed its antibacterial properties (Yaman Turan et al. 2021).
Several papers have associated propolis’s efficacy with the flavonoid contents in its structure. Bee species, region, season, and ecosystem affect all its chemical composition. Marcucci et al. (2001) and Shokrii et al. (2011) reported that antifungal and antibacterial activities stemmed from caffeic acid, an aromatic acid propolis contains. Moreover, Ota et al. (2001) showed that phenolic structures containing flavonoids, phenolic acids, and their esters all make propolis antifungal.
Flavonoids are effective in antimicrobial action mechanisms that damage cell walls and membranes, resulting in lysis, impaired energy transfer, and permeable microsomes and lysosomes (Cushnie et al. 2003; Echeverría et al. 2017; Kim and Chung 2011; Sanpa et al. 2015; Andre et al. 2022; Di Carlo et al. 1999). The hydroxyl groups in the structure of flavonoid compounds act on the organic components in the cell membrane through different mechanisms and transport toxic substances into the bacterial cells (Di Carlo et al. 1999; Takaisi-Kikuni and Schilcher 1994). Takaisi-Kikuni and Schilcher (1994) examined possible mechanisms of the antifungal activity of propolis and reported that propolis may have DNA replication inhibitory activity and indirectly interrupt cell division. We anticipate the results of our own study to be achieved by these mechanisms as well.
Numerous papers—especially ones on using alternative medicine in tissue engineering and dental applications—have investigated propolis-doped hydrogels. Few, however, have studied the design and evaluation of wound healing products featuring propolis, but none compared them with other common antibacterial agents. We therefore aimed to help fill that gap and examine the usability of environmentally friendly propolis-doped nanocomposites for antifungal and antibacterial textiles. We also wanted to compare its effectiveness with silver-doped nanocomposite materials, as they are common in standard antibacterial products. To do this, first we produced propolis-doped hydrogel nanocomposites and examined the textile materials and their antibacterial and antifungal properties. Then, we compared these products with silver from antibacterial and antifungal textiles, and assessed whether propolis could replace silver as an alternative. It has become necessary to analyse the washing resistance, colour changes, and some comfort properties of propolis and silver-doped hydrogels when they are applied to 100% cotton fabrics. This study is the first of its kind.
Experimental section
Materials
In this study, we supplied scoured and bleached 100% cotton fabric (127 g/m2, 27 picks/cm, 35 ends/cm) from Akkumas, a Turkish company. Sigma supplied us with PVA (Sigma Aldrich P-8136), NaCMC (CAS No.: 9004-32-4; MW: 359.4 g/mol; [C6H7O2(OH)x*(C2H2O3Na)y]n), silver nitrate (CAS No.: 7761-88-8; MW: 169.87 g/mol; AgNO3), and glutaraldehyde (CAS Number: 111-30-8; MW: 100.12 g/mol; [OHC(CH2)3CHO]n), whilst Merck gave us Ethanol (CAS No.: 64-17-5; 85%; MW: 46.07 g/mol; C2H5OH). Our propolis came from the Yalova Beekeepers’ Association. We used all of the chemicals in their original form without making any purification process.
Preparing hydrogel
We obtained a transparent PVA solution (4%) by mixing it in distilled water at 90 °C for two hours, at a stirring speed of 1500 rpm. We did the same for 4% NaCMC as well, except we stirred it at room temperature for 30 min, at 1500 rpm. The NaCMC was then transferred to the PVA one drop at a time, and then stirred—again at the same speed—for another two hours. We relied on preliminary trials and previous studies to figure out the working range of the active substances. Finally, different amounts of propolis extract (1, 2, 5, 10, 20, and 30 ml) and AgNO3 (0.01, 0.05, 0.1, 0.5, 1, and 5%) were added to this stock solution to create hydrogels. We also added 5% glutaraldehyde into the mixture and stirred it at 500 rpm for 24 h to ensure crosslinking of each solution on the fabric.
Padding hydrogel on the fabric
We regulated the pH levels in the hydrogels to 9 with NaOH and added an acrylate-based cross. Then, we soaked 100% cotton fabric samples in a padding solution for 10 min, and squeezed in on a rapid fulard machine (Model PA1, Labortex, Taiwan), at a cylinder pressure of 2 kg/cm2 with a pick-up of 85%. Afterwards, we dried the samples in a WTB binder oven (FN 120 Nuve Germany) at 110 ºC for 30 min. Table 1 shows the experimental set-up and sample codes.
Characterization
Determination of add-on
We weighed each fabric sample (10 × 10 cm2) before (W1) and after (W2) padding of hydrogel, and found the add on value using Eq. 1.
