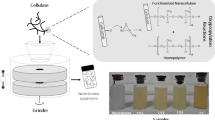
Abstract
Nata puree (NP)—obtained by disintegrating nata de coco (bacterial cellulose [BC]) using a household blender—can be combined with tamarind seed gum (TG) to generate NPTG. In this study, BC fibrils (BC-TG) were prepared by removing free TG from NPTG and characterized. BC-TG exhibited high water dispersibility and relatively long nanofibrils (> 20 μm). We examined the distribution of xyloglucan, the main component of TG, on BC nanofibrils using immunofluorescence staining with calcofluor white, which stains the hydrophilic cellulose surface, and found that xyloglucan was adsorbed at different sites along the fibers. This indicated that BC-TG was a composite nanofibril of xyloglucan and BC. Furthermore, BC-TG showed a higher degree of adsorption on hydrophobic plastic substrates than BC did, suggesting a change in the surface properties of BC. Because the BC-TG preparation process is simple, requires only water and raw materials, and does not involve chemical reactions, it is expected to be an environmentally friendly method for the preparation and modification of BC nanofibrils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bacterial cellulose (BC), produced by the gram-negative aerobic bacterium Gluconacetobacter, is a cellulose nanofiber with a diameter of approximately 50 nm (Haigler et al. 1982). Unlike plant cellulose, the BC is pure—free of hemicellulose, lignin, pectin, and other compounds found in plant pulp. In addition, BC exhibits several unique properties, including excellent purity, mechanical properties, crystallinity, water retention capacity, and a large surface area (Campano et al. 2016; Poddar and Dikshit 2021). These excellent properties make bacterial cellulose a suitable material for various applications, including tissue engineering, drug delivery, food applications, and preparation of different polymer nanocomposites.
Under static culture conditions, BC nanofibers form a three-dimensional network structure and generate gel-like membranes called pellicles, which are used in various applications (Czaja et al. 2006; Nogi and Yano 2008). Nata de coco, a derivative of the BC pellicle, is a traditional fermented food produced by certain strains of acetic acid bacteria as a gel composed of BC (Dourado et al. 2017; Azeredo et al. 2019). The disintegration of nata de coco can impart various properties to foods (Okiyama et al. 1993; Paximada et al. 2016; Guo et al. 2018), but can only be achieved under severe conditions using specialized equipment, such as high-pressure homogenizers and ultrasonic devices, which limits its application. The recently developed nata puree (NP) is obtained by disintegrating nata de coco using a household blender in the presence of water-soluble polysaccharides such as (1,3)(1,4)-glucan (BGL), tamarind seed gum (TG), and birch wood xylan (Tokuyasu et al. 2021, 2022). The preparation of NP is much simpler than the traditional methods of disintegration and, combined with 3D food-printing technology, allows for fine control of the 3D structure and texture in food powders (Tokuyasu et al. 2021, 2022).
TG is a polysaccharide-based substance found in the seeds of the tree Tamarindus indica (Khounvilay and Sittikijyothin 2012). TG mainly contains xyloglucan, which is widely used as a food thickener, stabilizer, and gelling agent (Picout et al. 2003). Xyloglucan presents a heterogeneous assembly of polysaccharides of various lengths and side chain patterns. Specifically, xyloglucans consist of a β-(1,4)-d-glucan backbone that is quasi-regularly substituted with β-d-xylosyl residues linked to glucose through the O-6 position (Park and Cosgrove 2015). Xyloglucan is present in the primary cell walls of plants and is involved in controlling plant elongation (Park and Cosgrove 2015; Cosgrove 2018). Experimental cultures of acetic acid bacteria in media containing TG have been conducted since the 1970s, and have indicated that BC and xyloglucans bind to each other through hydrophobic interactions (Hayashi et al. 1987; Whitney et al. 2006; Lopez et al. 2010; Zhao et al. 2014). However, most studies have focused on the cell wall and none have experimentally assessed the disruption of BC pellicles with xyloglucan and BC, as in the case of NP.
It has been suggested but not directly proven that NP interacts and forms complexes with water-soluble polysaccharides and BC (Tokuyasu et al. 2021, 2022). In this study, NP was prepared using TG, a water-soluble polysaccharide containing xyloglucan, which interacts with BC, to verify the complex formation between BC and TG. NP was prepared by disintegrating BC pellicles using a household mixer in the presence of a TG solution, followed by washing to remove free TG and purify the fiber, thereby generating BC-TG. In addition to characterizing the fibers in BC-TG, we investigated the effects of TG addition and the mixing treatment by comparing the characteristics of BC-TG with those of BC without TG but with water (BC-W) and BC-W mixed with TG (BC-TG’). First, the water dispersibility and morphological characteristics of the obtained fibers were evaluated. Complex formation between xyloglucan and BC was verified by direct visualization using immunostaining and confocal laser scanning microscopy. Finally, we evaluated the adsorption of the fibers on various hydrophobic substrates to investigate changes in the surface properties of BC.
