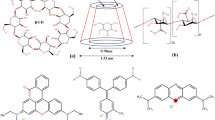
Abstract
A nanosized zirconium 1,4- dicarboxybenzene metal-organic framework (UiO-66-MOF) was synthesized and impregnated into cellulose acetate (CA) polymeric matrix to enhance the membrane characteristics for brackish water desalination. Phase inversion was used for the fabrication of CA/UiO-66 hybrid membranes (CAU-X), where X is the concentration of immobilized UiO-66 nanoparticles (UiO-66-NPs) into CA polymeric matrix. Morphological structure and functional groups were investigated through different characterization techniques to prove the successful synthesis of the prepared UiO-66-NPs, the blank CA membrane, and hybrid CAU-X membranes. For more CAU-X characteristics, porosity, contact angle, and tensile strength were measured. The obtained data demonstrated that the impregnation of zirconium-based-NPs had a positive influence on the blank CA membrane properties. Additionally, the performance of the fabricated membranes was investigated in reverse osmosis (RO) bench-scale unit. The performance results for the pristine CAU-0 membrane showed a high salt rejection (SR) of 99.8% and a permeate water flux (PWF) of 1.14 L/m2.h. In comparison to pristine CA membrane, CAU-X hybrid membranes have a slightly lower SR and a higher PWF. It was found that the hybrid CAU-0.02 membrane had almost a doubled PWF of 2.8 L/m2.h with only 2% sacrificed SR of 97.6% compared with CAU-0 membrane. Moreover, a much better PWF of 3.4 L/m2h and a sufficient SR of approximetly 92% were obtained by CAU-0.05 membrane. Thus, CAU-0.05 was selected to further test its performance under different operating parameters. Results revealed that the optimum parameters were recorded for a sodium chloride feed stock of 5000 ppm operating at 25 °C temperature and pressure up to 15 bar.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, there is a worldwide gap between the available clean fresh-waters and the population’s daily water demands (Elkady et al. 2016). Accordingly, municipal water with an acceptable quality has now become a rare commodity. Therefore, the current water stress situation has lightened the role of desalination processes in providing an alternative pure water source to meet the required global water needs. Thus, desalination has been widely expanding and spreading in the last decades to globally cover water needs (Angelakis et al. 2021; Shatat and Riffat 2014; Pistocchi et al. 2020; Elkady and Hassan 2021). Essentially, desalination is the process by which clean water is extracted from brackish or seawater (Huyen et al. 2021). The desalination process could be accomplished either by thermally or membrane driven techniques. Thermal desalination comprises multi-stage flash distillation (MSF), thermal vapor compression (TVC) and multi-effect distillation (MED). On the other side, membrane desalination includes membrane distillation (MD), pervaporation (PV), electrodialysis (ED), forward osmosis (FO) and reverse osmosis (RO) (Ba et al. 2019; Lotfy et al. 2022; Nthunya et al. 2022). For many years, RO has been considered as the prevalent desalination technique. It has been widely spread with around 69% of the total installed desalting plants because of its low energy requirement, lower footprints and relatively low cost compared to other desalination processes (Ritt et al. 2022; Feria-Díaz et al. 2021; Lim et al. 2021).
Accordingly, many researchers were devoted to investigating the enhancement of RO membranes using various materials and techniques. Biomimetic as well as silver nanoparticles (NPs) were impregnated into polyamide (PA) matrix to improve its performance (Huo et al. 2023). Additionally, covalent organic frameworks (COFs) were used to help in fabricating a thin film nanocomposite (TFN) with excellent properties (Qi et al. 2023). TiO2-NPs and polyethylene glycol were added to enhance polyimide based thin film composite (TFC) membrane (Hosseini et al. 2020). Multi-layer graphene oxide was used to modify conventional PA membrane permeability (Su et al. 2023). Another concept focused on studying the effect of solvent mixture and curing temperature on the membrane efficiency (Mokarinezhad et al. 2023). Amongst the most studied polymeric materials, CA is one of the most investigated and applied polymers for membrane fabrication. CA-based membranes are famous for their hydrophilic nature, biocompatibility, good chemical properties, chlorine tolerance, availability, ease of fabrication and low cost (Vatanpour et al. 2022; Lakra et al. 2021; Li et al. 2022a). Nonetheless, it still has some drawbacks that need to be improved to compete other RO membranes, for instance, mechanical stability, salt rejection and pure water permeability (Ghaseminezhad et al. 2019).
In accordance, many membrane enhancement approaches were deliberated to overcome CA membrane defects for different applications (Yang et al. 2019; De Guzman et al. 2021). Those approaches including but not limited to polymeric blending (Jamshaid et al. 2020; El-Gendi et al. 2021; Koriem et al. 2022b), surface grafting (Gebru and Das 2018; Xiang et al. 2019) and nanoparticles impregnation into polymeric materials forming mixed matrix membranes (MMMs) (Wei et al. 2020). Various investigations were considered with impregnating nano-sized materials within CA membranes such as candle soot (Abdelhamid and Khalil 2019), titanium and aluminum oxides (Baniasadi et al. 2021a; Shafiq et al. 2018), iron oxide (Evangeline et al. 2019), graphene oxide (GO) (Xu and Na 2020), silver (Xu et al. 2016), activated carbon (Koriem et al. 2022a) and Metal organic frameworks (MOFs) (Diab et al. 2021). MOFs are a class of hybrid organic-inorganic nano scaled materials with an extreme large surface area. Additionally, as a result to the presence of organic ligands, they have a much better affinity towards polymeric matrices comparing to the inorganic fillers (Zirehpour et al. 2016). Among the known MOFs, a zirconium-based MOF (UiO-66) is prominent for its water affinity, extraordinary chemical and thermal stability. Furthermore, its aperture size was estimated to be 6 Angstrom, making it a competitive candidate for sieving mono and divalent ions from salty waters (Gu et al. 2020; Kadhom et al. 2017; Ma et al. 2017).
The main purpose of the current study is to provide an efficient CA-RO membrane with an enhanced flux using a simple method. The promising results of our previous work (Koriem et al. 2022b) motivated us to further study the effect of MOF incorporation directly into CA membrane. Therefore, zirconium-based nanoparticles were impregnated at different concentrations into CA matrix to study their effect on improving the membrane water flux. Moreover, the performance of the optimum CAU-X membrane was investigated at various operating parameters. To the best of our knowledge, there is no other research concerned with impregnating UiO-66 nanoparticles (UiO-66-NPs) into CA membrane to be used for RO desalination. Consequently, in the current study, the hydrophilic porous zirconium-based UiO-66-NPs were selected to be synthesized and embedded into the CA polymeric cast membrane. The obtained NPs were characterized by various techniques to identify its functional and morphological structure, which in return confirms the successful preparation of the targeted MOF. The fabricated NPs were then incorporated into CA matrices at different concentrations and cast at certain conditions. The effect of UiO-66-NPs impregnation on the characteristics of the neat CA membrane was investigated via several characterization methods. After that, the desalination performance of all the fabricated CA-based membranes was tested with the aid of a lab-scale RO system. Lastly, the effect of operation conditions on the optimum membrane performance was further investigated by changing the initial feed water concentration, operating pressure, and temperature.