Swelling
The untreated and treated fabrics were subjected to swelling tests as stated by Abdel-Halim et al.. After we weighed the 5 × 5 cm2 fabric samples (Wd) for the test, we soaked them in distilled water for 30 minutes, removed excess water, weighed them again (Ww), and found the swelling rates using Eq. 2 (AbdelHalim et al. 2010).
Comfort properties of the fabric
To evaluate the comfort properties of fabrics after treatment, we examined their air permeability, water vapour permeability, and colour changes. Both the untreated and treated cotton fabrics were subjected to air permeability tests (SDL ATLAS, USA) according to TS 391 EN ISO 9237 standards. The breathability of the treated fabric was identified. They also underwent water vapour resistance tests on a sweat-guarded hotplate by TS EN 11092 standard to determine the permeability of the fabric. Any colour changes were later examined on a data colour measurement system (Datacolour Gretag Macbeth CE 7000, Spectrophotometer, USA), and using Kubelka-Munk equation (Eq. 3) to find their K/S values out.
Antibacterial and antifungal activity
We analysed the antibacterial activity of the samples with the AATCC 147-2004 Parallel Line Standard Method as a qualitative method. These microorganisms included Staphylococcus aureus AATCC 25923, Escherichia coli AATCC 25922, Klebsiella pneumoniae NRLLB4420, and Candida albicans. First, 1.0 ml of 24-hour cultures of microorganisms were transferred into nutrient broth (NB) and diluted in 0.5 McFarland (1.5 × 108 cfu). They then were inoculated into a petri dish containing nutrient agar (NA) via the loop-stretch method. Then, we placed the textile sample strips (3.5 × 5.5 ± 5mm) on the agar, and incubated the petri dishes at 37±2°C for 24 h. The untreated samples served as negative controls; whereas, the Ag-doped samples became positive controls (Sanli Yurudu et al. 2008). Later, we measured the width of their inhibition zones along a mean line on both sides of each sample using Eq. 4:
W: The width of the net inhibition zone I (mm), T: Total in the test sample, R: The diameter of the test sample in mm.
We measured the antimicrobial activity using the AATCC Test 100 protocol, albeit making minor modifications. We then added 1 ml of 0.5 McFarland bacteria solution [ corresponding to a culture density of approx. 1.5 × 108 cfu/ml (A0)] onto 3.5 × 5.5 ± 0.5 cm2 samples in a sterile petri dish to ensure that each piece of fabric absorbed. The samples were left to incubate at 37 ºC for 24 h, and then placed in isotonic sterile distilled water. Afterwards, the bacteria were shaken for one minute and transferred to the liquid. We took 1 μl of the mixture, inoculated it into a petri dish containing NA, left the resulting colonies to incubate at 37 °C for 24 hours, and then counted them (A24). We expressed the antimicrobial activity in percentage reduction in microorganisms after contact with the test samples—compared to number of microbial cells that survived after contact with the control. We expressed the results as the reduction (%) of microorganisms in the equation. We calculated the rate (%) of reduction in bacteria using Eq. 5.
Durability of antibacterial effects
We analysed how durable the antibacterial effects of the samples coated with microcapsules were after the fifth and tenth washing cycles. All the samples were washed in a bath containing 5 g/l ECE standard detergent at 40 °C for 30 minutes, in a washing fastness tester (Prowhite) at a liquor ratio of 50:1, and then rinsed in a cold distilled water, squeezed, and dried to dry at room temperature.
ATR-FTIR analysis
We made ATR-FTIR spectra images of the microcapsules (both with and without propolis) on an ATR-FTIR spectrometer (Perkin Elmer, Spectrum Two, USA). Each image included 25 scans at a resolution of 4 cm−1 (wave length: 4000–400 cm−1).
Scanning electron microscopy (SEM/EDX)
We examined the surface morphologies of both the untreated and treated samples under a scanning electron microscope (JXA-8230-SEM, Japan) gradually from 5 to 12 kV and then measured the silver-doped hydrogel-coated fabrics using SEM/EDX.
Results and Discussion
Antibacterial activity and durability
Antibacterial activity refers to the lack of bacterial colonies in and around the agar area the sample is in contact with (Sanli Yurudu et al. 2008). An increase in the diameter of the zone without growth indicates high antimicrobial activity. According to findings of the present study, the untreated samples and those treated with 1, 2, 5, 10, 25, and 30 ml propolis and 0.01, 0.05, 0.1, 0.5, 1, and 5% AgNO3 showed an antimicrobial activity against Staphylococcus aureus ATCC 25923, Escherichia coli, ATCC 25922, Klebsiella pneumoniae NRLLB4420, and Candida albicans. Table 2 shows the quantitative antibacterial results of all the samples before washing and after serial washing as well as Inhibition zone measurements were made in triplicate and their averages. The more antibacterial agent you had to fabrics, the more antibacterial they become—a result of there being more flavonoids and phenolic acids from the propolis. Fig. 1 shows the agar test results of the fabrics coated with a hydrogel containing the least amount of antibacterial agent (1 ml of propolis and 0.01 % AgNO3). The antibacterial effect was also achieved at the lowest levels.
