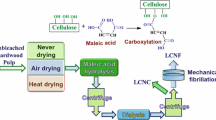
Abstract
The expanding field of lignin-containing nanocellulose offers a sustainable alternative to fossil-based substances in applications such as packaging, coatings, and composites. This has underscored the importance to explore the impact of raw materials due to the complexities of lignin structures and different raw fiber characteristics, which plays a significant role in determining the properties of the resultant lignin-rich cellulose materials. This study presents a detailed investigation and comparison on the production and structure-property relationships of lignin-containing microfibrillated cellulose (LMFC) fibers prepared from unbleached softwood and hardwood kraft pulps. The microfibrillation process was analyzed for both softwood and hardwood pulps, comparing the results across various stages of fibrillation. Distinguishing features of lignin structures in softwood and hardwood pulps were identified through Py-GC/MS analysis. Additionally, Digital Image Correlation was employed to investigate the varying failure patterns in LMFC films derived from different wood species. Softwood-derived LMFC films demonstrate less strain-concentrated regions and strain variation, attributed to the formation of more physical crosslinking joints by the elongated fibers. Consequently, softwood-origin LMFC films displayed superior load-sharing and enhanced tensile strength (287 MPa) compared to those derived from hardwood. Additionally, the denser lignin structures in unbleached softwood pulp further boosted the stiffness of resultant softwood-derived films. Upon recycling, LMFC films exhibited superior recovery of mechanical properties following drying, suggesting their significant potential for widespread commercial use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lignocellulosic biomass, the most abundant form of biomass on Earth, comprises nearly 90% of the planet’s fixed energy content. This has sparked a growing global interest in the conversion and utilization of lignocellulosic biomass for energy purposes, as well as the production of bio-based chemicals and polymers (Isikgor and Becer 2015). In conjunction with nanotechnology, lignocellulose has been used as feedstock to produce nanocellulose (Blanco et al. 2018). These bio-based materials are the best-known sustainable alternatives to petroleum-based materials, and they have shown great potential in films, coatings, packaging, absorbent, composites, and flexible electronic devices (Chen et al. 2021; Kurihara and Isogai 2014; Li et al. 2013; Xue et al. 2021; Zeng et al. 2017; Zhao et al. 2020), all of which benefit from the unique physical and chemical characteristics of nanocelluloses, such as large surface area, excellent strength, good biodegradability, and abundant hydroxyl groups.
Producing nanocelluloses from wood involves a deconstruction process on biomass cell walls. Most of the lignin and hemicellulose are removed to produce nanocelluloses with a high purity of cellulose. Different lignocellulosic biomass can be used as the raw material, with lignin-free pulp after bleaching being the most commonly used resource (Nechyporchuk et al. 2016). However, the bleaching step, intended to remove most of the remaining lignin after cooking, usually consumes much energy and chemicals, making the production of pure nanocelluloses an inherently non-sustainable procedure (Colodette et al. 2002; Hart & Kraft, 2013; Klugman et al. 2007).
Recently, a new type of nanocellulose, also known as lignin-containing nanocelluloses materials (LCNMs), has been reported (Solala et al. 2020). LCNMs can be prepared by directly converting the raw lignocellulosic feedstocks or low-cost unbleached pulps into nanoscale or microscale units without a complete delignification process. It has been reported that the preservation of lignin in nanocellulose networks can endow nanocelluloses with excellent moisture resistance and oxygen barrier properties (Huang et al. 2019; Rojo et al. 2015). LCNMs are up-and-coming candidates for packaging and composites since lignin helps to reduce moisture absorption in a watery environment. Furthermore, the thermal stability of nanocelluloses was also reported to be significantly improved due to the incorporation of lignin (Zhang et al. 2019). The inherent hydrophobic characteristic of lignin endows LCMF with better compatibility with other hydrophobic polymers in composites (Bian et al. 2018). Meanwhile, some studies reported that the mechanical properties of LCNM films could be significantly improved because of the binding function of lignin components (Jiang et al. 2020; Nair and Yan 2015; Oliaei et al. 2021).
The abundance of raw materials has greatly facilitated the production of nanocelluloses and many resources were utilized for producing nanocelluloses (Alemdar and Sain 2008; Nechyporchuk et al. 2016; Saito et al. 2009; Turbak et al. 1983; Zhao et al. 2013). So far, different lignocellulosic biomasses have also been reported for producing lignin-containing nanocellulose, including softwood pulps (Oliaei et al. 2021; Serra-Parareda et al. 2021; Turbak et al. 1983), hardwood pulps (Bian et al. 2017), bagasse (Zhang et al. 2019), wheat straw (Bian et al. 2019; Espinosa et al. 2020; Sánchez et al. 2016), and barks (Dou et al. 2019; Huang et al. 2019). Extraction of lignin-containing nanocelluloses from these lignocellulosic resources can involve different chemical or biological treatments, such as maleic acid hydrolysis (Bian et al. 2017), Tempo-oxidation (2,2,6,6 -Tetramethylpiperidine 1-oxyl) (Isogai et al. 2011; Saito et al. 2007; Wen et al. 2019), p-Toluenesulfonic (p – TsOH) acid hydrolysis (Dou et al. 2019), deep eutectic solvents (DES) treatment (Li et al. 2021; Liu et al. 2020) and enzymatic treatment (Han et al. 2021; Henriksson et al. 2007), afterwards, combining with mechanical fibrillation process to reduce the particle dimensions (Iwamoto et al. 2005; Turbak et al. 1983). Nevertheless, none of these chemicals or solvent treatments is an environmentally friendly method. They introduce extra cost to the production of LCNM and bring chemical pollution (Kargupta et al. 2021), meanwhile affecting the residual lignin content and structures. Alternatively, mechanical pretreatment can be a green and effective solution for the sustainable and scalable production of LCNM, minimizing potential pollution and consuming no chemicals or enzymes (Abe et al. 2009).
Different raw materials can vary significantly in fiber dimensions and chemical composition, all of which would affect the production efficiency of LCNM and properties of final products (Kargupta et al. 2021). Tarrés et al. reported that hemicellulose and lignin content could have a significant impact on the production of lignocellulosic nanofibers from triticale straws (Tarrés et al. 2017). A further study reported that the carboxylate groups and intrinsic viscosity of the initial pulps which are determined by their chemical composition were important factors for producing lignocellulosic nanofibers (Ehman et al. 2020). While existing research indicates that different chemical composition could significantly affect the fibrillation process and properties of resulting LCNM, few has explored further the possible impact of different lignin structures and fiber dimensions originated from distinct wood species on the properties of resulting nano- and microfibrillated cellulose with residual lignin present. Lignin, a complex polyphenolic polymer, has structures that vary greatly depending on the plant biomass source (Gioia et al. 2020; Tagami et al. 2019). Consequently, it can be anticipated that lignin-containing nanocelluloses produced from diverse wood species could have distinct properties, influenced by both lignin variations and different fiber morphology. There is a growing interest in the production of lignin-containing cellulose materials. Yet, there’s a clear knowledge gap in understanding the influence of pulp fibers, especially two of the essential industrial types of plants (softwood and hardwood), on the production and characteristics of lignin-containing cellulose nano/microfibers. This study intends to bridge this gap, offering insights important for both academia and industries utilizing lignocellulose-based products.