Materials and methods
Materials
The BC pellicle (Morinaga nata de coco plain) was purchased from Morinaga Milk Industry Co. Ltd. (Tokyo, Japan). TG and calcofluor white (CFW) was purchased from Tokyo Chemical Industry Co. Ltd. (Tokyo, Japan) and Sigma-Aldrich Japan Co. LLC. (Tokyo, Japan), respectively. LM15, a rat monoclonal antibody against xylosyl residues in the XXXG motif of xyloglucan, was purchased from Kerafast Inc. (Boston, MA, USA). A fluorescein isothiocyanate (FITC)-conjugated mouse anti-rat IgG2c secondary antibody was purchased from Thermo Fisher Scientific (K.K., Tokyo, Japan). 1/15 M phosphate buffer (PB, pH 7.0) and Blocking one®ฎ were purchased from Fujifilm Wako Pure Chemical Corp. (Osaka, Japan). The (PP), polyethylene (PE), and polyethylene terephthalate (PET) films were obtained from Toyobo Co. Ltd. (Osaka, Japan). The PS film was purchased from Nakatoshi Trading Co. Ltd. (Tokyo, Japan).
Preparing BC-TG, BC-W, and BC-TG’
The procedure for preparing each sample is depicted in Fig. 1. NP with TG as the water-soluble polysaccharide (NP-TG) was previously reported (Tokuyasu et al. 2021). Briefly, 20 g of de-syruped BC (corresponding to 6.9 mg dry cellulose/g) and 27 mL of aqueous solution of 0.35% (w/v) TG were mixed and then disintegrated at 25 000 rpm for 30 s using a Food mill TML180 (Tostem, Tokyo, Japan); disintegration was repeated three more times. The term ‘de-syruped’ refers to the process we applied to the BC pellicle. Originally, the nata de coco is steeped in syrup, and prior to its use in our experiments, we replaced this syrup with deionized water through a thorough rinsing process. To remove non-disintegrating BCs, the aqueous suspension was poured onto a testing sieve (aperture: 0.3 mm; diameter: 75 mm; Tokyo Screen Co., Ltd, Tokyo, Japan). At this point, the concentration of BC was 0.75 mg/mL and TG was 0.42 mg/mL. To remove the free TG from the NP-TG aqueous suspension, 1 mL of NP-TG was placed in a centrifuge tube and centrifuged at 12 000 rpm for 5 min at room temperature to obtain the precipitated fraction. The following washing operation was repeated three times: 1.0 mL of deionized water was added to the precipitated fraction, which was agitated by vortexing for 30 seconds and centrifuged at 12 000 rpm for 5 min at room temperature. After washing, 1.0 mL of deionized water was added to the precipitate fraction and stirred to obtain BC-TG. The concentration of BC at this point was calculated to be 0.75 mg/mL, which was used as the concentration of BC-TG. A control sample of BC-TG was prepared by adding water instead of TG solution (BC-W). That is, 20 g of de-syruped BC (corresponding to 6.9 mg dry cellulose/g) and 27 mL of deionized water were mixed and then disintegrated. The concentration of BC in BC-W after passing through the mesh was 0.35 mg/mL. Furthermore, 20 mL of BC-W (corresponding to 0.35 mg dry cellulose /mL) and 27 mL of aqueous solution of 0.35% (w/v) TG was mixed (BC-TG’) and used as a control sample. BC-TG’ was prepared by vortexing at room temperature for 3 min. Futhermore, the same procedure as for BC-TG was used for washing to remove free TG. The concentration of BC at this point was calculated to be 0.07 mg/mL, which was used as the conc entration of BC-TG’.
Scheme of the sample preparation. NP-TG was named according to a previous report (Tokuyasu et al. 2021). BC, bacterial cellulose; TG, aqueous solution of tamarind seed gum; dH2O, deionized water
Fourier transform infrared (FT-IR) spectroscopy
Freeze-dried BC-TG, BC-W, and BC-TG’ samples were subjected to FT-IR spectroscopy. All spectra were recorded using an FT/IR-4700 Type A instrument (JASCO, Corp., Tokyo, Japan) using the attenuated total reflectance (ATR) mode with a triglycine sulphate (TGS) detector. The spectra were obtained with a resolution of 1 cm−1, 20-fold integration, and normalized to 1162 cm−1.
Brief evaluation of water dispersibility in samples
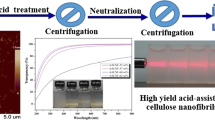
The aqueous dispersions of BC-TG, BC-W, and BC-TG’ at 1 × 10−3% (w/v) were placed in vials, irradiated with a 532 nm laser pointer at a perpendicular angle to the vial, and images were captured (Fig. 2a). The light-scattering variability was measured by calculating the intensity per pixel (gray value) from the plot profile of the laser lines in the resulting digital image (Fig. 2b). A histogram was created from measurements of > 1000 pixels per sample. The image analysis software ImageJ Fiji (Schindelin et al. 2012) was used for the measurements.