Quantitative tests were also applied to assess the antibacterial effect and the results are presented in Table 3. The results of these tests were validated with the quantitative results of the cotton fabrics containing propolis and AgNO3. 2 ml of propolis was antibacterial enough to alternate with AgNO3 (Table 3).
The antibacterial effects of the hydrogel-coated fabric samples after washing were assessed (Tables 2, 3). Their antimicrobial effect subsided with washing. The more propolis and AgNO3 there was, the better its washing resistance became. The hydrogels release themselves to some extent during each washing and the propolis/AgNO3 entrapped in the hydrogel diffuses into the water and thus gets away. However, when the amount of active agent in the hydrogel structure grows, the number of washes also increases as a natural result of the release (Pinho et al. 2011). However, the samples containing 30 ml of propolis after 10 cycles of washing had an antimicrobial effect against S. aureus and E. coli. The samples containing 30 ml of propolis subjected to 10 cycles of washing had an antimicrobial effect against K. pneumoniae NRLLB4420, but they did not show any antimicrobial effect C. albicans (Table 2 and Fig. 1a–d). The sample containing 0.5% AgNO3 did not lose its antimicrobial effect against Staphylococcus aureus even after 10 cycles of washing. The sample containing 5% AgNO3 had an antimicrobial effect against all the microorganisms tested except for Klebsiella pneumoniae, after 10 cycles of washing (Table 2, Fig.1 e–g).
Quantitative test also revealed that all the (treated) samples had effective antimicrobial activities against bacteria and fungi—which is compatible the qualitative test. The samples with 30 ml of propolis preserved their antimicrobial properties after 10 cycles of washing. The greatest effect was detected on S.aureus, as in the AATCC 100 test method (Fig. 2). Despite bacterial growth after 10 cycles of washing, the number of bacteria inoculated and the number of bacteria that grew after 10 cycles of washing (i.e. less than the initial inoculation) differed from one another (Fig. 2a). This indicated that the effect persisted but lost its effectiveness after washing. S. aureus in a 1% AgNO3 sample decreased by 98.67% even after 10 cycles of washing, while S. aureus in a 5% AgNO3 sample decreased by 99.02% even after 10 cycles of washing.
The biological activities of propolis have been widely investigated for a long time and there is plenty of evidence for its antimicrobial properties (Nefzi et al. 2023; Borozan et al. 2023; Altuntaş et al. 2023). The chemical composition of propolis plays an important role in dictating its antimicrobial effectiveness—hence explaining why activity against one bacterial species can vary greatly between propolis samples at the same concentration but different origins. In fact, its chemical composition can vary greatly depending on where and when it was collected, and what it is made from (Ozdal et al. 2019; Bankova et al. 2002).
The substances identified in the present study on the Anatolian propolis sample were flavonoids (pinocembrin, kaempferol, pinobanksin, apigenin), phenolic/aromatic acids (chlorogenic, caffeic, p-coumaric, ferulic, and trans-cinnamic acid) and phenolic aldehyde (vanillin). Several authors who studied the polyphenolic composition of Turkish and European propolis samples have already identified many of these substances (pinobanksin, kaempferol, ferulic acid, caffeic acid, and p-coumaric acid) (Altuntaş et al. 2023).
Assessment of fabric comfort properties
Post-treatment colour change, swelling, air permeability, and water vapour permeability are all important factors what textiles are to be used for. The quantity of application to the fabric (add-on) is also important for apparel and retention. Therefore, we made three measurements of each sample for K/S value, swelling ratio, add-on, air permeability, and water vapour permeability. Table 4 shows their mean values and standard deviations.