Experimental work
Materials
All the purchased chemicals, except of the commercial grade sodium chloride (NaCl, 99%) which was used for the preparation of saline feedwater solutions, were of a high analytical grade quality and used as they were purchased with no other modification or purification. For the synthesis of CA membranes, CA powder with 100,000 g/mol average molecular weight was obtained from Acros Organics. Acetone (\(\ge\)99%) and 1,4-dioxane (\(\ge\)99%) were supplied by Fisher, methanol (\(\ge\)99%) was provided by Sigma Aldrich and glacial acetic acid was purchased from Merck. To synthesize UiO-66-NPs, zirconium (IV) chloride (ZrCl4) (\(\ge\) 99.5%) and dimethylformamide (DMF) (\(\ge\)99%) were delivered by Merck and benzene-1,4-dicarboxylic acid (BDC) (>98%) was supplied by Acros Organics.
Synthesis of UiO-66 nanoparticles
UiO-66-NPs were synthetized via solvothermal method according to the schematic diagram illustrated in Fig. 1. According to literature, firstly, 0.15 g of ZrCl4 was stirred at room temperature and dissolved in 45 ml of DMF. Then, equimolar of BDC (0.11 g) was dissolved at 45 ml of DMF. After that, the two solutions were mixed, and 0.13 ml of distilled water was added as a modulator (Trinh et al. 2017; Zamidi Ahmad et al. 2018). The final solution was transferred to a Teflon cup, where it was well sealed in a stainless-steel autoclave jar. The jar was finally moved to a preheated furnace of 120 °C and left for 24 h (Valenzano et al. 2011). The resultant NPs were left to cool at room temperature, then they were separated using centrifugation at 6000 rpm for 60 min. Finally, the obtained NPs were washed three times using DMF, followed by another 3 times using methanol. The washed UiO-66-NPs were dried in a vacuum oven over night.
Fabrication of neat CA and CA/UiO-66 hybrid membranes
Flat sheet CA-based membranes either the blank or MMMs were fabricated via phase inversion technique and the detailed composition of each membrane is clearly illustrated in Table 1. As previously reported in literature, a dope solution of 14 wt% CA was prepared by dissolving certain amount of CA polymeric powder in a quaternary solvent mixture composed of (dioxane, acetone, acetic acid and methanol) (Morsy et al. 2016; Elkony et al. 2020a; Mohammed Ali et al. 2020). Each component of the mixture plays a vital role in the membrane synthesizing process. Dioxane and acetone were selected for their high CA dissolving ability (Aburideh et al. 2021), while acetic acid and methanol were added as softener and non-solvent (Duarte et al. 2006), respectively. For the synthesis of MMMs, various concentrations of 0.02, 0.05 and 0.1 wt% from UiO-66-NPs, relative to CA weight, were firstly well dispersed in the same quaternary solvent mixture. Then, same mass of CA powder was added to the UiO-66/solvent mixture. The neat CA and hybrid CA/UiO-66 dope solutions were continuously stirred at 20 °C for 12 h, then they were left for specific time in a cold temperature to discard defective micro air bubbles. Each dope solution was then cast at room temperature on a previously cleaned and dried glass plate with the help of an automatic film applicator, which was adjusted at 250 μm thickness and 50 mm/sec speed. Thenceforth, the cast film was left for 30 s to partially evaporate at room temperature before being immersed into a (0–4 °C) distilled water (DW) coagulation bath. The obtained flat sheet membranes were then moved and further washed in another cold DW bath to remove any residual solvents. As a final step, the fabricated membranes were thermally annealed for 10 min at 80 °C and the annealed membranes were preserved under DW for 24 h before being further characterized and tested in RO lab scale unit. The synthesized membranes were named (CAU-X), where X refers to UiO-66-NPs concentration.
Characterization techniques of UiO-66-NPs, neat CA membrane and CA/UiO-66 hybrid membranes
All the prepared samples were carefully dried in a drying oven prior to being characterized. The particle size of the obtained UiO-66-NPs was inspected by transmission electron microscope (TEM, JEOL JEM-2100 F). The Brunauer Emmet-Teller (BET) analyzer that is based on nitrogen adsorption/desorption was used to determine the fabricated nanoparticles surface area and pore volume. Samples were degassed under vacuum prior to characterization at 150 °C to remove any residuals or impurities. The functional groups of both MOF nanoparticles as well as the fabricated membranes were determined by Fourier transform infrared (FTIR, Bruker Vertex 60). The selected wave number was in the range of (400–4000 cm−1). Moreover, the crystalline nature of all the samples was investigated with the help of x-ray diffraction (XRD, Shimadzu XRD-6100) with a copper target (λ = 1.54 °A) which operating at 40 kV and 30 mA. A system consists of 1° diverging slit and 0.3 mm receiving slit was used. The diffraction peaks were acquired in a continuous scanning mode with a scanning range varying between 5° and 80° and a scanning speed of 12°/min. Furthermore, the thermal stability of the neat CA and MMMs was studied by thermal gravimetric analysis (TGA Q50). The morphology of NPs and membranes top, bottom and cross-section were investigated by scanning electron microscope (SEM, JEOL JSM-6010 LV). Membrane samples were cut under liquid nitrogen to obtain an obvious structure. All samples were coated with platinum prior to SEM characterization and the accelerating voltage used was 10 kV and 15 kV for UiO-66-NPs and membranes, respectively. To investigate the hydrophilic nature of pristine CA and CA/UiO-66 hybrid membranes, the contact angle of the synthesized membranes was measured with the help of (DSA 100, KRÜSS). For each membrane, various random locations were selected for the contact angle measuring to minimize any possible error. The mechanical strength of the membranes was evaluated by the tensile strength (4468, Instron). The membranes were tested under a 3 mm/min elongation rate.