Herein, this work focuses on the green and facile production of lignin-containing microfibrillated cellulose fibers (LMFC) from diverse wood species with high residual lignin content (> 10%) using a chemical-free method. Scandinavian pine (softwood) and Eucalyptus (hardwood) pulps were used as the raw materials, and detailed characterizations from the different raw materials to the final products were carried out, including fiber dimensions, chemical composition, total fiber charges, and fiber development during the microfibrillation process.
The thermal property, hydrophobicity and mechanical property of LMFC materials were evaluated and compared in the form of films. The relationships between the different lignin structures derived from different raw materials and the performances of resultant lignin-containing cellulose fibers has been extensively investigated to provide a fundamental understanding of the impact of raw fibers. Analytical pyrolysis was employed to study the residual lignin in raw pulps, providing better understanding of its chemical structure’s impact on the LMFC properties. Digital Image Correlation allows us methodically explore the differing failure patterns of LMFC films derived from various wood species. The role of lignin in the recyclability of LMFC films was further analyzed. These prepared bio-based materials which integrate chemically untreated natural wood components without any additives, can be of great interest in flexible electronic devices, food packaging, and bio-composite fields.
Experimental
Materials and chemicals
Laboratory unbleached softwood (Scandinavian pine) pulp and unbleached hardwood (Eucalyptus) pulp was produced by kraft cooking and kindly provided by a Swedish company. Due to the use of different raw materials (softwood vs. hardwood), the pulping processes differed, with the conditions detailed in Table S1. The raw pulps were denoted as LSKP and LHKP, respectively, representing lignin-rich softwood kraft pulp and lignin-rich hardwood kraft pulp. Afterwards, all pulp fibers were washed into sodium ion form for better swelling and dispersion (Oliaei et al. 2020). Sodium hydroxide (Sigma Aldrich), Hydrochloric acid (37%, Sigma Aldrich), and Sulfuric acid (72%, Alfa Aesar) were purchased and used without any purification.
Pulp analysis of LSKP and LHKP
Pulp fiber dimensions were measured using Lorentzen & Wettre fiber tester (Li et al. 2011). Fiber length, width, shape factor, and the number of kinks were analyzed. Each test was repeated twice, and the averages were reported. The curl index is calculated based on the shape factor value according to Page et al. (Esteves et al. 2021; Page 1985).
Chemical composition, including Klason lignin and carbohydrates composition of both LSKP and LHKP, were determined using acid hydrolysis. The monosaccharide composition of pulp fibers was measured according to standard SCAN-CM 71: 09 and the Klason lignin content was determined according to Tappi T-UM 250. After sulfuric acid digestion, the monosaccharide composition was tested using an anion-exchange chromatography Dionex ICS-3000 (HPAEC-PAD). Klason lignin content was determined gravimetrically based on the weight of insoluble residue after acid digestion (Carvalho et al. 2019). The total charge of pulp fibers was measured by conductometric titration according to Katz et al. (Katz and Beatson 1984) using a Metrohm titrator (Titrino 702SM). All pulp fibers were transformed to H+ protonated form before titration, and 0.1 M NaOH was used as the titration agent.
Py-GC/MS (Pyrolysis Gas Chromatography-Mass Spectrometry) is an analytical technique used to study the molecular fragments released during pyrolysis in combination with gas chromatography and mass spectrometry to identify the original substance of materials (Ponomarenko et al. 2015; Sitholé 2006). It can analyze insoluble materials with complex structures at trace concentrations, making it a powerful tool for analyzing lignocellulosic materials (Svärd et al. 2016). The Py-GC/MS analysis was performed using a Frontier Lab (Japan) Micro Double-shot Pyrolyser Py-2020iD directly coupled with Shimadzu GC/MS—QP 2010 apparatus (Japan) with capillary column RTX-1701 (Restec, USA), 60 m × 0.25 mm × 0.25 μm film (injector temperature 250 °C, ion source 250 °C with EI of 70 eV, MS scan range m/z 15–350, carrier gas helium at the flow rate of 1 mL min−1 and the split ratio 1:30). The pyrolysis was conducted at 500 °C. The weight of each sample (residual moisture content < 1%) was 1.00–2.00 mg. The oven program was isothermal at 60 °C for 1 min, then 6 K min−1 to 270 °C, and finally 270 °C for 10 min. The mass spectrometer was operated in the electron impact mode using 70 eV electron energy. The relative error of measurements was 1–3%.
Preparation of LMFC gels via mechanical fibrillation
All lignin-rich pulp fibers were first beaten in a PFI mill to a level of 30,000 revolutions and then mechanically fibrillated via Supermasscolloider (MKZA 10-15 J), each sample for 10 passes. Intermediate products of both LSKP and LHKP were collected at the 4th, 6th, and 8th passes during the microfibrillation process.
Fabrication of LMFC films
Firstly, LMFC gels were diluted to a concentration of 0.2 wt% and then stirred using Ultra Turrax for 10 min at 12,000 rpm for better dispersion of LMFC fibers. Afterward, all aqueous suspensions of LMFC samples were vacuum filtrated through a DVPP membrane (0.65 μm, Durapore) and followed by drying in a sheet former Rapid Köthen at 93 ± 2 °C for 15 min. The LMFC films prepared from softwood and hardwood were named LMFC-SW and LMFC-HW, respectively.
Characterizations of LMFC gels and LMFC films
DLS measurement
LMFC-SW and LMFC-HW gels were diluted to a concentration of 0.01wt% to avoid particle agglomeration at higher concentrations (Reid et al. 2017). The hydrodynamic radius of micro-sized lignin-containing cellulose fibers was measured at 25℃ using Malvern Zetasizer. Three measurement runs were set for each test using 173 backscattering modes, and all samples were measured 15 times in each run.
Concentration determination
The concentration of LMFC-SW and LMFC-HW gel samples were measured at different grinding passes. For the concentration determination, 1 mL of each sample in an Eppendorf tube was weighed (w1) and then freeze-dried. The concentration was then calculated gravimetrically based on the weight of the freeze-dried sample (w2) according to the following equation:
Surface charge measurement
The surface charge of intermediate gels was measured by Stabino polyelectrolyte titration using Poly-DADMAC (Poly-diallyldimethylammonium chloride) as the titration agent (Horvath 2003). Each sample was measured at least twice.
Water contact angle measurement
A contact angle analyzer (Theta Lite, Biolin Scientific, Finland) was used to measure the water contact angle of films. A 4 µL droplet was dropped at different locations on the surface, and the contact angle was reported at 10 s. All measurements were conducted in triplicate.
Thermogravimetric analysis
Thermogravimetric analysis (TGA) was conducted using a Mettler Toledo TGA/DSC instrument. 5–8 mg samples were loaded in crucibles and pressed to ensure good contact with the bottom. The experiments were performed from 30 to 700 °C under a nitrogen atmosphere with a heating rate of 10 °C min−1. A decomposition temperature is defined as the intersection of the line (connection of weight loss percentages of 20% and 50%) and the extension line of the TGA baseline. A maximum weight loss temperature Tmax is defined as the maximum peak value of DTG curves.