Field emission scanning electron microscopy (FE-SEM)
BC-TG, BC-W, and BC-TG’ were diluted to achieve solid concentrations of 1 × 10−4% (w/v) in deionized water, and 50 µL of the aqueous suspensions were dropped onto a silicone-coated cover glass and air-dried in an oven at 60 °C. The cover glass was fixed with carbon tape on the SEM sample holders, and the samples were coated with osmium tetroxide using an osmium coater (Neoc, Meiwafosis Co., Ltd., Tokyo, Japan). The coating time was 20 s, corresponding to a film thickness of 5 nm. The samples were examined using FE-SEM SU8000 (Hitachi High-Technologies Co., Ltd., Tokyo, Japan; accelerating voltage: 1.5 kV). The apparent fiber width was measured from the FE-SEM images and the actual fiber width was determined by subtracting the thickness of the osmium coating (10 nm). A histogram was created from measurements at 250 points per sample. The image analysis software ImageJ Fiji (Schindelin et al. 2012) was used for the measurements.
Fluorescence microscopy
The BC-TG, BC-W, and BC-TG’ were diluted to achieve the solids concentrations of 1 × 10−5% (w/v) in deionized water, and 50 µl of the aqueous suspensions were dropped onto a silicone-coated cover glass and air-dried in an oven at 60 °C. The samples were stained with 0.001% (w/v) CFW aqueous solution at room temperature for 10 min and washed with deionized water. The stained samples were analyzed using a BX51 fluorescence microscope (Olympus Co., Ltd., Tokyo, Japan) after immersion in PB. We used an oil-immersion 100× objective lens with a numerical aperture (NA) of 1.3, an excitation filter with an excitation wavelength of 330–385 nm, and an absorption filter with a detection fluorescence wavelength of 420 nm. Fiber length was measured from fluorescence images. A histogram was created from the measurements of > 100 fibers per sample using ImageJ Fiji software (Schindelin et al. 2012).
Immunofluorescence microscopy with confocal laser scanning microscope (CLSM)
A drop of BC-TG, BC-W, or BC-TG’ aqueous dispersion (1 × 10−4% [w/v]) was mounted on silicon-coated cover glass and air-dried. The samples were incubated with LM15, a primary antibody against xyloglucan, in blocking buffer (Blocking One, Nacalai Tesque, Inc., Kyoto, Japan) at room temperature for 1 h, and washed with 1/15 M PB (pH 7.0). LM15 was prepared by 100-fold dilution of the undiluted solution with a blocking solution. Before staining with LM15, BCs on the coverslips were treated with blocking buffer at room temperature for 30 min as a blocking treatment to suppress non-specific adsorption. As a secondary antibody, FITC isothiocyanate-conjugated mouse anti-rat IgG2c was incubated in blocking buffer at room temperature for 1 h and then washed with PB. CFW was used as a site-specific probe depending on fiber hydrophilic planes (Tsuji et al. 2021). The BCs were secondarily stained with 0.001% (w/v) CFW aqueous solution at room temperature for 30 min and washed with PB. After double staining with CFW and LM15 with FITC, the BCs were observed by CLSM (TCS SP8, Leica Microsystems, Wetzlar, Germany) using an HCX PL APO oil objective lens (× 63, NA = 1.4) with excitation wavelengths of 405 and 488 nm for CFW and LM15-FITC, respectively. The fluorescence emissions of CFW and LM15-FITC were detected at 415–450 nm and 515–600 nm, respectively. CLSM images were obtained using Leica Application Suite X (LAS X) software (Leica Microsystems).
Evaluation of fiber adsorption on plastic substrate
Using PP, PE, PET, and PS plastic films as adsorption substrates, a drop of BC-TG, BC-W, or BC-TG’ aqueous dispersion (1 × 10−4% [w/v]) was dropped onto each substrate and allowed to incubate at room temperature for 10 min. After the droplets were removed, deionized water was gently dripped onto each substrate and immediately removed. This process was repeated five times to remove unadsorbed fibers. After cleaning, the substrate was dried in a thermostatic oven at 60 °C for 10 min. The CFW solution was then dropped onto the treated substrate and allowed to stand for 10 min at room temperature. After removing the CFW solution used to stain the cellulose fibers, a drop of PB was gently dropped onto the substrate and immediately removed. This process was repeated three times to remove excess CFW.
The stained samples were subjected to fluorescence microscopy BX51 (Olympus) after immersion in PB. We used an oil-immersion 100× objective lens (NA = 1.3), an excitation filter with an excitation wavelength of 330–385 nm, and an absorption filter with a detection fluorescence wavelength of 420 nm.
Background fluorescence was removed from the acquired fluorescent images using ImageJ Fiji software (Schindelin et al. 2012), and the images were binarized after light and dark adjustments were applied. The percentage of white areas on the screen of the binarized image was calculated, and five images were captured per film. The measurements were performed on three films to calculate the mean and standard deviation (SD).