RO performance evaluation of the neat CA and hybrid CA/UiO-66 membranes
The permeate water flux (PWF) as well as salt rejection (SR) ability were investigated in a bench scale RO crossflow setup (CF042, Sterlitech, USA). Each tested membrane had a rectangular surface area of 42 cm2, which is similar to the active area of the testing cell. A saline solution of 5000 ppm NaCl was fed at room temperature to the cell by a high-pressure pump (HPP) (Hydra-cell) and the pressure was gradually increased until certain pressure was obtained. Later, the effect of initial feed water concentration and the applied pressure on the optimum membrane performance was observed. The solute concentration was detected in the permeate water by a TDS measuring device (Adwa, AD32).
The membrane performance was evaluated based on the rejected salts (R%) and the flux of the produced water (J) as illustrated in Eqs. (1 & 2) (Al-Hobaib et al. 2015; Anjum et al. 2020):
Where, R (%) is the salt rejection of each membrane, Cp and Ci are the concentrations (ppm) of permeated and feed waters, respectively.
Where, J (L/m2 h) is the permeated flux, Q (L) is the permeated water volume, A (m2) is the membrane active area, and t (h) is the time.
Results and discussion
Characterization of the synthesized UiO-66 NPs
The characteristics of the fabricated UiO-66 NPs were indicated via various methods. FTIR was used to confirm the successful preparation of UiO-66-NPs by determining the functional groups of the prepared NPs. As shown in Fig. 2a, the peak appeared at 1655 cm−1 is assigned to C = O of terephthalic acid. Moreover, the band observed at 1578 cm−1 give an indication to the carboxylate group of the organic ligand (Zhang et al. 2019; Elhussein et al. 2020). The weak peak at 1502 cm−1 refers to the presence of C = C bond of the benzene ring in BDC (Nasrabadi et al. 2019). In addition, the peaks detected at 743 and 659 cm−1 are attributed to O-H and C-H bonds of the organic BDC ligand. Whereas the shown band at 548 cm−1 is assigned to the (Zr-OC) asymmetric stretch (Koriem et al. 2021).
XRD was investigated in order to understand the crystalline nature of the fabricated NPs and the main diffraction peaks of UiO-66-NPs are illustrated in Fig. 2b. As it can be seen, at 2\({\uptheta }\) the appearance of peaks at 7.24°, 8.42° and 25.62° gives an indication to the successful fabrication of UiO-66. The results also indicated that the nanoparticles of UiO-66 are highly crystalline in nature. The studied crystallographic structure of synthetized UiO-66 was in accordance with the previously mentioned in literature (Molavi et al. 2018; Chen et al. 2018).
SEM images were observed to understand the morphology and size of the prepared UiO-66-NPs. As it can be seen in Fig. 2c, UiO-66 has a very small particle size of around 50 nm. However, large particles are presented, and this might be due to agglomerations caused by the small size.
Thus, TEM images were studied to determine the average particle size of the synthesized UiO-66-NPs. As it is illustrated in Fig. 2d, the obtained particles can be considered homogenous in size and shape. Additionally, the measured average size of UiO-66-NPs based on the TEM images was found to be in the range of 35 nm. Clearly, the prepared particles are nano-sized materials, which is advantageous for many applications (Li and Bie 2017).
BET results showed that the fabricated UiO-66-NPs has a high value of surface area of 1013.4 m2/g and a pore volume of 0.584 cm3/g. Thus, this porous structure is expected to provide water paths for a better permeability. The obtained results agree to previously published results (Su et al. 2017; Mesgarian et al. 2020).
Characterization of the fabricated neat CA and hybrid CA/UiO-66 membranes
FTIR Analysis
The functional groups of the fabricated blank and hybrid membranes were examined by FTIR. As it can be noticed in Fig. 3, for the neat CAU-0 membrane, the wide band between 3670 and 3160 cm−1 represents the cellulosic characteristic OH peak, whilst the recorded value of 2970 cm−1 could be attributed to C-H stretching vibration, while the peak at 1724 cm−1 refers to the C = O stretching vibration.
Additionally, the value at 1332 cm−1 gives an indication to the presence of C–H bending and the bands at 1037 and 1128 cm−1 represent respectively the C–O symmetric and asymmetric vibration (Kumar et al. 2019; Namjoufar et al. 2021; Li et al. 2022b). On the other hand, the addition of UiO-66 NPs did not have a noticeable change on CA functional groups. However, the intensity of OH peak was decreased after UiO-66 impregnation, this might refer that the added NPs have formed a hydrogen bond with the CA polymer (Tanvidkar et al. 2022).
XRD Analysis
XRD patterns of the pristine CAU-0 and mixed CAU-0.02, CAU-0.05 and CAU-0.1 membranes were investigated to verify the impregnation of UiO-66-NPs into CA matrix. Figure 4 illustrates the amorphous nature of the blank CAU-0 membrane. However, with the incorporation of UiO-66 NPs, the intensity of characteristic diffraction peaks of Zr-MOF starts to slightly increase within the composite membranes at around 2θ = 7.2° and 8.4°. Additionally, with the escalating concentration of MOF, the top of the characteristic CA wide peak between 15° and 30° slightly shifted towards the distinctive peak of UiO-66 at 25.8° (Al-Shaeli et al. 2021; Liang et al. 2021). The previous findings prove the successful impregnation of UiO-66 NPs with CA polymeric matrix.
SEM analysis
To inspect the influence of Zr-based MOF on the pristine CA membrane morphological structure, SEM images of each membrane surface, bottom and cross section were examined. As clearly demonstrated in Fig. 5a the surface of the neat CAU-0 membrane consists of a smooth non-porous structure (Peixoto et al. 2020). Nonetheless, this surface started to corrugate to some extent with the addition of UiO-66-NPs. Moreover, small white particles became visible on the surface of CA/UiO-66 hybrid membranes and this might be a confirmation of the presence of UiO-66-NPs in the structure of the cast CAU-X membranes. However, with increasing the concentration of NPs some large agglomeration was found on the surface and this might have a negative effect on the membrane performance.
The bottom surface of the neat CAU-0 and hybrid CA/UiO-66 membranes is shown in Fig. 5b. As displayed, no pores were present on the bottom surface of the neat CAU-0 membrane. Yet, some small holes were detected after the impregnation of UiO-66-NPs as shown in CAU-0.05 bottom surface. Same observation for another filler was mentioned elsewhere (Ali et al. 2021b).
The membranes cross-sectional morphologies were clearly identified in Fig. 5c. As it can be seen, the blank CAU-0 membrane had nearly a free macro-void morphology, which might be due to the low exchange rate of solvent and non-solvent (El-Ghaffar et al. 2020). On the other side, an obvious asymmetric structure can be seen in the hybrid CA/UiO-66 membranes. This asymmetric morphology is characterized by the presence of a thin top active layer on a porous-thick layer and the pores of this supporting layer could have a tear or finger like shape (Ali et al. 2021b;Elkony et al. 2020b;Ebrahim et al. 2016). It is clearly illustrated that the addition of UiO-66-NPs influenced the morphology of blank CAU-0 membrane and thus it is expected to provide an alternative water way and in return this will affect the membrane permeability. In addition, from cross-section SEM images, the presence of UiO-66 NPs has developed the shape of the existed finger-like pores. Compared to CAU-0 membrane the impregnation of UiO-66-NPs have enhanced the rate solvent/non-solvent exchange causing the formation of a porous structure (Norahim et al. 2019; Baniasadi et al. 2021b).