Our results demonstrated that—as expected—the colour change was lower in propolis-added hydrogels and higher in silver-containing fabrics. The increase in the amount of propolis and silver also yielded higher K/S values. While there were higher swelling rate values in the treated fabrics due to both the hydrophilic structure of the monomers used and the network structure of the hydrogels. However, the hydrophilic groups in the structure of the propolis itself led a higher swelling rate. On the other hand, the more propolis there was, the less swelling occurred thanks to the amount of unremovable wax in it. The spike in add-on values after treatment confirmed that the fabric surface was coated. Pores between fibres and yarns, alongside voids (amorphous regions) in the fibre structure all dictate how permeable a fabric is. Hydrogel coats the fibre, and masks amorphous regions, thereby reducing air permeability. On the other hand, the hydrophilic structure of hydrogels supports water vapour transmission—a positive quality. SEM images taken after coating indicated the presence of hydrogel both on the fibre surface and in the spaces between the fibres (Fig. 4-b). The latter reduced the number and size of the fabric pores, thus impairing the air permeability and improving the water vapour permeability resistance, which is compatible with the literature (Kokabi et al. 2007; Cavalu et al. 2018).
Assessment of surface parameters
SEM evaluation
We analysed the microstructure of untreated, propolis-treated (F-P6), and silver-treated (F-S6) cotton fabric samples under scanning electron microscope (SEM) (Figs. 3, 4).
The hydrogel padding gave the samples a smoother surface. SEM images show the hydrogel structures between the fibres as well as the coating on the fabric surface, changed in terms of permeability. We also took high-resolution (2000 X) images to view the morphological change on the cotton surface. SEM images show that cotton-specific traces in the untreated samples dropped due to the hydrogel coating.
To determine whether silver was added to the hydrogel structure or not, we applied SEM/EDX to the silver-doped coatings (See Fig. 4). The uncoated samples contained 0 wt.% of silver; whereas, the coated samples had 15.46 wt.% of silver.
ATR-FTIR evaluation
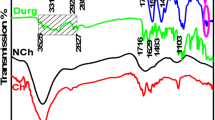
We measured untreated samples and F-P6 and F-S6 coded ones on an ATR-FTI and the results are presented in Fig. 5.
Figure 5 shows the properties of FTIR spectra for propolis-doped hydrogel (with a straight line), Ag-doped (with a dashed line), and untreated cotton (with a dotted line) fabrics. The FTIR spectrum detected characteristic peaks for cellulose at about 3350 cm−1 for O–H stretching, 2900 cm−1 for C–H trying, 1400 cm−1 for CH2 bending, 1035 cm−1 for C–O stretching, and 875 cm−1 for C–O–C stretching of glycoside. The peaks between the 3400–3100 cm−1, 2900–2800 cm−1, 1800–1600 cm−1, 1400−1300 cm−1, and 1100–900 cm−1 all explained the hydrogel structure (Fig. 3). The peaks were related to hydrogel-structured materials, plus supported phenolic acid and flavonoids arising from propolis. A wide band between 3400 and 3100 cm−1 represents the stretching of the –OH groups in the Na-CMC, PVA, phenolic structure, and carboxylic acid (FTIR and FTIR 1). The marker bands of propolis-doped hydrogel appear at about 2900 cm−1 because of the stretching vibrations of C–H bonds in CH2 and CH3 groups. The bands at 2944 cm−1 and 2820 cm−1 also represent asymmetric and symmetric CH stretching in PVA and Na-CMC structures, respectively. A band between 1800 cm−1−1300 cm−1 represents the C=O stretching structures of ester carbonyl structures. The peaks at 1756 cm−1 and 1426 cm−1 represent C=O asymmetric and symmetric stretching, respectively. All gradients of hydrogel structures such as Na-CMC, PVA, and propolis have C=O stretching vibrations. While 1290 cm−1 is assigned to C–O groups in polyols (such as hydroxyl flavonoids), while the strong band at 1160 cm−1 is assigned to the C–O and C–OH vibrations of polyols (Yaman Turan et al. 2021; Oliveira et al. 2016; Franca et al. 2014; Haghdoosta et al. 2016).
4. Conclusion
In this study, it was determined that propolis extract had a biocidal effect on both gram positive and gram negative bacteria and on C. albicans from yeasts. Haghdoosta et al. (2016), Aral and Yigit (2022), Cushnie and Lamb (2005) obtained similar results on propolis, as well. AgNO3 and propolis both exhibited antibacterial and antifungal effects. The more propolis were used, the more phenolics and flavonoids there were, and the more antibacterial and washing resistant fabric was rendered (Table 2, Fig 1). Similarly, the more substance you have to remove, the more washing cycles you need (Table 3, Fig. 2).
While the hydrogel structure was visible in the SEM images, the SEM-EDX data also revealed the applied silver. FTIR analyses showed an increase in the band intensity representing Na-CMC and PVA and proved the hydrogel coating. On the other hand, in propolis-doped hydrogels, the bands representing flavonoid and phenolic structures exhibited greater density.