Physical-mechanical properties
The thickness of films was measured at least on 10 points using a digital thickness tester (Mituyoko S112SB, Japan), and the averages were reported and used for density calculation. The mechanical properties were measured according to ISO 527-2. Specifically, 5 mm strips were cut from each sample and loaded into Instron 5944 tensile machine equipped with a video extensometer. A constant elongation rate of 5 mm min−1 was used for the test, and at least 10 specimens of each sample were tested.
Microscopy
A Hitachi S-4800 field emission was used for morphological studies on both LMFC suspensions and films. To investigate the morphology development following different grinding passes, each gel sample of LMFC-SW and LMFC-HW at the 4th, 6th, 8th and 10th cycles was diluted and then freeze-dried. Subsequently, samples were attached to carbon tape and sputter coated with a platinum-palladium layer for observation. To statically analyze the diameter distribution of LMFC fibers, diluted suspensions of microfibers were filtrated via aluminum oxide membranes with nanopores of around 20 nm (FlexiPor 20 nm; SmartMembranes GmbH, Halle, Germany) to reduce the loss of fibers in filtration as well as to alleviate the aggregation of fibers in the sample preparation step (Larsson et al. 2019). Afterwards, the membranes were attached on a carbon tape and then sputter coated using 208 h Cressington Sputter Coater (SmartMembranes GmbH, Germany). The SEM images were analyzed in ImageJ, and at least 150 fibrils were collected and analyzed for diameter distribution. For morphological observation on LMFC films, the sample was cut and then put on the substrate horizontally and vertically to obtain images of the surface and fracture line.
Digital image correlation (DIC)
Digital image correlation (Chen and Coppieters 2023; Schreier et al. 2009), a full-field optical method, was used to measure the strain distribution on LMFC-SW and LMFC-HW samples. Three strips of each sample were prepared with a width of 5 mm and a gauge length of 25 mm. Their surfaces were sprayed with random white speckle patterns as the carrier of deformation information. All these samples are loaded with a constant speed of 5 mm/min. The images on the sample were recorded by the stereo-digital image correlation (stereo-DIC) system equipped with two Basler acA4096-30 μm cameras (resolution of 4096 \(\times\) 2168 pixels) with a frequency of 5 Hz/s. The recorded images were processed using VIC-3D (Correlated Solutions, USA) with a subset size of 31 \(\times\) 31 pixels, step size of 5 pixels, and strain window size of 11 \(\times\) 11 points. The Poisson’s ratio was estimated from the retrieved strain fields.
Results and discussion
Pulp Properties
Lignin-containing cellulose gel products were mechanically produced from unbleached softwood (Scandinavian pine) and hardwood (Eucalyptus) kraft pulps, as shown in Fig. 1a. To understand the microfibrillation process that transforms raw pulp fibers into lignin-containing cellulose gels, characteristics of raw pulp fibers were studied, such as chemical composition, fiber dimensions, and total fiber charges, which potentially influence the raw fibers’ fibrillation efficiency.
Softwood and hardwood pulp fibers are inherently various in fiber morphology, chemical composition, and intrinsic strength. Figure 1b and c showed the morphology of unbleached softwood and hardwood raw pulp fibers, and LHKP fibers showed a more rigid structure with a lower curl index (Fig. 1f), indicating the presence of a greater number of straight fibers compared to softwood. Furthermore, LSKP has a larger mean length, around three times that of LHKP and the average width of LSKP is around twice as wide as LHKP fibers. Both LSKP and LHKP showed a high pulp yield (Table S2), this makes the production of nanocellulose from lignin-rich pulps very competitive economically. The higher pulp yield of hardwood is likely resulted from the higher percentage of lignin component preserved in LHKP.
In Table 1, the chemical composition of raw pulps is determined using acid hydrolysis. LHKP and LSKP showed a hemicellulose content of 10.2% and 15.1%, respectively. In the eucalyptus pulp, xylans are the main components of hemicelluloses, whereas, in pine pulp, the content of hemicelluloses consists mainly of xylans and mannans, but other monosugars (arabinose and galactose) also account for small percentages (Ehman et al. 2020). As shown in Table 1, the Klason lignin content of LSKP and LHKP is 10.9% and 14.3%, indicating a large amount of residual lignin preserved in both samples.
To further analyze the chemical composition of LHKP and LSKP, Py-GC/MS was conducted. The results from Py-GC/MS analysis are shown in Table 2. Accordingly, the relative carbohydrate composition of softwood kraft pulp is 59.8% and 47.1% for hardwood kraft pulp. During analytical pyrolysis, with a typically higher temperature (in a range of 500 °C), carbohydrates can further degrade to various compounds, for example, acids, furans, aldehydes and ketones, anhydrosugars (details in Table S3). Specifically, the main thermal degradation product of carbohydrates obtained by Py-GC/MS is aldehyde/ketone, taking up 45.8% of carbohydrates from chromatogram in LSKP. Aldehydes and ketones are formed from the dehydration, fragmentation, or rearrangement of cellulose and hemicellulose during pyrolysis. Around 15.9% of the carbohydrates in LSKP are decomposed to furan, mainly furfural and furanone, which is attributed to the degradation of furanose rings present in carbohydrates, particularly in hemicellulose (Ansari et al. 2019). For hardwood kraft pulp, around 52.3% and 14.3% of carbohydrates in LHKP are decomposed to aldehyde/ketone and furan, respectively.
Kleen et al. reported that Py-Gc/Ms method showed good reproducibility in predicting the main composition in pulp, i.e., glucose, xylose, mannose, and lignin (Kleen et al. 1993). As shown in Table 2, the quantitative analysis of the relative content of lignin and carbohydrate composition of LSKP is similar to the chemical composition determined by the acid hydrolysis method shown in Table 1. However, the relative composition of carbohydrates and lignin in LHKP did not correlate well with results from acid hydrolysis shown in Table 1. A lower percentage of Klason lignin in LHKP was detected from wet chemistry measurement, probably due to the higher amount of acid-soluble lignin in hardwood samples. The amount of soluble lignin is reported to be about 0.2 to 0.5% in softwoods (coniferous woods) and sulfate pulps, while in hardwoods (deciduous woods) and non-wood fibers, the content of soluble lignin is up to 3 to 5%. In pulps with a lignin content of more than 1%, the soluble lignin content could constitute about one-half or more of the total lignin content (TAPPI 2006). It is worth mentioning that Py-Gc/Ms and sugar analysis are two essentially different analytical methods, where the lignin content is determined based on the identification and quantification of lignin-derived compounds after pyrolysis in Py-Gc/Ms analysis, while the determination of lignin content using sugar analysis is based on the obtained residue after acid hydrolysis of carbohydrates into monosugars. However, a similar trend was observed in these two methods that LHKP showed a higher lignin content compared to LSKP. A high percentage of residual lignin was preserved in both unbleached pulps.