Contact angle and membrane porosity
Table 2 demonstrates the effect of UiO-66-NPs impregnation on the hydrophilic nature and porosity of the neat CAU-0 membrane. As demonstrated, the obtained contact angle of the pristine CAU-0 membrane was found to be 58.1°, which is in accordance with the recorded value in literature (Lee 2020; Ali et al. 2021a; Qi et al. 2022). This value has slightly declined for the fabricated hybrid CA/UiO-66 membranes. This could be attributed to the hydrophilic nature of UiO-66 NPs that facilitate the passage of water molecules through the membrane. The measured contact angle of CAU-0.02 and CAU-0.05 was found to be 54.6° and 53.2°, respectively. However, the value was found to be 56.3° for CAU-0.1 membrane. The slight increase in contact angle of the later membrane could be due to the nanoparticles agglomeration, which in return has slightly increased the roughness of the membrane surface (El-Ghaffar et al. 2020;Ali et al. 2021a, 2021b). It could be expected that with enhancing the hydrophilic nature of the CA/UiO-66 MMMs the permeate water flux would also enhance eventually. In addition, the membrane porosity increased gradually from 71.4% for the neat CA membrane to 79.3% for CAU-0.1 hybrid membrane. The increased porosity can be interpreted as the presence of a super hydrophilic nanofiller in the dope polymeric solution increases the exchange rate between solvent and non-solvent phases in the coagulation bath. This rapid exchange could influence the formation of a longer finger like pores (Ma et al. 2020; Emadzadeh et al. 2014).
Mechanical strength
In order to study the effect of UiO-66 on the mechanical stability of the blank CAU-0 membrane, the tensile strength properties were investigated in Fig. 6. As it can be seen, the addition of UiO-66-NPs has positive impact onto the mechanical strength of CAU-0 membrane. The tensile strength was elevated from 6.05 MPa for CAU-0 up to 10.7 for CAU-0.05 membrane, then it decreased with further UiO-66-NPs addition to be 6.8 MPa for CAU-0.1 membrane. The filler/polymer interaction could be the reason of the increase in the mechanical property of the membrane. However, if the filler concentration has further increased this might cause aggregation of NPs, which in return forms stress points in the membrane structure (Asiri et al. 2022). In addition, as previously illustrated by SEM images, the presence of UiO-66-NPs has affected the porous structure of the hybrid membranes and larger pores were formed with higher MOF-NPs concentration. This also might be the reason behind the lower membrane mechanical stability of CAU-0.1. Same trend was previously reported (Gzara et al. 2016; Zahid et al. 2021).
RO membrane performance
In the experimental work, the permeate water started to flow at 10 bars for the pristine membrane. However, the permeate water started to flow at lower pressure of 8 bars after the impregnation of UiO-66-NPs, same observation was previously reported for other filler (Ali et al. 2021b). The experiments were conducted at 10 bars for all membranes to provide a fair comparison at the same conditions. Figure 7 exhibits a comparison of the performance of blank CA and hybrid CA/UiO-66 membranes at the same initial concentration, operating temperature and operating pressure. As clearly illustrated, the blank CAU-0 membrane has the highest salt rejection and the lowest water flux of 99.8% and 1.14 L/m2h,respectively. These results are in consistent with the previously explained dense structure in SEM images of CAU-0 membrane.
Compared to the blank CAU-0 membrane, the hybrid CAU-X membranes were found to have lower salt rejection and better permeability. This might be caused by the presence of nonselective voids formed between UiO-66-NPs and CA chains (Ali et al. 2021a). As it can be seen, CAU-0.02 membrane’s salt rejection has slightly diminished to 97.6%, while the PWF has almost doubled to be 2.8 L/m2h. Additionally, with further increase in UiO-66 concentration in the membrane matrix, the salt rejection has further decreased by 5.7%, while the PWF has increased by almost 20% for the CAU-0.05 membrane in comparison to CAU-0.02 membrane. Generally, the impregnation of the hydrophilic UiO-66-NPs has enhanced the hydrophilic nature of the membranes, as previously explained by contact angle, and in return the membrane PWF due to the interaction between water molecules and the surface of membrane. Furthermore, the addition of the hydrophilic nanofiller has improved the membrane porosity and pores distribution, as aforementioned in Fig. 5, and consequently the hybrid membrane permeability compared to the neat CAU-0 membrane. Additionally, UiO-66-NPs have increased the membrane pores to some extent causing some salts to escape as well as water molecules to pass. Same observations were previously reported with other nanofillers (Ali et al. 2021a, 2021b;Nyamiati et al. 2021;Shi et al. 2017). Surprisingly, with further addition of UiO-66 the salt rejection was raised from 92% for CAU-0.05 to 93% for CAU-0.1 membrane, while the PWF was slightly reduced from 3.4 L/m2h for CAU-0.05 to 2.8 L/m2h for CAU-0.1 membrane. This might be a result to UiO-66-NPs agglomeration and blocking some membrane pores (Ghaseminezhad et al. 2019). Accordingly, the fabricated CAU-0.05 composite membrane was considered as an optimum membrane for RO operation.
The results of this study were compared to previously published research articles that focused on the enhancement of CA-based RO membranes with numerous nanoparticles. As illustrated in Table 3, the blending of UiO-66-NPs with the pristine membrane had a satisfying performance either in salt rejection or permeability compared to some nanoparticles.
Influence of RO operation parameters onto the performance of CAU-0.05 composite membrane
In order to assess the membrane performance under different RO conditions, salt rejection and PWF of CAU-0.05 membrane were measured at diverse feedwater concentrations, pressure and temperatures. As it can be seen in Fig. 8, the membrane salt rejection was affected by the increased feedwater concentration, where the rejection decreased from 96.74 to 72.3% with the increase of feedwater TDS from 1000 to 13,000 ppm. This is due to the increased concentration of feedwater caused an increased osmotic pressure at the surface of the membrane. In this case, a layer of concentrated rejected ions is built at the membrane surface, which impeded the transport of lower molecular solutes. Additionally, the permeate water flux decreased from 3.4 L/m2h to 2 L/m2h with the increase of feedwater salt concentration from 1000 to 13,000 ppm, respectively. This might be explained as an effect for the concentration polarization phenomenon, which means the presence of greater concentration of the rejected ions at the membrane surface than that of the bulk solution. These results agree with previous studies (Alsalhy et al. 2013; Shigidi et al. 2022).