Ideal wound dressings should be antibacterial and have enhanced water vapour permeability and air permeability. As Table 4 demonstrates, the air permeability of the treated and untreated fabrics dropped at acceptable level, while the water vapour permeability improved. Colour change was minimal, and therefore did not negatively impact wound follow-up in the areas where it was used. Moreover, acceptable modifications in fabric properties and washing resistance show that hydrogel application offers efficient medicinal solutions.
In conclusion, we were able to achieve desirable antibacterial effect even at very low amounts of propolis. We feel that it would make a great alternative core material to silver, particularly in disposable products, therefore rendering outstanding results for daily padding applications due to its rich high propolis content, particularly if treating fungi.
Availability of data and materials
Not applicable.
References
Abdel-Halim ES, Konczewicz W, Zimniewska M, Al-Deyab SS, El-Newehy MH (2010) Enhancing hydrophilicity of bioscoured flax fabric by emulsification post-treatment. Carbohyd Polym 82:195–201. https://doi.org/10.1016/j.carbpol.2010.04.065
Abdel-Mohsen EAM, Aly AS, Hrdina R, Montaser AS, Hebeish A (2011) Co-synthesis of PVA/chitosan hydrogels for biomedical application. J Polym Environ 19:1005–1012. https://doi.org/10.1007/s10924-011-0334-0
Altuntaş Ü, Güzel İ, Özçelik B (2023) Phenolic Constituents, Antioxidant and antimicrobial activity and clustering analysis of propolis samples based on PCA from different regions of Anatolia. Molecules 28:1121–1139. https://doi.org/10.3390/molecules28031121
Andre A, Arief I, Apriantini A, Jayanegara A, Budiman C (2022) Antimicrobial activity of propolis extract and their application as a natural preservative in livestock products: a meta-analysis. Food Sci Anim Resour 42:280–294. https://doi.org/10.5851/kosfa.2022.e4
Aral N, Yiğit I (2022) Antibacterial nonwoven with propolis for use in surgical masks. Mater Sci Forum 1063:63–69. https://doi.org/10.4028/p-rfd11q
Arıkan HK, Solak HH (2017) Propolis extract-PVA nanocomposites of textile design: antimicrobial effect on gram positive and negative bacterias. Int J Second Met 4:218–224. https://doi.org/10.21448/ijsm.371563
Bankova V, Popova M, Bogdanov S, Sabatini AG (2002) Chemical composition of European propolis: expected and unexpected results. Z Nat C J Biosci 57:530–533. https://doi.org/10.1515/znc-2002-5-622
Borozan AB, Popescu S, Madosa E, Ciulca A, Moldovan C, Gergen I (2023) Comparative study on the antimicrobial activity of propolis, catechin, quercetin and gallic acid. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 51:12826–12846. https://doi.org/10.15835/nbha51212826
Bothra A (2014) Preparation and characterization of Poly Vinyl Alcohol - Gelatin- Carboxy Methyl Chitosan Polymer Films. Dissertation, National Institute of Technology.
Cao Y, Gu J, Wang S, Zhang Z, Chen S (2020) Guanidine-functionalized cotton fabrics for achieving permanent antibacterial activity without compromising their physicochemical properties and cytocompatibility. Cellulose 27:6027–6036. https://doi.org/10.1007/s10570-020-03137-2
Cavalu S, Bisboaca S, Mates IM, Pasca PM, Laslo V, Costea T, Fritea L, Vicas S (2018) Novel formulation based on chitosan-Arabic gum nanoparticles entrapping propolis extract, production, physico-chemical and structural characterization. Rev De Chimie 69:3756–3760. https://doi.org/10.37358/RC.18.12.6836
Chirila L, Constantinescu GC, Danila A, Popescu A, Constantinescu RR, Sandulache IM (2020) Functionalization of textile materials with bioactive polymeric systems based on propolis and cinnamon essential oil. Industria Textila 71:186–192. https://doi.org/10.35530/IT.071.02.1793
Costa-Júnior ES, Barbosa-Stancioli EF, Mansur AAP, Vasconcelos WL, Mansur HS (2009) Preparation and characterization of chitosan/poly (vinyl alcohol) chemically crosslinked blends for biomedical applications. Carbohyd Polym 76:472–481. https://doi.org/10.1016/j.carbpol.2008.11.015
Cushnie TP, Lamb AJ (2005) Antimicrobial activity of flavonoids. Int J Antimicrob Agents 26:343–356. https://doi.org/10.1016/j.ijantimicag.2005.09.002
Cushnie TPT, Hamilton ESV, Lamb AJ (2003) Assessment of the antibacterial activity of selected flavonoids and consideration of discrepancies between previous reports. Microbiol Res 158:281–289. https://doi.org/10.1078/0944-5013-00206
Dantas TNC, Silva HSRC, Neto AAD, Marcucci MC, Maciel MAM (2010) Development of a new propolis microemulsion system for topical applications. Rev Bras 20:368–375. https://doi.org/10.1590/S0102-695X2010000300013
Davis KA, Anseth KS (2002) Controlled release from crosslinked degradable networks. Crit Rev Ther Drug Carrier Syst 19:385–423. https://doi.org/10.1615/critrevtherdrugcarriersyst.v19.i45.30
Demirci N (2010) Improving of anti-microbial and hydrophilic polymer coatings for using of catheter production. Dissertation, Gazi University.