Figure 1g shows the total charge or total carboxylate content of raw pulps. LHKP exhibited a higher total fiber charge of 160 µeq/g, indicating their higher swelling ability compared to LSKP. In a previous study, fiber charges of unbleached pulps were reported to be correlated with glucuronic acids in hemicellulose (xylan) and carboxylic acids from residual lignin (Laine et al. 1994). Most carboxyl groups in unbleached kraft pulp fibers originate from methylglucuronic acid side groups in xylan, while some aliphatic carboxylic acids connected to cellulose or lignin structures could also provide extra charges, explaining the higher total charge of unbleached pulps (Sjostrom 1989). Generally, the fiber charge decreases with a decreased kappa number. In the bleaching step, most of the charged groups are removed along with lignin components. Therefore, a lower charge is achieved in fully bleached pulps (Esteves et al. 2022). Generally, a higher fiber charge is more readily for the fibrillation of fibers (Afra et al. 2013; Ehman et al. 2016, 2020). This can lead to electrostatic repulsion between fibers with a better dispersal capacity of raw fibers (Oliaei et al. 2020).
Microfibrillation process
The above-characterized LSKP and LHKP samples were used to produce lignin-containing microfibrillated cellulose products. To investigate the impact of different wood species on the fibrillation process of high lignin content pulps, concentration and surface charge of gel products at 4th, 6th, 8th and 10th fibrillation passes were studied, and the curves were plotted in Fig. 2a and b. A decrease in the concentration of both LSKP and LHKP can be seen in Fig. 2b from the 6th to 8th microfibrillation pass as a result of the successful separation and breaking down of fibers into smaller fibrils. Surface charge measurement can be determined as a method to assess the degree of fibrillation owing to its sensitivity to the specific surface area, thus, the particle size of cellulose fibrils (Larsson et al. 2019). Espinosa et al. further calculated the diameter of LCNF fibers based on the poly-DADMAC surface adsorption on the fibers which allows an approximate estimation of the fiber dimensions (Espinosa et al. 2016). In Fig. 2a, the surface charge of LHKP increased significantly from the 4th pass to the 8th pass, indicating a higher degree of fibrillation of LHKP compared to LSKP. Overall, hardwood pulp fibers are more readily disintegrated than softwood fibers. On the one hand, it can attribute to the smaller dimensions of LHKP raw fibers. On the other hand, the higher fiber charge of raw fibers can make them more susceptible to outside forces and facilitate their disintegration into smaller-sized fragments in the fibrillation step (Tagami et al. 2019).
The freeze-drying method was used to dry the gel samples at a diluted concentration to study the fiber morphology during different fibrillation passes. Figure 2c-j show the images of softwood and hardwood pulp fibers at the 4th, 6th, 8th, and 10th grinding passes. Due to the presence of high-content lignin components, which act as the binder between cellulose fibrils in raw plants, few fibrillated lignin-containing cellulose fibers can be seen in Fig. 2c and g at the initial grinding passes. However, with increased grinding passes, in other words, higher energy input, a larger number of free fibers can be obtained (Fig. 4e and i). This trend also matches the drastic growth of surface charge in Fig. 2a from the 6th to 8th passes, indicating the successful fibrillation of raw pulp fibers from the 6th pass.
The size distribution of LMFC suspensions obtained from softwood and hardwood pulps after the 10th grinding pass was analyzed and presented in Fig. 3. Both LMFC-SW and LMFC-HW showed a wide diameter distribution range. Figure 3a and c show that more aggregates were presented in LMFC-SW samples. The diameter distribution is further fitted by log-norm distribution; see the blue curves in Fig. 3b and d. The diameter’s mean value and standard deviation are 74 and 69 nm for LMFC-SW, 53 and 57 nm for LMFC-HW samples, which confirms the smaller diameter and diameter distribution for LMFC-HW fibers. This difference is attributed to the lower ease of fibrillation in LMFC-SW samples compared to LMFC-HW with the same energy input. Moreover, hardwood’s intrinsic smaller fiber dimensions would also affect the size distribution of LMFC products.
González et al. compared the widths distribution of microfibrillated cellulose fibers obtained from different pulps using different techniques. Their findings highlight that MFC size determinations might not accurately represent the actual distribution of dimensions. This is because various microscopy techniques measure only some fractions of fibrils which is limited by the techniques (González et al. 2022). Consequently, the methods employed to assess MFC width distribution can significantly influence the calculated width. It’s important to note that the method used in our study might also introduce discrepancies from the actual MFC dimensions due to potential loss of finer fibrils during filtration and the constraints of the SEM technique.
Properties of LMFC-SW and LMFC-HW films
To evaluate the lignin-containing microfibrillated cellulose products produced from softwood and hardwood pulps, films were made from both LMFC-SW and LMFC-HW samples. As shown in Fig. 4, the high amount of lignin in both samples resulted in a yellowish appearance. The surface and fracture morphology of LMFC-SW and LMFC-HW films are also shown in Fig. 4. Compared to LMFC-HW films, LMFC-SW showed a more intertwined network of lignin-containing cellulose fibers from the surface. Both LMFC-HW and LMFC-SW films showed layer structures formed by the cellulose fibrils, as shown in Fig. 4.
In Fig. 5a and Table S4, LMFC films produced from hardwood showed a higher water contact angle compared to softwood, which is likely caused by the higher content of lignin components remained in LMFC-HW films, as lignin is a more hydrophobic polymer due to the aromatic moieties in the three-dimensional structures of lignin. As shown in Fig. 5d and e, LMFC-SW showed higher thermal stability with a decomposition temperature of 306 o C and T max of 349 o C, compared to LMFC-HW films with a decomposition temperature of 293 o C and T max of 340 o C (Table S5). A similar trend was also observed between softwood and hardwood kraft lignin. Ayumu et al. reported that softwood kraft lignin showed a higher T max of 395.4 o C than 376.4 o C compared to hardwood kraft lignin (Tagami et al. 2019). The higher thermal stability of both LMFC-SW and softwood kraft lignin is likely attributed to the higher portion of guaiacyl units presented in softwood with higher thermal stability (Poletto 2017).
LMFC gels from different wood species were further evaluated by measuring their mechanical properties as self-standing films. The tensile strength of LMFC-SW and LMFC-HW were tested, and the results are shown in Fig. 5b and c. Compared to LMFC-HW, LMFC-SW showed a much higher tensile strength of 287.8 MPa and a higher Young’s modulus. To examine the variations in the mechanical characteristics of cellulose films with high lignin content derived from diverse wood types, a series of investigations were conducted to delve deeper into how varying raw pulp fibers influence the properties of microfibrillated cellulose films with high lignin content.
Effect of raw materials on strength of LMFC-SW and LMFC-HW films
After the same fibrillation process, the produced LMFC-SW and LMFC-HW showed distinct fiber length and width due to the different intrinsic pulp fiber dimensions originated from softwood and hardwood, as shown in Fig. 1b and c. Figure S7 shows the relative particle size of both LMFC samples from the DLS test. Due to the branched structures and larger particle dimensions of LMFC, it is challenging to conduct statistical analysis on the fibril length based on SEM or AFM images. DLS determines particle size based on the scattering intensity from particles undergoing Brownian motion, and it provides insights into the relative fibril length when performing the same testing conditions and concentrations for all samples (Reid et al. 2017). In Figure S7, LMFC-SW showed a relatively higher mean fiber length of 7.4 μm than HW, measured at 5.0 μm. Longer fibers could form more joints within the network of lignin-containing nanocellulose fibers, and the shear-lag effect (Kulachenko et al. 2012) is less pronounced, transforming into greater strength (Motamedian et al. 2019). Meanwhile, they have more contact points for forming joints and interconnected networks, as shown in Figure S8. More joints within networks can benefit stress transferring during a tensile test. Herein, LMFC-SW films showed a much higher tensile strength than LMFC-HW under comparable film densities (Table S6).