For the same feedwater salinity, the effect of the applied pressure on CAU-0.05 membrane performance was illustrated in Fig. 9. It was found that, the PWF increased from 3.4 L/m2h to 10.47 L/m2 h with increasing the pressure from 10 to 30 bars. This increase was expected as the elevated applied pressure forces more water molecules to pass through the semi-permeable membrane. However, with increasing the pressure the membrane salt rejection ability decreased from 92 to 70.5% when the operating pressure raised from 10 to 30 bars. This decline might refer to the concentration polarization and the formation of NaCl salt layer near to the membrane surface, which caused more salts to escape through the membrane. These results are in agreement with literature (Elkony et al. 2020a).
The temperature of the feedwater is considered one of the most important parameters that affects the RO membrane performance (Gedam 2012). Figure 10 shows the effect of various feedwater temperatures on the performance of CAU-0.05 membrane. As it was demonstrated, the membrane salt rejection declined from 92 to 85.6% as the feedwater temperature increased from 25°C to 40 °C, respectively. On the other hand, the PWF increased as the feedwater temperature rose. This could be explained that as temperature increase the viscosity decrease and this facilitate the water permeation rate through the membrane. Additionally, the salt solubility increases too and thus a higher diffusion rate could take place through the membrane. This trend was previously reported in literature (Gedam 2012; Abdulmuttaleb et al. 2014).
Conclusions
The present work investigated the effect of UiO-66-NPs impregnation on the characteristics and performance of CA blank membrane for brackish water desalination. UiO-66 MOF was fabricated via solvothermal method, while neat CA and hybrid CA/UiO-66 membranes were synthesized by NIPS technique. The data of the characterized samples proved the successful preparation of UiO-66-NPs as well as the successful incorporation of UiO-66 nano-MOF into CA membrane matrix. The RO membrane performance was conducted, and the results revealed that the PWF was enhanced by the addition of UiO-66-NPs. It was found that CAU-0 membrane has a 99.8% and 1.14 LMH salt rejection and permeability, respectively. However, with a small reduction of salt rejection to be almost 97.6%, the permeability incremented to become 2.8 LMH for CAU-0.02 membrane. The same trend continued for CAU-0.05 membrane with a salt rejection of 93% and PWF of 3.4 LMH. With further addition of UiO-66-NPs, salt rejection slightly increased by only 1%, while membrane permeability decreased by around 17% for CAU-0.1 membrane. The optimum membrane was selected to be further tested under various feedwater concentrations, operating temperatures, and pressures. After compromising between the membrane PWF and MSR, the optimum membrane was found to be CAU-0.05 with PWF of 3.4 L/m2h, which is almost three times higher than that of CAU-0 blank membrane. Furthermore, the CAU-0.05 membrane mechanical stability was enhanced compared with the neat CA membrane, which indicates a better pressure stability. The performance results showed that CAU-0.05 membrane can reject salts from brackish water up to 5000 ppm NaCl by 92%. Additionally, the same membrane can reject salts with the same efficiency and even better permeability of 5 LMH when working under pressure up to 15 bar. The optimum operating pressure to maintain the membrane efficiency was found to be 25 °C.
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
Abdelhamid AE, Khalil AM (2019) Polymeric membranes based on cellulose acetate loaded with candle soot nanoparticles for water desalination. J Macromol Sci Part A 56:153–161. https://doi.org/10.1080/10601325.2018.1559698
Abdulmuttaleb S, Dalaf A, Sabri L et al (2014) Effect of operating conditions on reverse osmosis. RO) Membrane Performance
Aburideh H, Tigrine Z, Aoudjit L et al (2021) Development of acid modified cellulose acetate membranes for salt water treatment. Cellul Chem Technol 55:1153–1161. https://doi.org/10.35812/CelluloseChemTechnol.2021.55.99
Al-Hobaib AS, Al-Sheetan KM, Shaik MR et al (2015) Characterization and evaluation of reverse osmosis membranes modified with Ag2O nanoparticles to improve performance. Nanoscale Res Lett. https://doi.org/10.1186/s11671-015-1080-3
Al-Shaeli M, Smith SJD, Jiang S et al (2021) Long-term stable metal organic framework (MOF) based mixed matrix membranes for ultrafiltration. J Memb Sci. https://doi.org/10.1016/j.memsci.2021.119339
Ali ASM, Soliman MM, Kandil SH et al (2021) Tailoring nanocomposite membranes of cellulose of acetate Silica Nanoparticles for Desalination. J Materiomics. https://doi.org/10.21203/rs.3.rs-820853/v1
Ali ASM, Soliman MM, Kandil SH, Khalil MMA (2021) Emerging mixed matrix membranes based on zeolite nanoparticles and cellulose acetate for water desalination. Cellulose 28:6417–6426. https://doi.org/10.1007/s10570-021-03924-5
Alsalhy QF, Albyati TM, Zablouk MA (2013) A study of the effect of operating conditions on reverse osmosis membrane performance with and without air sparging technique. Chem Eng Commun 200:1–19. https://doi.org/10.1080/00986445.2012.685529
Angelakis AN, Valipour M, Choo KH et al (2021) Desalination: from ancient to present and future. Water (Switzerland). https://doi.org/10.3390/w13162222
Anjum T, Tamime R, Khan AL (2020) Mixed-matrix membranes comprising of polysulfone and porous uio-66, zeolite 4a, and their combination: preparation, removal of humic acid, and antifouling properties. Membranes (Basel) 10:1–21. https://doi.org/10.3390/membranes10120393
Asiri AM, Petrosino F, Pugliese V et al (2022) Synthesis and characterization of blended cellulose acetate membranes. Polym (Basel). https://doi.org/10.3390/polym14010004
Ba X, Wang X, Cui N et al (2019) Preparation, characterisation, and desalination performance study of cellulose acetate membranes with MIL-53(fe) additive. J Memb Sci. https://doi.org/10.1016/j.memsci.2019.04.061