Results and discussion
Fiber dispersion of BC-TG
The objective of this study was to characterize the BC fibers in NP-TG. NP-TG was washed with deionized water after passing through a mesh, and centrifuged to eliminate the effects of free TGs—hereafter referred to as BC-TG. We used samples of BC-W and BC-TG’ for comparison. The FT-IR results (Supplementary Fig. 1) indicated that BC-TG, BC-W, and BC-TG’ all contained cellulose, and were thus assumed to contain BC fibers.
Dispersibility in water is an important factor affecting the use of BC fibers. Therefore, we first evaluated the dispersibility of each sample in water.
Figure shows the Tyndall phenomenon, which occurs when the path of light passing through a liquid is affected by an object of the same or larger wavelength than that of the light. In BC-W, light was diffusely reflected, whereas in BC-TG and BC-TG’, light was clearly observed in a linear pattern. To quantify the degree of light scattering, a plot profile and the gray value per pixel were obtained by image analysis (Fig. 2c). These results indicated that BC-W had the largest variation in light scattering, and BC-TG’ had a larger variation than that of BC-TG (Table 1), suggesting that the water dispersion of BC-W and BC-TG’ is more heterogeneous than that of BC-TG. The high water dispersibility of the BC-TG fibers was confirmed by fluorescence microscopy (Fig. 3); that is, BC-TG could be observed as a single fiber, whereas agglomerates were observed in BC-W and BC-TG’. Notably, BC-TG’ was observed as a loosened fiber compared to BC-W. Similar results were observed in the FE-SEM images (Supplementary Fig. 2). Previous studies have shown that defibrillated BC fibers can reaggregate (Lin et al. 2015; Tokuyasu et al. 2021). The dispersibility of BC-TG and BC-TG was better than that of BC-W, suggesting that the addition of TG prevents BC fiber reaggregation and improves the dispersibility of BC fibers. In addition, we observed differences in the dispersibility of the BC fibers after washing with water, suggesting that TG was adsorbed and remained on the BC.
Size characterization of BC-TG fibers
Fiber width and length are important factors in material design (Fukuzumi et al. 2013). Particle size measurements of NP prepared using BC and BGL have been performed in previous studies (Tokuyasu et al. 2021). The addition of BGL was suggested to reduce the particle size of BC in NP (Tokuyasu et al. 2021). However, the length and width of BC fibers remain unclear. Therefore, we analyzed the distributions of the fiber length and width of BC-TG. Simultaneously, size measurements of BC-W and BC-TG’ were performed to investigate the effects of blender treatment and TG addition; extreme aggregates were avoided in BC-W and BC-TG’ measurements.
Figure 4a illustrates the lengths of the BC fibers in each sample measured by fluorescence microscopy (Tagawa et al. 2021). The average length of BC-TG, BC-W, and BC-TG’ fibers was 23.4 (SD = 16.8), 6.0 (SD = 4.4), and 16.9 (SD = 10.8) µm, respectively. Student’s t-test showed a significant difference among all samples; specifically, BC-TG tended to have longer fibers than BC-W and BC-TG, and the fiber length of BC-TG’ tended to be longer than that of BC-W. Figure 4b shows the width of the BC fibers in each sample measured using FE-SEM. The average width of the BC-TG, BC-W, and BC-TG’ fibers were 71 (SD = 17), 90 (SD = 35), and 78 (SD = 42) nm, respectively. Student’s t-test showed significant differences between the fiber widths of BC-TG and BC-W, and those of BC-TG’ and BC-W. BC-W tended to have wider fibers than BC-TG and BC-TG’. However, no significant differences were observed between the fiber widths of BC-TG and BC-TG’.
In summary, BC-TG tended to have longer fiber lengths and narrower fiber widths than BC-W. The addition of TG tended to increase the fiber length and decrease the fiber width. Because the addition of TG suppressed BC fiber reaggregation (Figs. 2 and 3), it is plausible that BC-TG’ had the original fiber width of BC-W. These results suggest that the mixing treatment reduces the fiber width of the BC to 70–80 nm and cuts the fiber direction to a few to several tens of micrometers. We observed an increase in fiber length after blender treatment in the presence of TG. These results indicate that the addition of TG suppresses the aggregation of long fibers and increases the apparent number of long fibers. In addition, based on the understanding that xyloglucan interacts with cellulose fibers within plant cell walls (Park and Cosgrove 2015; Cosgrove2018), similar interactions in BC-TG and BC-TG’ possibly contributed to an increase in fiber length. However, these hypotheses require further investigation, specifically by measuring fiber length after xyloglucanase treatment.
This study revealed that the BC-TG nanofibrils in NP had relatively long fiber lengths. For example, BC nanofibers based on the aqueous counter collision method has a length of ca. 3 μm and width of ca. 30 nm (Kose et al. 2011). The wide fiber width of the BC nanofibrils in this study may be due to the lower energy of the household blender compared to that in previous studies. Remarkably, we obtained long inherently aggregated fibers.