a Original specimen with speckle pattern; b Fractured specimen at the end of the test; c Strain fluctuation along the vertical middle lines as recorded by DIC; d Strain fields at time 7.8 s, corresponding to tensile strain of 2,6 %; e Strain fields at time 19.8 s, corresponding to tensile strain of 6.6 %; f COV of tensile strain as a function of applied strain
The mechanical behavior of LMFC-SW and LMFC-HW samples was further studied in the uniaxial tensile test using digital image correlation (DIC) to verify the assumption further. Figure 6a and b show the images of sample surface at the initial and fracture state, respectively, and the whole loading progress was showcased in the video (Supporting S9). The Poisson’s ratio was estimated from the retrieved strain fields, as shown in Figure S10. They are measured to be around 0.3 for both samples with minimal deviation, suggesting they were close to being isotropic in plane (Kulachenko et al. 2012). The images recorded for all strips were further processed by DIC algorithm to retrieve the full-field surface strain distribution on all strips. Figure 6d and e show the tensile strain fields at two states, i.e., at 7.8 and 19.8 s. More significant strain concentration regions are present at both states on the LMFC-HW sample surface; see the red regions on the strain maps. The large local strain (see the red regions) dedicates a smaller modulus related to inadequate fiber reinforcement and joints in these regions. In contrast, the blue regions have a larger modulus.
More red regions appeared in LMFC-HW strips during the tensile process, indicating that more strain-concentrated regions were exhibited in HW samples, while in SW samples, few strain-concentrated regions were observed, meaning better stress transfer during the tensile test. The same trend can also be seen at 19.8 s. The red regions expanded considerably in HW strips, whereas SW samples maintained a desirable strain distribution under a constant elongation rate, as shown in Fig. 6e.
LMFC-SW samples exhibited a more uniform strain distribution, likely attributed to the better fiber physical crosslinking concentration at those regions formed by the longer fibrils. The strain inhomogeneity shown on the map reflects the differences in fiber physical-crosslinking distribution between LMFC-HW and SW samples. SW samples showed fewer red regions than HW because more fiber joints formed from the longer fibrils. The SEM images on surface structures of both SW and HW films further verified the entanglement between longer softwood fibrils and the formation of more integrated networks, as shown in Figure S8. Consequently, it demonstrates a better strain distribution within SW fibers networks in the substrate.
When comparing the strain fields at 7.8 and 19.8 s, with measured applied strains of 2.6% and 6.6%, respectively, the areas of strain inhomogeneity remained consistent, suggesting that the stretching of the samples did not markedly alter the microstructure, including aspects such as fiber orientation and concentration. The majority of the deformation appears to be due to fibers’ elongation. Inspecting the strain fields (Fig. 6d and e) and comparing them to the fractured samples (Fig. 6b), It is found that hardwood samples fractured near the strain concentration areas, while the fracture in the SW samples was not aligned with the regions of high local strain. Furthermore, the crack was aligned normal to the applied stress. This suggests the failure mode in softwood was likely dominated by the fracture of the fibers rather than еру shear-dominated pull-out mechanisms, meaning that the crosslinking between the softwood fibers was exceptionally high, and the limiting factor was the fibers’ strength.
To better evaluate the strain inhomogeneity, the strain fluctuation along the vertical middle lines was extracted and shown in Fig. 6c. Both materials have strain fluctuation phenomenon, but LMFC-HW films show larger amplitudes (only the strain of HW exceed the two dashed lines, see Fig. 6c) on the strain curves. This is also a demonstration of the more significant strain inhomogeneity in the HW films. More fiber joints formed from the physical crosslinking and longer LMFC-SW fibers can reduce the impact of uniform strain distribution. To evaluate the evolution of the strain distribution during the uniaxial tensile test, the coefficient of variation (COV), defined as the ratio between the standard deviation and the mean of the strain, was measured for all samples (Fig. 6f). The LMFC-HW samples held larger strain heterogeneous than LMFC-SW samples during the whole loading progress suggesting that the load sharing was better in LMFC-SW owing to longer fibers.
Effect of raw materials on stiffness of LMFC-SW and LMFC-HW films
Owing to the presence of a significant portion of residual lignin components in both LMFC-SW and HW films, the different chemical structures of lignin components could also affect the final properties of films. Herein, it is important to investigate the connections between different lignin structures and their potential impact on the final properties of lignin-containing films.
Structures of residual lignin after the kraft pulping process differ significantly from native lignin structures due to the reactions in the pulping step. The native lignin structure is known for its complexity, where β-O-4’ linkages are largely present. However, they are less common in residual lignin because of the breakage of β-O-4’ linkages in the kraft process, which could create more phenolic structures in remaining lignin. Meanwhile, some reduced structures may also be formed (Fig. 7). To further investigate the potential impact of different residual lignin structures derived from the different wood species (unbleached softwood and hardwood raw pulps) on the mechanical properties of the fabricated LMFC films. Py-GC/MS method was used to analyze the structures of residual lignin from the original raw pulps.
In Table 2, the lignin derivates of both LSKP and LHKP samples were analyzed using analytical pyrolysis, more specifically, phenyl and benzyl derivates, guaiacyl derivates, and syringyl derivates of each sample. The phenyl and benzyl derivates account for 21.0% and 7.2% of SW and HW raw pulps, respectively. A high amount of syringyl derivates was exhibited in HW samples, accounting for 73.2% of lignin derivates, while residual lignin preserved in SW samples was almost composed of guaiacyl-based units. These distinctions result from the different monolignols composition in native softwood and hardwood lignins, namely, coniferyl alcohol and sinapyl alcohol. Sinapyl-based lignin does not form condensed structures (monolignols connected via carbon-carbon linkages at the C5 position) due to a methoxy group at the C5 position, preventing the coupling reactions at this position. Hence, a smaller portion of C5 condensed structures appears in LMFC-HW samples because of the different lignin structures originating from different wood species.
As illustrated in Fig. 5f, LMFC-SW exhibited an elevated elastic modulus of 15 GPa, in contrast to the modulus of 13 GPa in HW films. This disparity is likely a result of the combined effects of the formation of an increased number of condensed C5 linkages in SW samples and their longer fibers preserved. The higher amount of condensed C5 linkages in SW samples could give rise to a more complicated and rigid three-dimensional lignin structure in LMFC-SW samples because of the high amount of guaiacyl units (Table 1). The high aromatic density and low molecular mobility of condensed C5 structures led to a higher stiffness owing to the lack of free rotation between monomers (Gioia et al. 2020). Furthermore, SW’s longer fibers can also enhance films’ stiffness through the shear-lag effect (Kulachenko et al. 2012), leading to a lower fraction of free unstressed fiber segments. This further suggests that different raw materials can have a significant impact on the mechanical properties of resultant lignin-rich cellulose films due to different lignin structures and fiber morphology displayed. A further investigation can be done to conduct a comparative analysis of films from the same raw material with selective delignification to remove lignin while preserving the fiber length (Table S11). This method can serve as an ideal approach to further isolate the impact of lignin content/structure from fiber length effects.