Baniasadi J, Shabani Z, Mohammadi T, Sahebi S (2021) Enhanced performance and fouling resistance of cellulose acetate forward osmosis membrane with the spatial distribution of TiO2 and Al2O3 nanoparticles. J Chem Technol Biotechnol 96:147–162. https://doi.org/10.1002/jctb.6521
Chen S, Zhang L, Zhang Z et al (2018) Study on the Desorption process of n-Heptane and methyl cyclohexane using UiO-66 with hierarchical pores. ACS Appl Mater Interfaces 10:21612–21618. https://doi.org/10.1021/acsami.8b04931
De Guzman MR, Andra CKA, Ang MBMY et al (2021) Increased performance and antifouling of mixed-matrix membranes of cellulose acetate with hydrophilic nanoparticles of polydopamine-sulfobetaine methacrylate for oil-water separation. J Memb Sci 620:118881. https://doi.org/10.1016/j.memsci.2020.118881
Diab KE, Salama E, Hassan SH, et al (2021) Biocompatible MIP-202 Zr-MOF tunable sorbent for cost-effective decontamination of anionic and cationic pollutants from waste solutions. Sci Rep 11:6619. https://doi.org/10.1038/s41598-021-86140-2
Duarte AP, Cidade MT, Bordado JC (2006) Cellulose acetate reverse osmosis membranes: optimization of the composition. J Appl Polym Sci 100:4052–4058. https://doi.org/10.1002/app.23237
Ebrahim S, Morsy A, Kenawy E et al (2016) Reverse osmosis membranes for water desalination based on cellulose acetate extracted from Egyptian rice straw. Desalin Water Treat 57:20738–20748. https://doi.org/10.1080/19443994.2015.1110052
El-Gendi A, Abdallah H, Amin A (2021) Economic study for blend membrane production. Bull Natl Res Cent 45:126. https://doi.org/10.1186/s42269-021-00584-0
El-Ghaffar MAA, Elawady MM, Rabie AM, Abdelhamid AE (2020) Enhancing the RO performance of cellulose acetate membrane using chitosan nanoparticles. J Polym Res 27:1–12. https://doi.org/10.1007/s10965-020-02319-7
Elhussein EAA, Şahin S, Bayazit ŞS (2020) Removal of carbamazepine using UiO-66 and UiO-66/graphene nanoplatelet composite. J Environ Chem Eng 8:2–9. https://doi.org/10.1016/j.jece.2020.103898
Elkady MF, Shokry Hassan H (2021) Photocatalytic degradation of malachite green dye from aqueous solution using environmentally compatible Ag/ZnO polymeric nanofibers. Polymers 13:2033. https://doi.org/10.3390/polym13132033
Elkady MF, Shokry Hassan H, Salama E (2016) Sorption profile of phosphorus ions onto ZnO nanorods synthesized via sonic technique. J Eng 2016:2308560. https://doi.org/10.1155/2016/2308560
Elkony Y, Mansour ES, Elhusseiny A et al (2020) Novel Grafted/Crosslinked Cellulose Acetate Membrane with N-isopropylacrylamide/N,N-methylenebisacrylamide for Water Desalination. Sci Rep 10:1–13. https://doi.org/10.1038/s41598-020-67008-3
Elkony Y, Mansour ES, Elhusseiny A, Ebrahim S (2020) Effect of cellulose acetate/cellulose triacetate ratio on reverse osmosis blend membrane performance. Polym Eng Sci 60:2852–2863. https://doi.org/10.1002/pen.25517
Emadzadeh D, Lau WJ, Matsuura T et al (2014) A novel thin film composite forward osmosis membrane prepared from PSf–TiO2 nanocomposite substrate for water desalination. Chem Eng J 237:70–80. https://doi.org/10.1016/j.cej.2013.09.081
Evangeline C, Pragasam V, Rambabu K et al (2019) Iron oxide modified polyethersulfone/cellulose acetate blend membrane for enhanced defluoridation application. Desalin Water Treat 156:177–188. https://doi.org/10.5004/dwt.2018.23174
Feria-Díaz JJ, Correa‐Mahecha F, López‐Méndez MC et al (2021) Recent desalination technologies by hybridization and integration with reverse osmosis: a review. Water (Switzerland) 13:1–40. https://doi.org/10.3390/w13101369
Gebru KA, Das C (2018) Humic acid removal using cellulose acetate membranes grafted with poly(methyl methacrylate) and aminated using tetraethylenepentamine. J Environ Manage 217:600–610. https://doi.org/10.1016/j.jenvman.2018.03.131
Gedam VV (2012) Performance evaluation of Polyamide Reverse Osmosis membrane for removal of contaminants in Ground Water Collected from Chandrapur District. J Membr Sci Technol. https://doi.org/10.4172/2155-9589.1000117
Ghaseminezhad SM, Barikani M, Salehirad M (2019) Development of graphene oxide-cellulose acetate nanocomposite reverse osmosis membrane for seawater desalination. Compos B Eng 161:320–327. https://doi.org/10.1016/j.compositesb.2018.10.079
Gu Z, Yu S, Zhu J et al (2020) Incorporation of lysine-modified UiO-66 for the construction of thin-film nanocomposite nanofiltration membrane with enhanced water flux and salt selectivity. Desalination 493:114661. https://doi.org/10.1016/j.desal.2020.114661
Gzara L, Ahmad Rehan Z, Khan SB et al (2016) Preparation and characterization of PES-cobalt nanocomposite membranes with enhanced anti-fouling properties and performances. J Taiwan Inst Chem Eng 65:405–419. https://doi.org/10.1016/j.jtice.2016.04.012
Hosseini SS, Jalili Palandi MAR, Mokarinezhad N (2020) Exploring the characteristics, performance, and modification of Matrimid for development of thin-film composite and thin-film nanocomposite reverse osmosis membranes. Polym Adv Technol 31:2209–2221. https://doi.org/10.1002/pat.4941
Huo HQ, Mi YF, Yang X et al (2023) Polyamide thin film nanocomposite membranes with in-situ integration of multiple functional nanoparticles for high performance reverse osmosis. J Memb Sci. https://doi.org/10.1016/j.memsci.2022.121311
Huyen Trang Do Thi, Tibor Pasztor, Daniel Fozer, Flavio Manenti AJT (2021) Comparison of desalination technologies using renewable energy sources with life cycle, PESTLE, and multi-criteria decision analyses. Water (Basel) 13:3023. https://doi.org/10.3390/w13213023
Jamshaid F, Dilshad MR, Islam A et al (2020) Synthesis, characterization and desalination study of polyvinyl chloride-co-vinyl acetate/cellulose acetate membranes integrated with surface modified zeolites. Microporous Mesoporous Mater 309:110579. https://doi.org/10.1016/j.micromeso.2020.110579