Adsorption of xyloglucan on BC nanofibrils
The previous our results showed that BC-TG had better water dispersibility and longer BC nanofibril fiber lengths. TG is mainly composed of xyloglucan, which previous studies have suggested interacts with BC (Hayashi et al. 1994; Zhao et al. 2014). These findings suggest that TG components such as xyloglucan are adsorbed on BC nanofibers. Therefore, we performed FT-IR and visualized the distribution of xyloglucan in BC by CLSM using LM15 to clarify whether xyloglucan is adsorbed on BC or not.
Kacˇura´kova´ et al (2002) reported that the FT-IR spectra of composites from xyloglucan and BC were slightly different compared to BC. However, the FT-IR measurements we performed showed no significant difference in both BC-TG and BC-TG’ compared to BC-W (Supplementary Fig. 1). Therefore, next, we visualized the distribution of xyloglucan in BC using CLSM with LM15.
BC-W, BC-TG, and BC-TG’ were immunostained using the primary antibody LM15 and a FITC-fused secondary antibody, and double-stained using CFW, which stains cellulose (Fig. 5a). No FITC fluorescence was observed for BC-W, indicating that xyloglucan was not present in the fibrous form of BC (Supplementary Fig. 3a). In contrast, in BC-TG and BC-TG’, FITC fluorescence was observed on the BC nanofibrils (Supplementary Fig. 3b), suggesting that xyloglucan was adsorbed onto the BC nanofibrils. In a magnified image of the BC nanofibrils of BC-TG, the fluorescence of CFW (magenta) and FITC (green) was mostly colocalized, but there were some non-overlapping areas. The fluorescence intensities of CFW and FITC on BC nanofibrils are plotted in Fig. 5b, which showed that CFW and FITC fluorescence did not overlap completely. Similar results were observed for BC-TG’ (Supplementary Fig. 3b and 3c).
These results indicated that xyloglucan was adsorbed on BC-TG and BC-TG’. We hypothesized that the xyloglucan adsorbed on the surface of the BC fibrils prevented them from reaggregating. Fang et al. (2016) reported that the addition of xyloglucan to cellulose nanocrystals (CNCs) improved CNC dispersion.
It was suggested that xyloglucan recognizes specific surfaces rather than completely covering the BC fibrils. In cellulose, the parallel packing of β-1,4-glucans into fibrils results in hydrophilic and hydrophobic faces populated by polar –OH and nonpolar –CH groups, respectively. Computational studies have shown that xyloglucan adsorbs onto the hydrophobic surfaces of cellulose microfibrils (CMFs) (Zhao et al. 2014; Benselfelt et al. 2016), likely through the activities of carbohydrate-binding module 3 (CBM3) (Lehtiö et al. 2003). A recent study on onion cell walls reported a more than 5-fold increase in the amount of CBM3–nanogold binding to CMF after cell wall degradation by xyloglucanase (Zheng et al. 2018). CFW has been confirmed to adsorb on the hydrophilic surface of cellulose (Tsuji et al. 2021); the fact that the fluorescent surfaces of CFW and LM15-FITC did not always overlap suggests that LM15-FITC adsorbs on the hydrophobic surface of cellulose (Supplementary Fig. 4). In the future, we plan to use a label-fused CBM and fluorescent protein to elucidate whether xyloglucan is adsorbed onto the hydrophobic surface of cellulose.
Localization of cellulose and xyloglucan in BC-TG fiber. a Fluorescence images acquired using CLSM. Magenta indicates the fluorescence of CFW, which stains cellulose, and green indicates the fluorescence of FITC, which recognizes LM15, an antibody specific for xyloglucan. Below images indicate expanded view of the area indicated by the dashed line in upper images. White in the merged image indicates the overlap of CFW and FITC fluorescence. b A plot profile of fluorescence intensity of BC-TG fibrils in the inset image. Magenta and green indicate the fluorescence intensities of CFW and FITC, respectively
Adsorption ability of BC-TG on hydrophobic substrates
The results in Fig. 5 indicate that xyloglucan was adsorbed onto the BC fibrils. In other words, the BC-TG in NP-TG is a hybrid nanofibril of xyloglucan and BC. Previous reports suggested that xyloglucan binds to the hydrophobic surface of cellulose (Zhao et al. 2014; Benselfelt et al. 2016). More specifically, Zhao et al. (2014) reported that (XXXG)3 was found to bind more favorably to the (1 0 0) and (2 0 0) hydrophobic surfaces of (higher plant) cellulose Iβ microfibrils than to the (1-10) and (110) hydrophilic plane surfaces. (The counterpart cellulose Iα surfaces for BC are the hydrophobic (110) and the hydrophilic (100) and (010) planes.) Here, each [1,4]-d-glucosyl residue in the backbone was assigned a one-letter code according to its substituents: G = β-d-Glc; X = α-d-Xyl-[1,6]-β-d-Glc). This behavior was attributed to the topography of the hydrophobic CMF surface, which stabilized (XXXG)3 in a flat conformation. Based on these findings, we hypothesized that the adsorption of xyloglucan onto BC fibrils would alter their surface properties. Furthermore, because xyloglucan was not removed by washing with water, we speculated that xyloglucan adsorbed on BC fibrils may be more compatible with hydrophobic molecules than with water molecules. Therefore, we evaluated the adsorption of BC-TG on various plastic films used as hydrophobic substrates.