Recyclability
To assess the recyclability of LMFC films, dried leftover of LMFC films were cut into small pieces and then dispersed in deionized water, followed by magnetic stirring and Turrax mixer stirring. As displayed in Fig. 8a, the recycled LMFC-SW and LMFC-HW films registered tensile strengths of 206 MPa and 148 MPa, respectively. Even after recycling, the softwood samples retained a higher Young’s modulus than the hardwood samples.
The biological compatibility of lignin polymers with cellulose did not destroy the recyclability of cellulose materials. Lignin-containing cellulose fibers can be well dispersed after drying and then fabricated into films, as shown in Fig. 8b and c. The recovery of tensile strength for LMFC-SW and LMFC-HW films was 71.5% and 63.2%, respectively, both of which are higher than the reported MFC films, with a recovery of tensile strength of 50.5% when dried at 100 o C (Silva et al. 2021).
Hornification is a well-known process that occurs during cellulose fiber drying due to the proximity of cellulose chains coming into close contact (Nordenström et al. 2021; Silva et al. 2021). The formation of intermolecular bonding and irreversible aggregation of fibers usually lead to reduced internal volume and lower water absorption capacity after hornification. This will lead to a decreased swelling of those fibers when exposed to a water environment compared to the original fibers (Ballesteros et al. 2017). LMFC films showed a higher recovery degree of mechanical properties than MFC films, which is likely caused by the residual lignin between fibers that help reduce the formation of hydrogen bonds during the drying process. It is believed that hemicellulose and hemicellulose-lignin matrix could help to sustain the contact between cellulosic surfaces, preventing the occurrence of hornification (Wan et al. 2010). Herein, LMFC could have a better redispersibility compared to MFC materials. High recovery of tensile properties is vital in the commercialization and transportation of nanocellulose production since MFC may need to be dried for transportation and then redispersed for further use. When MFC is dewatered, the occurrence of morphological and chemical changes between fibers usually cannot be recovered completely in redispersion (Ämmälä et al. 2021; Moser et al. 2018). This trend is observed in both pure nanocellulose and lignin-containing nanocellulose materials. However, the higher recovery of mechanical properties for LMFC makes them more promising candidates in the commercialization of the nanocellulose market.
Conclusions
In this study, we investigated the impact of different raw fibers on their mechanical fibrillation to produce lignin-containing microfibrillated cellulose (LMFC) fibers from lignin-rich softwood and hardwood kraft pulps. Hardwood pulp was more readily fibrillated than softwood when subjected to the same energy input, while softwood-derived LMFC demonstrated superior thermal stability and mechanical properties.
We employed strain field measurements with Digital Image Correlation to examine the failure mechanisms of LMFC films derived from varied wood species. Less strain-concentrated regions were shown within softwood-derived LMFC films due to the increased physical crosslinking joints formed by longer fibers sourced from softwood pulp. Failure modes of LMFC-SW were predominantly governed by fibril fracture, signifying exceptional crosslinking strength between fibers. These softwood-derived films exhibited better stress transfer, more uniform stress distribution and higher strength (287 MPa) compared to hardwood counterparts.
The distinct lignin structures in softwood and hardwood species leads to the presence of more condensed structures in LMFC-SW films. The synergistic effect of the denser lignin structures and SW’s longer fibers contribute to the higher stiffness (Young’s modulus) of LMFC-SW films. Furthermore, recyclability tests revealed the higher mechanical property recovery (> 60%) in lignin-containing films compared to traditional MFC films post-drying. The findings established the relationship between raw fibers and the performances of resulting lignin-containing cellulose materials, offering crucial insights into lignin-containing nanocellulose production and contributing to the development of a broader, more sustainable, and high-yielding market for these materials.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
Abe K, Nakatsubo F, Yano H (2009) High-strength nanocomposite based on fibrillated chemi-thermomechanical pulp. Compos Sci Technol 69(14):2434–2437. https://doi.org/10.1016/j.compscitech.2009.06.015
Afra E, Yousefi H, Hadilam MM, Nishino T (2013) Comparative effect of mechanical beating and nanofibrillation of cellulose on paper properties made from bagasse and softwood pulps. Carbohydr Polym 97(2):725–730
Alemdar A, Sain M (2008) Isolation and characterization of nanofibers from agricultural residues—wheat straw and soy hulls. Bioresour Technol 99(6):1664–1671
Ämmälä A, Sirviö JA, Liimatainen H (2021) Energy consumption, physical properties and reinforcing ability of microfibrillated cellulose with high lignin content made from non-delignified spruce and pine sawdust. Ind Crops Prod 170(May):113738. https://doi.org/10.1016/j.indcrop.2021.113738
Ansari KB, Arora JS, Chew JW, Dauenhauer PJ, Mushrif SH (2019) Fast pyrolysis of cellulose, hemicellulose, and lignin: effect of operating temperature on bio-oil yield and composition and insights into the intrinsic pyrolysis chemistry. Ind Eng Chem Res 58(35):15838–15852
Ballesteros JEM, dos Santos V, Mármol G, Frías M, Fiorelli J (2017) Potential of the hornification treatment on eucalyptus and pine fibers for fiber-cement applications. Cellulose 24(5):2275–2286. https://doi.org/10.1007/s10570-017-1253-6
Bian H, Chen L, Dai H, Zhu JY (2017) Integrated production of lignin containing cellulose nanocrystals (LCNC) and nanofibrils (LCNF) using an easily recyclable di-carboxylic acid. Carbohydr Polym 167:167–176. https://doi.org/10.1016/j.carbpol.2017.03.050
Bian H, Gao Y, Luo J, Jiao L, Wu W, Fang G, Dai H (2019) Lignocellulosic nanofibrils produced using wheat straw and their pulping solid residue: from agricultural waste to cellulose nanomaterials. Waste Manag 91:1–8. https://doi.org/10.1016/j.wasman.2019.04.052
Bian H, Gao Y, Wang R, Liu Z, Wu W, Dai H (2018) Contribution of lignin to the surface structure and physical performance of cellulose nanofibrils film. Cellulose 25(2):1309–1318. https://doi.org/10.1007/s10570-018-1658-x