Kadhom M, Hu W, Deng B (2017) Thin film nanocomposite membrane filled with metal-organic frameworks UiO-66 and MIL-125 nanoparticles for water desalination. Membranes (Basel). https://doi.org/10.3390/membranes7020031
Koriem OA, El-Shazly AH, El-Kady MF (2021) A study of the effect of reaction time on the preparation of zirconium based UiO-66 MOF. Key Eng Mater 897:57–62. https://doi.org/10.4028/www.scientific.net/kem.897.57
Koriem OA, Kamel AM, Shaaban W, Elkady MF (2022) Enhancement of dye separation performance of eco-friendly cellulose acetate-based membranes. Sustainability 14:14665. https://doi.org/10.3390/su142214665
Koriem OA, Showman MS, El-Shazly AH, Elkady MF (2022) Cellulose acetate/polyvinylidene fluoride based mixed matrix membranes impregnated with UiO-66 nano-MOF for reverse osmosis desalination. Cellulose. https://doi.org/10.1007/s10570-022-04889-9
Kumar M, Isloor RTS et al (2019) Use of cellulose acetate/polyphenylsulfone derivatives to fabricate ultrafiltration hollow fiber membranes for the removal of arsenic from drinking water. Int J Biol Macromol 129:715–727. https://doi.org/10.1016/j.ijbiomac.2019.02.017
Lakra R, Balakrishnan M, Basu S (2021) Development of cellulose acetate-chitosan-metal organic framework forward osmosis membrane for recovery of water and nutrients from wastewater. J Environ Chem Eng 9:105882. https://doi.org/10.1016/j.jece.2021.105882
Lee SE (2020) Surface modifications of cellulose acetate Film for the application of Face Shield. J Mater Sci Chem Eng 08:41–45. https://doi.org/10.4236/msce.2020.88004
Li D, Bie Z (2017) Metal-organic framework incorporated monolithic capillary for selective enrichment of phosphopeptides. RSC Adv 7:15894–15902. https://doi.org/10.1039/c7ra00263g
Li T, Wang Y, Wang X et al (2022b) Desalination Characteristics of Cellulose Acetate FO Membrane Incorporated with ZIF-8 nanoparticles. Membranes (Basel). https://doi.org/10.3390/membranes12020122
Liang Z, Wang J, Zhang Q et al (2021) Composite PVDF ultrafiltration membrane tailored by sandwich-like GO@UiO-66 nanoparticles for breaking the trade-off between permeability and selectivity. Sep Purif Technol 276:119308. https://doi.org/10.1016/j.seppur.2021.119308
Lim YJ, Goh K, Kurihara M, Wang R (2021) Seawater desalination by reverse osmosis: current development and future challenges in membrane fabrication – a review. J Memb Sci 629:119292. https://doi.org/10.1016/j.memsci.2021.119292
Lotfy HR, Staš J, Roubík H (2022) Renewable energy powered membrane desalination: review of recent development. Environ Sci Pollut Res 29:46552–46568. https://doi.org/10.1007/s11356-022-20480-y
Ma D, Peh SB, Han G, Chen SB (2017) Thin-film nanocomposite (TFN) membranes Incorporated with super-hydrophilic metal-organic framework (MOF) UiO-66: toward enhancement of water flux and salt rejection. ACS Appl Mater Interfaces 9:7523–7534. https://doi.org/10.1021/acsami.6b14223
Ma W, Li T, Zhang Q et al (2020) Preparation of hybrid membranes by incorporating hydrophilic UiO-66 nanoparticles for high-performance pervaporation dehydration of aprotic solvents. J Nanopart Res 22:64. https://doi.org/10.1007/s11051-020-4778-9
Mansor ES, Abdallah H, Shaban AM (2023) Development of TiO2/polyvinyl alcohol-cellulose acetate nanocomposite reverse osmosis membrane for groundwater-surface water interfaces purification. Mater Sci Eng B. https://doi.org/10.1016/j.mseb.2022.116222
Mansor ES, Abdallah H, Shalaby MS, Shaban AM (2023b) Enhancement of reverse osmosis membranes for groundwater purification using cellulose acetate incorporated with ultrathin graphitic carbon nitride nanosheets. Environ Nanotechnol Monit Manag. https://doi.org/10.1016/j.enmm.2022.100760
Mesgarian R, Heydarinasab A, Rashidi A, Zamani Y (2020) Adsorption and growth of water clusters on UiO-66 based nanoadsorbents: a systematic and comparative study on dehydration of natural gas. Sep Purif Technol 239:116512. https://doi.org/10.1016/j.seppur.2020.116512
Mohammed Ali AS, Fadl EA, Soliman MM, Kandil SH (2020) Optimization of the evaporation step in cellulose acetate membranes preparation by dry–wet phase inversion technique for water desalination applications. Desalin Water Treat 174:63–70. https://doi.org/10.5004/dwt.2020.24862
Mokarinezhad N, Hosseini SS, Nxumalo EN (2023) Development of polyamide/polyacrylonitrile thin film composite RO membranes by interfacial polymerization assisted with an aromatic/aliphatic organic solvent mixture. J Appl Polym Sci 140:e53811. https://doi.org/10.1002/app.53811
Molavi H, Zamani M, Aghajanzadeh M et al (2018) Evaluation of UiO-66 metal organic framework as an effective sorbent for Curcumin’s overdose. Appl Organomet Chem 32:1–10. https://doi.org/10.1002/aoc.4221
Morsy A, Ebrahim S, Kenawy ER et al (2016) Grafted cellulose acetate reverse osmosis membrane using 2-acrylamido-2-methylpropanesulfonic acid for water desalination. Water Sci Technol Water Supply 16:1046–1056. https://doi.org/10.2166/ws.2016.025
Namjoufar M, Farzi A, Karimi A (2021) Removal of Acid Brown 354 from wastewater by aminized cellulose acetate nanofibers: experimental and theoretical study of the effect of different parameters on adsorption efficiency. Water Sci Technol 83:1649–1661. https://doi.org/10.2166/wst.2021.070
Nasrabadi M, Ghasemzadeh MA, Monfared MRZ (2019) The preparation and characterization of UiO-66 metal-organic frameworks for the delivery of the drug ciprofloxacin and an evaluation of their antibacterial activities. New J Chem 43:16033–16040. https://doi.org/10.1039/c9nj03216a
Norahim N, Faungnawakij K, Quitain AT, Klaysom C (2019) Composite membranes of graphene oxide for CO2/CH4 separation. J Chem Technol Biotechnol 94:2783–2791. https://doi.org/10.1002/jctb.5999
Nthunya LN, Bopape MF, Mahlangu OT et al (2022) Fouling, performance and cost analysis of membrane-based water desalination technologies: a critical review. J Environ Manage 301:113922. https://doi.org/10.1016/j.jenvman.2021.113922