Figure 6a shows the CFW fluorescence images of BC-W, BC-TG, and BC-TG’ adsorbed on the PET film. BC-W showed no aggregates, as observed by direct fluorescence observation of the dispersion (Fig. 3b), and only nanofibers were adsorbed; BC-TG was adsorbed to a greater degree than BC-W. BC-TG’ was also adsorbed to a greater extent than BC-W, and the aggregates observed in the direct fluorescence observation of the BC-TG’ dispersion (Fig. 3c) were also adsorbed. To evaluate the amount of adsorption on PP, PE, PET, and PS, the area fractions of fluorescence in binarized fluorescence images were calculated (Fig. 6b). For all plastic films, BC-TG showed higher values than BC-W. BC-TG’ also showed a higher adsorption capacity than BC-W, but less so than that of BC-TG. BC-W tended to be adsorbed less on polymers containing aromatic rings, such as PET and PS, than on polymers containing mainly carbon chains, such as PP and PE, and the addition of TG improved the adsorption capacity.
The adsorption of BC nanofibrils onto plastic polymers was previously reported (Ishikawa et al. 2021), and is thought to involve the hydrophobic surfaces of cellulose. Since the micro-sized aggregates present in BC-W were not adsorbed onto the substrate, we hypothesized that the exposed hydrophobic surfaces were again hidden in the aggregates. In other words, the reaggregation of BC after dissociation may be related to the interaction between the hydrophobic surfaces of the cellulose; the enhanced adsorption by BC-TG and BC-TG’ was probably due to changes in BC surface properties resulting from the adsorption of xyloglucan. The difference in the amounts of adsorbed BC-TG and BC-TG’ could be due to two complex factors: (1) The difference in the ratio of nanofibrillated fibers and (2) the difference in the interaction between BC and xyloglucan. In other words, the interaction between BC and TG may be different when mixed with BC under disturbed conditions and when mixed under mild conditions. Future studies will analyze this interaction using nuclear magnetic resonance and other techniques.
The addition of TG resulted in a significant increase in the adsorption of BC fibrils onto PET and PS films. From a different perspective, this suggests that BC-W recognizes and selectively adsorbs to the polymer molecules of plastics. This also suggests that, as hypothesized, xyloglucan adsorbed on BC is more similar to hydrophobic polymers. Unlike cellulose, xyloglucan has side chains that allow it to adsorb more flexibly onto various polymers. This issue should be investigated in future studies.
Adsorption ability of different BC samples to various plastic substrates. a indicates representative fluorescence images of CFW-stained BC-W, BC-TG, and BC-TG’ adsorbed on PET film. b indicates a graph of the measured values of the area fraction of fluorescence in the binarized fluorescent images to determine the adsorption capacity of the sample. The number indicates the ratio compared to the amount of adsorption to BC-W on the same substrate. The pairs of lower-case letters indicate no significant difference (p > 0.05, Student’s t-test)
Conclusions
In this study, BC nanofibrils in fibrillated BC (NPTG) combined with TG were purified (BC-TG) and characterized. BC-TG showed high dispersibility in water and suppressed fiber reaggregation (Figs. 2 and 3). BC-TG had relatively long nanofibrils (exceeding 20 μm) with a width of 70 nm (Fig. 4). Labeling of xyloglucan by immunostaining revealed that BC-TG was a hybrid fiber of xyloglucan and BC nanofibrils (Fig. 5); xyloglucan was probably adsorbed on the hydrophobic surface of cellulose in BC nanofibrils and was not removed by washing with water. Therefore, we hypothesized that xyloglucan adsorbed on BC would have a greater affinity for hydrophobic materials, and evaluated the adsorption capacity of BC-TG on various hydrophobic substrates. Our results suggested that BC-TG has a higher adsorption capacity for hydrophobic polymer films than that of BC-W. These effects of TG addition were observed even after mixer defibrillation. However, to defibrillate BC in high yield and improve its dispersion, it is best to add TG during blender processing.
A notable finding from this study was the distribution of xyloglucan on the BC fibers, which showed positionally selective adsorption rather than covering the entire BC. Considering that different areas were stained compared to those with CFW staining, which stains hydrophilic surfaces, it is likely that xyloglucan was also adsorbed on hydrophobic surfaces. Further studies in combination with CBM are required. Our study confirmed that fluorescence observation using BC-TG is an effective method for determining the distribution of xyloglucan on BC fibers and could be used to characterize the interactions between cellulose and xyloglucan.