Di Carlo G, Mascolo N, Izzo AA, Capasso F (1999) Flavonoids: old and new aspects of a class of natural therapeutic drugs. Life Sci 65:337–353. https://doi.org/10.1016/S0024-3205(99)00120-4
Echeverría J, Opazo J, Mendoza L, Urzúa A, Wilkens M (2017) Structure-activity and lipophilicity relationships of selected antibacterial natural flavones and flavanones of chilean flora. Molecules 22:608. https://doi.org/10.3390/molecules22040608
El Salmawi MK (2007) Application of polyvinyl alcohol (PVA)/carboxymethyl cellulose (cmc) hydrogel produced by conventional crosslinking or by freezing and thawing. J Macromol Sci Part A Pure Appl Chem 44:619–624. https://doi.org/10.1080/10601320701285045
El Hage S, Lajoie B, Stigliani J, Furiga-Chusseau A, Roques C, Baziard G (2014) Synthesis antimicrobial activity and physico-chemical properties of some n- alkyldimethylbenzylammonium halides. J Appl Biomed 12:245–253. https://doi.org/10.1016/j.jab.2014.02.002
Franca JR, De Luca MP, Tribeiro T, Castilho RO (2014) Propolis based chitosan varnish: drug delivery, controlled release and antimicrobial activity against oral pathogen bacteria. Alter Med 14:478–482. https://doi.org/10.1186/1472-6882-14-478
Gabriel GJ, Madkour AE, Dabkowski JM, Nelson CF, Nüsslein K, Tew GN (2008) Synthetic mimic of antimicrobial peptide with nonmembrane-disrupting antibacterial properties. Biomacromol 9:2980–2983. https://doi.org/10.1021/bm800855t
Ganji F, Abdekhodaie MJ, Ramazany-Sadtabadi A (2007) Gelation time and degradation rate of chitosan as a thermosensitive injectable hydrogel. J Sol-Gel Sci Technol 42:47–53. https://doi.org/10.1007/s10971-006-9007-1
Glampedaki P, Jocic D, Warmoeskerken MCG (2011) Moisture absorption capacity of polyamide 6,6 fabrics surface functionalised by chitosan-based hydrogel finishes. Prog Org Coat 72:562–571. https://doi.org/10.1016/j.porgcoat.2011.06.019
Gonçalves IS, Lima LR, Berretta AA, Amorim NA, Pratavieira S, Corrêa TQ, Nogueira FAR, Barud HS (2023) Antimicrobial formulation of a bacterial nanocellulose/propolis-containing photosensitizer for biomedical applications. Polymers 15:987. https://doi.org/10.3390/polym15040987
Haghdoosta NS, Salehib TZ, Khosravia A, Sharifzadeha A (2016) Antifungal activity and influence of propolis against germ tube formation as a critical virulence attribute by clinical isolates of Candida albicans. J De Mycologie Médicale 26:298–305. https://doi.org/10.1016/j.mycmed.2015.11.004
Hosseinkhani H, Hosseinkhani M, Khademhosseini A (2006) Enhanced angiogenesis through controlled release of basic fibroblast growth factor from peptide amphiphile for tissue regeneration. Biomaterials 27:5836–5844. https://doi.org/10.1016/j.biomaterials.2006.08.003
http://slabo.org.br/cont_anais/anais_9_colaob/abstract/13-006.pdf.