Nyamiati RD, Devi BC, Febriansyah BA et al (2021) Effect of graphene oxide on the performance of cellulose acetate/polyethylene glycol membrane with blending method for desalination of Brackish Water. IOP Conf Ser Mater Sci Eng 1143:012059. https://doi.org/10.1088/1757-899x/1143/1/012059
Peixoto I, Faria M, Gonçalves MC (2020) Synthesis and characterization of novel integral asymmetric monophasic cellulose–acetate/silica/titania and cellulose–acetate/titania membranes. Membranes (Basel) 10:1–26. https://doi.org/10.3390/membranes10090195
Pistocchi A, Bleninger T, Breyer C et al (2020) Can seawater desalination be a win-win fix to our water cycle? Water Res 182:115906. https://doi.org/10.1016/j.watres.2020.115906
Qi J, Chen Y, Zhang W-T et al (2022) Imparting Cellulose Acetate Films with hydrophobicity, high transparency, and self-cleaning function by constructing a Slippery Liquid-Infused Porous Surface. Ind Eng Chem Res 61:7962–7970. https://doi.org/10.1021/acs.iecr.2c00776
Qi H, Peng Y, Lv X et al (2023) Synergetic effects of COFs interlayer regulation and surface modification on thin-film nanocomposite reverse osmosis membrane with high performance. Desalination 548:116265. https://doi.org/10.1016/j.desal.2022.116265
Ritt CL, Stassin T, Davenport DM et al (2022) The open membrane database: synthesis–structure–performance relationships of reverse osmosis membranes. J Memb Sci. https://doi.org/10.1016/j.memsci.2021.119927
Shafiq M, Sabir A, Islam A et al (2018) Cellulaose acetate based thin film nanocomposite reverse osmosis membrane incorporated with TiO2 nanoparticles for improved performance. Carbohydr Polym 186:367–376. https://doi.org/10.1016/j.carbpol.2018.01.070
Shatat M, Riffat SB (2014) Water desalination technologies utilizing conventional and renewable energy sources. Int J Low Carbon Technol 9:1–19. https://doi.org/10.1093/ijlct/cts025
Shi Y, Li C, He D et al (2017) Preparation of graphene oxide–cellulose acetate nanocomposite membrane for high-flux desalination. J Mater Sci 52:13296–13306. https://doi.org/10.1007/s10853-017-1403-0
Shigidi I, Harharah RH, Abdalla GMT et al (2022) Studying different operating conditions on reverse osmosis performance in the treatment of wastewater containing nickel(II) ions. Membranes (Basel) 12:1163. https://doi.org/10.3390/membranes12111163
Su Z, Miao YR, Zhang G et al (2017) Bond breakage under pressure in a metal organic framework. Chem Sci 8:8004–8011. https://doi.org/10.1039/c7sc03786d
Su T, Li X, Yang ZM, Liu LF (2023) A novel polyamide thin-film nanocomposite reverse osmosis membrane constructed by a 3D multi-layer graphene oxide assembled with 1,3-diamino-2-propanol. J Memb Sci 681:121773
Tanvidkar P, Jonnalagedda A, Kuncharam BVR (2022) Fabrication and testing of mixed matrix membranes of UiO-66-NH2 in cellulose acetate for CO2 separation from model biogas. J Appl Polym Sci. https://doi.org/10.1002/app.53264
Trinh DX, Tran TPN, Taniike T (2017) Fabrication of new composite membrane filled with UiO-66 nanoparticles and its application to nanofiltration. Sep Purif Technol 177:249–256. https://doi.org/10.1016/j.seppur.2017.01.004
Valenzano L, Civalleri B, Chavan S et al (2011) Disclosing the complex structure of UiO-66 metal organic framework: a synergic combination of experiment and theory. Chem Mater 23:1700–1718. https://doi.org/10.1021/cm1022882
Vatanpour V, Pasaoglu ME, Barzegar H et al (2022) Cellulose acetate in fabrication of polymeric membranes: a review. Chemosphere. https://doi.org/10.1016/j.chemosphere.2022.133914
Wei W, Liu J, Jiang J (2020) Atomistic simulation study of polyarylate/zeolitic-imidazolate framework mixed-matrix membranes for water desalination. ACS Appl Nano Mater 3:10022–10031. https://doi.org/10.1021/acsanm.0c02004
Xiang L, Yang J, Luo D et al (2019) Construction of efficient desalting layer on a cellulose acetate membrane by acetalized surface crosslinking treatment. Polym Eng Sci 59:913–918. https://doi.org/10.1002/pen.25036
Xu P, Na N (2020) Study on Antibacterial properties of cellulose acetate seawater desalination reverse-osmosis membrane with Graphene Oxide. J Coast Res 105:246–251. https://doi.org/10.2112/JCR-SI105-052.1
Xu F, Weng B, Materon LA et al (2016) Fabrication of cellulose fine fiber based membranes embedded with silver nanoparticles via Forcespinning. J Polym Eng 36:269–278. https://doi.org/10.1515/polyeng-2015-0092
Yang Z, Zhou Y, Feng Z et al (2019) A review on reverse osmosis and nanofiltration membranes for water purification. Polymer (Basel) 11:1–22. https://doi.org/10.3390/polym11081252
Zahid M, Akram S, Rashid A et al (2021) Investigating the antibacterial activity of polymeric membranes fabricated with aminated graphene oxide. Membr (Basel). https://doi.org/10.3390/membranes11070510
Zamidi Ahmad M, Navarro M, Lhotka M et al (2018) Enhancement of CO2/CH4 separation performances of 6FDA-based co-polyimides mixed matrix membranes embedded with UiO-66 nanoparticles. Sep Purif Technol 192:465–474. https://doi.org/10.1016/j.seppur.2017.10.039
Zhang Q, Yang T, Liu X et al (2019) Heteropoly acid-encapsulated metal-organic framework as a stable and highly efficient nanocatalyst for esterification reaction. RSC Adv 9:16357–16365. https://doi.org/10.1039/c9ra03209f
Zirehpour A, Rahimpour A, Khoshhal S et al (2016) The impact of MOF feasibility to improve the desalination performance and antifouling properties of FO membranes. RSC Adv 6:70174–70185. https://doi.org/10.1039/c6ra14591d
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Conceptualization, OAK, MSS, AHE and ME; methodology, OAK, MSS, AHE and ME; analysis, OAK, ME; investigation, OAK; supervision, MSS, AHE and ME, writing-review and editing, OAK, MSS, AHE and ME. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Koriem, O.A., Showman, M.S., El-Shazly, A.H. et al. Synthesis of high-performance biocompatible polymeric membranes incorporated with zirconium-based MOF for an enhanced brackish water RO desalination. Cellulose 31, 2309–2325 (2024). https://doi.org/10.1007/s10570-023-05723-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-023-05723-6