In this study, we found that composite nanofibers of BC and xyloglucan could be obtained by preparing NP. This process reinforces the inherent amphiphilic hydrophobic properties of BC fibers and is expected to be an environmentally friendly method for the surface modification of cellulose fibers without chemical reactions.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding.
References
Azeredo HMC, Barud H, Farinas CS, Vasconcellos VM, Claro AM (2019) Bacterial cellulose as a raw material for food and food packaging applications. Front Sustain Food Syst 3:7. https://doi.org/10.3389/fsufs.2019.00007
Benselfelt T, Cranston ED, Ondaral S, Johansson E, Brumer H, Rutland MW, Wågberg L (2016) Adsorption of xyloglucan onto cellulose surfaces of different morphologies: an entropy-driven process. Biomacromolecules 17:2801–2811. https://doi.org/10.1021/acs.biomac.6b00561
Campano C, Balea A, Blanco A, Negro C (2016) Enhancement of the fermentation process and properties of bacterial cellulose: a review. Cellulose 23:57–91. https://doi.org/10.1007/s10570-015-0802-0
Cosgrove DJ (2018) Nanoscale structure, mechanics and growth of epidermal cell walls. Curr Opin Plant Biol 46:77–86. https://doi.org/10.1016/j.pbi.2018.07.016
Czaja W, Krystynowicz A, Bielecki S, Brown RM Jr (2006) Microbial cellulose–the natural power to heal wounds. Biomaterials 27:145–151. https://doi.org/10.1016/j.biomaterials.2005.07.035
Dourado F, Gama M, Rodrigues AC (2017) A review on the toxicology and dietetic role of bacterial cellulose. Toxicol Rep 4:543–553. https://doi.org/10.1016/j.toxrep.2017.09.005
Fang W, Arola S, Malho J-M, Kontturi E, Linder MB, Laaksonen P (2016) Noncovalent dispersion and functionalization of cellulose nanocrystals with proteins and polysaccharides. Biomacromolecules 17:1458–1465. https://doi.org/10.1021/acs.biomac.6b00067
Fukuzumi H, Saito T, Isogai A (2013) Influence of TEMPO-oxidized cellulose nanofibril length on film properties. Carbohydr Polym 93:172–177. https://doi.org/10.1016/j.carbpol.2012.04.069
Guo Y, Zhang X, Hao W, Xie Y, Chen L, Li Z, Zhu B, Feng X (2018) Nano-bacterial cellulose/soy protein isolate complex gel as fat substitutes in ice cream model. Carbohydr Polym 198:620–630. https://doi.org/10.1016/j.carbpol.2018.06.078
Haigler CH, White AR, Brown RM Jr, Cooper KM (1982) Alteration of in vivo cellulose ribbon assembly by carboxymethylcellulose and other cellulose derivatives. J Cell Biol 94:64–69. https://doi.org/10.1083/jcb.94.1.64
Hayashi T, Marsden MPF, Delmer DP (1987) Pea xyloglucan and cellulose: VI. Xyloglucan-cellulose interactions in vitro and in vivo. Plant Physiol 83:384–389. https://doi.org/10.1104/pp.83.2.384
Hayashi T, Ogawa K, Mitsuishi Y (1994) Characterization of the adsorption of xyloglucan to cellulose. Plant Cell Physiol 35:1199–1205. https://doi.org/10.1093/oxfordjournals.pcp.a078714
Ishikawa G, Tsuji T, Tagawa S, Kondo T (2021) Adsorption of Janus-type amphiphilic cellulose nanofibrils onto microspheres of semicrystalline polymers. Macromolecules 54:9393–9400. https://doi.org/10.1021/acs.macromol.1c01163
Kačuráková M, Smith CA, Gidley JM, Wilson HR (2002) Molecular interactions in bacterial cellulose composites studied by 1DFT-IR and dynamic 2D FT-IR spectroscopy. Carbohydr Res 337:1145–1153. https://doi.org/10.1016/S0008-6215(02)00102-7
Khounvilay K, Sittikijyothin W (2012) Rheological behaviour of tamarind seed gum in aqueous solutions. Food Hydrocoll 26:334–338. https://doi.org/10.1016/j.foodhyd.2011.03.019
Kose R, Mitani I, Kasai W, Kondo T (2011) Nanocellulose’ as a single nanofiber prepared from pellicle secreted by Gluconacetobacter xylinus using aqueous counter collision. Biomacromolecules 12:716–720. https://doi.org/10.1021/bm1013469
Lehtiö J, Sugiyama J, Gustavsson M, Fransson L, Linder M, Teeri TT (2003) The binding specificity and affinity determinants of family 1 and family 3 cellulose binding modules. Proc Natl Acad Sci U S A 100:484–489. https://doi.org/10.1073/pnas.212651999
Lin D, Li R, Lopez-Sanchez P, Li Z (2015) Physical properties of bacterial cellulose aqueous suspensions treated by high pressure homogenizer. Food Hydrocoll 44:435–442. https://doi.org/10.1016/j.foodhyd.2014.10.019
Lopez M, Bizot H, Chambat G, Marais MF, Zykwinska A, Ralet MC, Driguez H, Buléon A (2010) Enthalpic studies of xyloglucan-cellulose interactions. Biomacromolecules 11:1417–1428. https://doi.org/10.1021/bm1002762