Islama A, Yasin T, Gull N, Maqsood Khan S, Munawar MA, Shafiq M, Sabir A, Jamil T (2016) Evaluation of selected properties of biocompatible chitosan/poly(vinyl alcohol) blends. Int J Biol Macromol 82:551–556. https://doi.org/10.1016/j.ijbiomac.2015.09.073
Jastrzębska-Stojko ZR, Stojko A, Rzepecka-Stojko A, Kabała J (2013) Biological activity of propolis-honey balm in the treatment of experimentally-evoked burn wounds. Molecules 18:14397–14413. https://doi.org/10.3390/molecules181114397
Kim YH, Chung HJ (2011) The effects of Korean propolis against foodborne pathogens and transmission electron microscopic examination. Biotechnol 28:713–718. https://doi.org/10.1016/j.nbt.2010.12.006
Kokabi M, Sirousazar M, Muhammad Hassan Z (2007) PVA–clay nanocomposite hydrogels for wound dressing. Eur Polym J 43:773–781. https://doi.org/10.1016/j.eurpolymj.2006.11.030
Kurek-Górecka A, Górecki M, Rzepecka-Stojko A, Balwierz R, Stojko J (2020) Bee products in dermatology and skin care. Molecules 25:556–572. https://doi.org/10.3390/molecules25030556
Latif IA, Abdullah HM, Saleem MH (2016) Electrical and swelling study of different prepared hydrogel. Am J Polym Sci 6:50–57. https://doi.org/10.5923/j.ajps.20160602.04
Marcucci MC, Ferreres F, Garcıa-Viguera C, Bankova VS, De Castro SL, Dantas AP (2001) Phenolic compounds from Brazilian propolis with pharmacological activities. J Ethnopharmacol 74:105–112. https://doi.org/10.1016/s0378-8741(00)00326-3
Nefzi N, Pagliari S, Campone L, Megdiche-Ksouri W, Giarratana F, Cicero N, Ziino G, Nalbone L (2023) Chemical composition and comprehensive antimicrobial activity of an ethanolic extract of propolis from Tunisia. Antibiotics 12:802–822. https://doi.org/10.3390/antibiotics12050802
Oliveira RN, Mancini MC, De Oliveira FCS, Passos TM (2016) FTIR analysis and quantification of phenols and flavonoids of five commercially available plants extracts used in wound healing. Rev Mater 21:767–779. https://doi.org/10.1590/S1517-707620160003.0072
Ota C, Unterkircher C, Fantinato V, Shimizu MT (2001) Antifungal activity of propolis on different species of Candida. Mycoses 44:375–378. https://doi.org/10.1046/j.1439-0507.2001.00671.x
Ozdal T, Ceylan FD, Eroglu N, Kaplan M, Olgun EO, Capanoglu E (2019) Investigation of antioxidant capacity, bioaccessibility and LC-MS/MS phenolic profile of Turkish propolis. Food Res Int 122:528–536. https://doi.org/10.1016/j.foodres.2019.05.028
Pinho E, Magalhães L, Henriques M (2011) Antimicrobial activity assessment of textiles: standard methods comparison. Ann Microbiol 61:493–498. https://doi.org/10.1007/s13213-010-0163-8
Ramadan MA, Elkhateeb E, Nassar S (2019) Printed cotton fabrics with antibacterial properties based on honey gum containing printing paste formulation. Egypt J Chem 62:2175–2182. https://doi.org/10.21608/ejchem.2019.18110.2105
Rogina-Car B, Rogina J, Govorčin Bajsić E, Budimir A (2020) Propolis-eco-friendly natural antibacterial finish for nonwoven fabrics for medical application. J Ind Text 49:1100–1119. https://doi.org/10.1177/1528083718805711
Salama A, El-Sakhawy M (2023) Polysaccharides/propolis composite as promising materials with biomedical and packaging applications: a review. Bioref Biomass Conv. https://doi.org/10.1007/s13399-022-02814-5
Saleh S, Salama A, Ali AM, Saleh AK, Elhady BA, Tolba E (2023) Egyptian propolis extract for functionalization of cellulose nanofiber/poly (vinyl alcohol) porous hydrogel along with characterization and biological applications. Sci Rep 13:7739–7754. https://doi.org/10.1038/s41598-023-34901-6
Sanli Yurudu NO, Kimiran EA, Sanli Yurudu N (2008) The evaluation of antibacterial activity of fabrics impregnated with dimethyltetradecyl (3-(trimethoxysilyl) propyl) ammonium chloride. Eur J of Biol 67:115–122. https://dergipark.org.tr/en/pub/iufsjb/issue/9051/112883
Sankarganesh P, Parthasarthy V, Ganesh Kumar A, Ragu S, Saraniye M, Udaykumari N, Anbarasan R (2022) Preparation of cellulose-PVA blended hydrogels for wound healing applications with controlled release of the antibacterial drug: an in vitro anticancer activity. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-022-02586-y