Blanco A, Monte MC, Campano C, Balea A, Merayo N, Negro C (2018) Nanocellulose for industrial use: cellulose nanofibers (CNF), cellulose nanocrystals (CNC), and bacterial cellulose (BC). In: Handbook of nanomaterials for Industrial Applications. Elsevier Inc. https://doi.org/10.1016/B978-0-12-813351-4.00005-5
de Carvalho DM, Moser C, Lindström ME (2018) Sevastyanova O (2019) Impact of the chemical composition of cellulosic materials on the nanofibrillation process and nanopaper properties. Ind Crops Prod 127:203–211. https://doi.org/10.1016/j.indcrop.2018.10.052
Chen B, Coppieters S (2023) Meshfree digital image correlation using element free galerkin method: theory, algorithm and validation. Exp Mech 63(3):517–528
Chen Y, Zhang L, Yang Y, Pang B, Xu W, Duan G, Jiang S, Zhang K (2021) Recent progress on nanocellulose aerogels: Preparation, modification, composite fabrication, applications. Adv Mater 33(11):2005569. https://doi.org/10.1002/adma.202005569
Colodette JL, Gomide JL, Girard R, Jääskeläinen A-S, Argyropoulos DS (2002) Influence of pulping conditions on eucalyptus kraft pulp yield, quality, and bleachability. Tappi J 1(1):14–20
Dou J, Bian H, Yelle DJ, Ago M, Vajanto K, Vuorinen T, Zhu J (2019) Lignin containing cellulose nanofibril production from willow bark at 80 °C using a highly recyclable acid hydrotrope. Industrial Crops and Products 129:15–23. https://doi.org/10.1016/j.indcrop.2018.11.033
Ehman NV, Lourenço AF, McDonagh BH, Vallejos ME, Felissia FE, Ferreira PJT, Chinga-Carrasco G, Area MC (2020) Influence of initial chemical composition and characteristics of pulps on the production and properties of lignocellulosic nanofibers. Int J Biol Macromol 143:453–461. https://doi.org/10.1016/j.ijbiomac.2019.10.165
Ehman NV, Tarrés Q, Delgado-Aguilar M, Vallejos ME, Felissia F, Area MC, Mutjé P (2016) From pine sawdust to cellulose nanofibres. Cellul Chem Technol 50(3–4):361–367
Espinosa E, Rol F, Bras J, Rodríguez A (2020) Use of multi-factorial analysis to determine the quality of cellulose nanofibers: effect of nanofibrillation treatment and residual lignin content. Cellulose 27(18):10689–10705. https://doi.org/10.1007/s10570-020-03136-3
Espinosa E, Tarrés Q, Delgado-Aguilar M, González I, Mutjé P, Rodríguez A (2016) Suitability of wheat straw semichemical pulp for the fabrication of lignocellulosic nanofibres and their application to papermaking slurries. Cellulose 23(1):837–852. https://doi.org/10.1007/s10570-015-0807-8
Esteves CV, Sevastyanova O, Östlund S, Brännvall E (2021) Differences and similarities between kraft and oxygen delignification of softwood fibers: effects on chemical and physical properties. Cellulose 28(5):3149–3167. https://doi.org/10.1007/s10570-021-03713-0
Esteves CVG, Sevastyanova O, Östlund S, Brännvall E (2022) The impact of bleaching on the yield of softwood kraft pulps obtained by high alkali impregnation. Nord Pulp Pap Res J 37(4):593–608. https://doi.org/10.1515/npprj-2022-0035
Gioia C, Colonna M, Tagami A, Medina L, Sevastyanova O, Berglund LA, Lawoko M (2020) Lignin-based epoxy resins: unravelling the relationship between structure and material properties. Biomacromol 21(5):1920–1928. https://doi.org/10.1021/acs.biomac.0c00057
González G, Ehman NV, Aguerre YS, Henríquez-Gallegos S, da Fonte APN, Muniz GIB, Pereira M, Carneiro ME, Vallejos ME, Felissia FE, Area MC (2022) Quality of microfibrillated cellulose produced from unbleached pine sawdust pulp as an environmentally friendly source. Waste Biomass Valoriz 13(3):1609–1626. https://doi.org/10.1007/s12649-021-01615-7
Han X, Bi R, Khatri V, Oguzlu H, Takada M, Jiang J, Jiang F, Bao J, Saddler JN (2021) Use of endoglucanase and accessory enzymes to facilitate mechanical pulp nanofibrillation. ACS Sustain Chem Eng 9(3):1406–1413. https://doi.org/10.1021/acssuschemeng.0c08588
Hart РW, Kraft ECF (2013) Pulp bleaching: a review of the development and use of techno-economic models to optimize cost, performance, and justify capital expenditures. Tappi J 12(10):19–29
Henriksson M, Henriksson G, Berglund LA, Lindström T (2007) An environmentally friendly method for enzyme-assisted preparation of microfibrillated cellulose (MFC) nanofibers. Eur Polymer J 43(8):3434–3441
Horvath E (2003) Appropriate conditions for polyelectrolyte titration to determine the charge of cellulosic fibers, Licentiate Thesis, KTH, Sweden. http://kth.diva-portal.org/smash/record.jsf?pid=diva2:7656
Huang Y, Nair SS, Chen H, Fei B, Yan N, Feng Q (2019) Lignin-rich nanocellulose fibrils isolated from parenchyma cells and fiber cells of western red cedar bark. ACS Sustain Chem Eng 7(18):15607–15616. https://doi.org/10.1021/acssuschemeng.9b03634
Isikgor FH, Becer CR (2015) Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers. Polym Chem 6(25):4497–4559. https://doi.org/10.1039/c5py00263j
Isogai A, Saito T, Fukuzumi H (2011) TEMPO-oxidized cellulose nanofibers. Nanoscale 3(1):71–85. https://doi.org/10.1039/c0nr00583e
Iwamoto S, Nakagaito AN, Yano H, Nogi M (2005) Optically transparent composites reinforced with plant fiber-based nanofibers. Appl Phys A: Mater Sci Process 81(6):1109–1112. https://doi.org/10.1007/s00339-005-3316-z
Jiang B, Chen C, Liang Z, He S, Kuang Y, Song J, Mi R, Chen G, Jiao M, Hu L (2020) Lignin as a wood-inspired binder enabled strong, water stable, and biodegradable paper for plastic replacement. Adv Funct Mater 30(4):1–11. https://doi.org/10.1002/adfm.201906307
Kargupta W, Seifert R, Martinez M, Olson J, Tanner J, Batchelor W (2021) Sustainable production process of mechanically prepared nanocellulose from hardwood and softwood: a comparative investigation of refining energy consumption at laboratory and pilot scale. Ind Crops Prod 171(July):113868. https://doi.org/10.1016/j.indcrop.2021.113868
Katz S, Beatson RP (1984) The determination of strong and weak acidic groups in sulfite pulps. Svensk Papperstidning 87(6):48–53
Kleen M, Lindblad G, Backa S (1993) Quantification of lignin and carbohydrates in kraft pulps using analytical pyrolysis and multivariate data analysis. J Anal Appl Pyrol 25:209–227
Klugman S, Karlsson M, Moshfegh B (2007) A scandinavian chemical wood pulp mill. Part 1. Energy audit aiming at efficiency measures. Appl Energy 84(3):326–339. https://doi.org/10.1016/j.apenergy.2006.07.003
Kulachenko A, Denoyelle T, Galland S, Lindström SB (2012) Elastic properties of cellulose nanopaper. Cellulose 19(3):793–807. https://doi.org/10.1007/s10570-012-9685-5
Kurihara T, Isogai A (2014) Properties of poly (acrylamide)/TEMPO-oxidized cellulose nanofibril composite films. Cellulose 21(1):291–299
Laine J, Lövgren L, Stenius P, Sjöberg S (1994) Potentiometric titration of unbleached kraft cellulose fibre surfaces. Colloids Surf a 88(2–3):277–287. https://doi.org/10.1016/0927-7757(94)02834-6