Nogi M, Yano H (2008) Transparent nanocomposites based on cellulose produced by bacteria offer potential innovation in the electronics device industry. Adv Mater 20:1849–1852. https://doi.org/10.1002/adma.200702559
Okiyama A, Motoki M, Yamanaka S (1993) Bacterial cellulose III. Development of a new form of cellulose. Food Hydrocoll 6:493–501. https://doi.org/10.1016/S0268-005X(09)80073-8
Park YB, Cosgrove DJ (2015) Xyloglucan and its interactions with other components of the growing cell wall. Plant Cell Physiol 56:180–194. https://doi.org/10.1093/pcp/pcu204
Paximada P, Tsouko E, Kopsahelis N, Koutinas AA, Mandala I (2016) Bacterial cellulose as stabilizer of o/w emulsions. Food Hydrocoll 53:225–232. https://doi.org/10.1016/j.foodhyd.2014.12.003
Picout DR, Ross-Murphy SB, Errington N, Harding SE (2003) Pressure cell assisted solubilization of xyloglucans: tamarind seed polysaccharide and Detarium gum. Biomacromolecules 4:799–807. https://doi.org/10.1021/bm0257659
Poddar MK, Dikshit PK (2021) Recent development in bacterial cellulose production and synthesis of cellulose based conductive polymer nanocomposites. Nano Select 2:1605–1628. https://doi.org/10.1002/nano.202100044
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. https://doi.org/10.1038/nmeth.2019
Tagawa S, Ishida K, Tsuji T, Kondo T (2021) Facile size evaluation of cellulose nanofibrils adsorbed on polypropylene substrates using fluorescence microscopy. Cellulose 28:2917–2929. https://doi.org/10.1007/s10570-021-03759-0
Tokuyasu K, Matsuki J, Yamagishi K, Ike M (2022) Characterization of the interactions between cereal flour and nata puree in batter. J Appl Glycosci Advpub:Jag. https://doi.org/10.5458/jag.jag.JAG-2022_0007. 0007:JAG-2022
Tokuyasu K, Yamagishi K, Matsuki J, Nei D, Sasaki T, Ike M (2021) ‘Nata puree,’ a novel food material for upgrading vegetable powders, made by bacterial cellulose gel disintegration in the presence of. J Appl Glycosci (1999) 68:77–87. https://doi.org/10.5458/jag.jag.JAG-2021_0009
Tsuji T, Tsuboi K, Yokota S, Tagawa S, Kondo T (2021) Characterization of an amphiphilic Janus-type surface in the cellulose nanofibril prepared by aqueous counter collision. Biomacromolecules 22:620–628. https://doi.org/10.1021/acs.biomac.0c01464
Whitney SEC, Wilson E, Webster J, Bacic A, Reid JSG, Gidley MJ (2006) Effects of structural variation in xyloglucan polymers on interactions with bacterial cellulose. Am J Bot 93:1402–1414. https://doi.org/10.3732/ajb.93.10.1402
Zhao Z, Crespi VH, Kubicki JD, Cosgrove DJ, Zhong L (2014) Molecular dynamics simulation study of xyloglucan adsorption on cellulose surfaces: effects of surface hydrophobicity and side-chain variation. Cellulose 21:1025–1039. https://doi.org/10.1007/s10570-013-0041-1
Zheng Y, Wang X, Chen Y, Wagner E, Cosgrove DJ (2018) Xyloglucan in the primary cell wall: assessment by FESEM, selective enzyme digestions and nanogold affinity tags. Plant J 93:211–226. https://doi.org/10.1111/tpj.13778
Acknowledgments
We thank Mr. Shintaro Abe and Mr. Ayaka Kikuchi for their technical assistance. This work was supported by the Cabinet Office, Government of Japan, Moonshot R&D Program for Agriculture, Forestry, and Fisheries (funding agency: Bio-Oriented Technology Research Advancement Institution), Grant Number JPJ009237. We would like to thank Editage (www.editage.com) for English language editing.
Funding
Open Access funding partially provided by Shinshu University. This work was supported by the Cabinet Office, Government of Japan, Moonshot R&D Program for Agriculture, Forestry, and Fisheries (funding agency: Bio-Oriented Technology Research Advancement Institution), Grant Number JPJ009237.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, Data collection and analysis were performed by Satomi Tagawa. The first draft of the manuscript was written by Satomi Tagawa and All authors commended on previous versions of the manuscript. Masahiro Mizuno read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tagawa, S., Tokuyasu, K., Yamagishi, K. et al. Characterization of hybrid nanofibrils composed of xyloglucan and disintegrated bacterial cellulose. Cellulose 31, 2239–2249 (2024). https://doi.org/10.1007/s10570-023-05712-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-023-05712-9