Sanpa S, Popova M, Bankova V (2015) Antibacterial compounds from propolis of Tetragonula laeviceps and Tetrigona melanoleuca (Hymenoptera:Apidae) from Thailand. PLoS ONE 10:e0126886. https://doi.org/10.1371/journal.pone.0126886
Sharaf S, Higazy A, Hebeish A (2013) Propolis induced antibacterial activity and other technical properties of cotton textiles. Inter J of Biological Macromolecules 59:408–416. https://doi.org/10.1016/j.ijbiomac.2013.04.030
Shin JY, Jeong H, Yong Lee D (2018) Synthesis and biocompatibility of PVA/NaCMC hydrogels crosslinked by cyclic freezing/thawing and subsequent gamma-ray irradiation. J of Biomedical Engin Research 39:161–167. https://doi.org/10.9718/JBER.2018.39.4.161
Shokrii H, Khosravi AR, Yalfani R (2011) Antifungal efficacy of propolis against fluconazole-resistant Candida glabrata isolates obtained from women with recurrent vulvo vaginal candidiasis. Int J Gynaecol Obstet 114:158–159. https://doi.org/10.1016/j.ijgo.2011.02.019
Takaisi-Kikuni NB, Schilcher H (1994) Electron microscopic and microcalorimetric investigations of the possible mechanism of the antibacterial action of a defined propolis provenance. Planta Med 60:222–227. https://doi.org/10.1055/s-2006-959463
Tavakol M, Vasheghani-Farahani E, Dolatabadi- Farahani T, Hashemi-Najafabadi S (2009) Sulfasalazine release from alginate-N, O-carboxymethyl chitosan gel beads coated by chitosan. Carbohydr Polym 77:326–330. https://doi.org/10.1016/j.carbpol.2009.01.005
Vílchez S, Miras J, Molina R, Fages E, Ferrándiz M, Erra P, Esquena J (2020) Functional polyamide fabrics by application of Ph stimuli-responsive chitosan hydrogels. J Ind Text 50:526–542. https://doi.org/10.1177/1528083719835762
Wang W, Wat E, Hui CL, Chan BSF, Kan C, Wang X, Hu H, Wong ECW, Lau CBS, Leung P (2016) Dual-functional transdermal drug delivery system with controllable drug loading based on thermosensitive poloxamer hydrogel for atopic dermatitis treatment. Sci Rep 6:24112. https://doi.org/10.1038/srep24112
Wikanta T, Erizal A, Tjahyono B, Sugiyono C (2012) Synthesis of polyvinyl alcohol-chitosan hydrogel and study of its swelling and antibacterial properties. Squalen 7:1–10. https://doi.org/10.15578/squalen.v7i1.10
Xiao Z, Li Q, Liu H, Zhao Q, Niu Y, Zhao D (2022) Adhesion mechanism and application progress of hydrogels. Eur Polym J 173:111277. https://doi.org/10.1016/j.eurpolymj.2022.111277
Yaman Turan N, Turker E, Inşaatçı Ö (2021) Microparticles loaded with propolis to make antibacterial cotton. Cellulose 28:4469–4483. https://doi.org/10.1007/s10570-021-03783-0
Yin Y, Yang Y, Xu H (2002) Swelling behavior of hydrogels for colon-site drug delivery. J Appl Polym Sci 83:2835–2842. https://doi.org/10.1002/app.10259
Yu Q, Song Y, Shi X, Xu C, Bina Y (2011) Preparation and properties of chitosan derivative/poly(vinyl alcohol) blend film crosslinked with glutaraldehyde. Carbohyd Polym 84:465–470. https://doi.org/10.1016/J.CARBPOL.2010.12.006
Zhang D, Zhou W, Wei B, Wang X, Tang R, Nie J, Wang J (2015) Carboxyl-modified poly(vinyl alcohol)- crosslinked chitosan hydrogel films for potential wound dressing. Carbohyd Polym 125:189–199. https://doi.org/10.1016/j.carbpol.2015.02.034
Zou W, Chen Y, Zhang X, Li J, Sun Gui Z, Du B, Chen S (2018) Cytocompatible chitosan based multi-network hydrogels with antimicrobial, cell anti-adhesive and mechanical properties. Carbohyd Polym 202:246–257. https://doi.org/10.1016/j.carbpol.2018.08.124
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). Not applicable.
Author information
Authors and Affiliations
Contributions
NYT generated the idea, conducted experiment, data curation, data validation, written initial draft and final draft review. SEK and BA cooperated for antimicrobial testing and their evaluation, written draft.
Corresponding author
Ethics declarations
Conflict of interest
Not applicable.
Ethical approval
Not applicable
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yaman Turan, N., Korcan, E. & Aydin, B. Comparing the antimicrobial properties of propolis and silver particle-doped cotton fabric. Cellulose 31, 3259–3273 (2024). https://doi.org/10.1007/s10570-024-05790-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-024-05790-3