Larsson PA, Riazanova AV, Ciftci C, Rojas G, Øvrebø R, Wågberg HH, Berglund LA (2019) Towards optimised size distribution in commercial microfibrillated cellulose: a fractionation approach. Cellulose 26(3):1565–1575. https://doi.org/10.1007/s10570-018-2214-4
Li B, Bandekar R, Zha Q, Alsaggaf A, Ni Y (2011) Fiber quality analysis: OpTest fiber quality analyzer versus L&W fiber tester. Ind Eng Chem Res 50(22):12572–12578
Li F, Biagioni P, Bollani M, Maccagnan A, Piergiovanni L (2013) Multi-functional coating of cellulose nanocrystals for flexible packaging applications. Cellulose 20(5):2491–2504. https://doi.org/10.1007/s10570-013-0015-3
Li X, Ning C, Li L, Liu W, Ren Q, Hou Q (2021) Fabricating lignin-containing cellulose nanofibrils with unique properties from agricultural residues with assistance of deep eutectic solvents. Carbohydr Polym 274(July):118650. https://doi.org/10.1016/j.carbpol.2021.118650
Liu C, Li MC, Chen W, Huang R, Hong S, Wu Q, Mei C (2020) Production of lignin-containing cellulose nanofibers using deep eutectic solvents for UV-absorbing polymer reinforcement. Carbohydr Polym 246(June):116548. https://doi.org/10.1016/j.carbpol.2020.116548
Moser C, Henriksson G, Lindström M (2018) Improved dispersibility of once-dried cellulose nanofibers in the presence of glycerol. Nord Pulp Pap Res J 33(4):647–650. https://doi.org/10.1515/npprj-2018-0054
Motamedian HR, Halilovic AE, Kulachenko A (2019) Mechanisms of strength and stiffness improvement of paper after PFI refining with a focus on the effect of fines. Cellulose 26:4099–4124
Nair SS, Yan N (2015) Effect of high residual lignin on the thermal stability of nanofibrils and its enhanced mechanical performance in aqueous environments. Cellulose 22(5):3137–3150. https://doi.org/10.1007/s10570-015-0737-5
Nechyporchuk O, Belgacem MN, Bras J (2016) Production of cellulose nanofibrils: a review of recent advances. Ind Crops Prod 93:2–25. https://doi.org/10.1016/j.indcrop.2016.02.016
Nordenström M, Kaldéus T, Erlandsson J, Pettersson T, Malmström E, Wågberg L (2021) Redispersion strategies for dried cellulose nanofibrils. ACS Sustain Chem Eng 9(33):11003–11010. https://doi.org/10.1021/acssuschemeng.1c02122
Oliaei E, Berthold F, Berglund LA, Lindström T (2021) Eco-friendly high-strength composites based on hot-pressed lignocellulose microfibrils or fibers. ACS Sustain Chem Eng 9(4):1899–1910. https://doi.org/10.1021/acssuschemeng.0c08498
Oliaei E, Lindén PA, Wu Q, Berthold F, Berglund L, Lindström T (2020) Microfibrillated lignocellulose (MFLC) and nanopaper films from unbleached kraft softwood pulp. Cellulose 27(4):2325–2341. https://doi.org/10.1007/s10570-019-02934-8
Page DH (1985) The mechanism of strength development of dried pulps by beating [fiber kinking, crimping, upgrading]. Svensk Papperstidning.
Poletto M (2017) Assessment of the thermal behavior of lignins from softwood and hardwood species. Maderas. Ciencia y Tecnología 19(1):63–74
Ponomarenko J, Dizhbite T, Lauberts M, Volperts A, Dobele G, Telysheva G (2015) Analytical pyrolysis–A tool for revealing of lignin structure-antioxidant activity relationship. J Anal Appl Pyrol 113:360–369
Reid MS, Villalobos M, Cranston ED (2017) Benchmarking cellulose nanocrystals: from the laboratory to industrial production. Langmuir 33(7):1583–1598. https://doi.org/10.1021/acs.langmuir.6b03765
Rojo E, Peresin MS, Sampson WW, Hoeger IC, Vartiainen J, Laine J, Rojas OJ (2015) Comprehensive elucidation of the effect of residual lignin on the physical, barrier, mechanical and surface properties of nanocellulose films. Green Chem 17(3):1853–1866. https://doi.org/10.1039/c4gc02398f
Saito T, Hirota M, Tamura N, Kimura S, Fukuzumi H, Heux L, Isogai A (2009) Individualization of nano-sized plant cellulose fibrils by direct surface carboxylation using TEMPO catalyst under Neutral conditions. Biomacromol 10(7):1992–1996
Saito T, Kimura S, Nishiyama Y, Isogai A (2007) Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromol 8(8):2485–2491. https://doi.org/10.1021/bm0703970
Sánchez R, Espinosa E, Domínguez-Robles J, Loaiza JM, Rodríguez A (2016) Isolation and characterization of lignocellulose nanofibers from different wheat straw pulps. Int J Biol Macromol 92:1025–1033. https://doi.org/10.1016/j.ijbiomac.2016.08.019
Schreier H, Orteu J-J, Sutton MA (2009) Image correlation for shape, motion and deformation measurements: basic concepts, theory and applications, vol 1. Springer, Cham
Serra-Parareda F, Aguado R, Tarrés Q, Mutjé P, Delgado-Aguilar M (2021) Chemical-free production of lignocellulosic micro- and nanofibers from high-yield pulps: synergies, performance, and feasibility. J Clean Prod 313(March):1–10. https://doi.org/10.1016/j.jclepro.2021.127914
Silva LE, dos Santos A, de Torres A, McCaffrey L, Klamczynski Z, Glenn A, Sena Neto G, de Wood AR, Williams D, Orts T, Damásio W, Tonoli GHD (2021) Redispersion and structural change evaluation of dried microfibrillated cellulose. Carbohydr Polym 252:117165. https://doi.org/10.1016/j.carbpol.2020.117165
Sitholé BB (2006) Pyrolysis in the pulp and paper industry. Encyclop Anal Chem: Appl Theory Instrum. https://doi.org/10.1002/9780470027318.a2210
Sjostrom E (1989) The origin of charge on cellulosic fibers. Nord Pulp Pap Res J 4(2):90–93
Solala I, Iglesias MC, Peresin MS (2020) On the potential of lignin-containing cellulose nanofibrils (LCNFs): a review on properties and applications. Cellulose 27(4):1853–1877. https://doi.org/10.1007/s10570-019-02899-8
Svärd A, Sevastyanova O, Dobele G, Jurkjane V, Brännvall E (2016) COST action FP1105: effect of raw materials and pulping conditions on the characteristics of dissolved kraft lignins. Holzforschung 70(12):1105–1114
Tagami A, Gioia C, Lauberts M, Budnyak T, Moriana R, Lindström ME (2018) Sevastyanova O (2019) Solvent fractionation of softwood and hardwood kraft lignins for more efficient uses: Compositional, structural, thermal, antioxidant and adsorption properties. Ind Crops Prod 129:123–134. https://doi.org/10.1016/j.indcrop.2018.11.067
TAPPI T (2006) Acid-insoluble lignin in wood and pulp. TAPPI Test Methods
Tarrés Q, Ehman NV, Vallejos ME, Area MC, Delgado-Aguilar M, Mutjé P (2017) Lignocellulosic nanofibers from triticale straw: the influence of hemicelluloses and lignin in their production and properties. Carbohydr Polym 163:20–27. https://doi.org/10.1016/j.carbpol.2017.01.017
Turbak AF, Snyder FW, Sandberg KR (1983) Microfibrillated cellulose, a new cellulose product: properties, uses, and commercial potential. J Appl Polym Sci Appl Polym Symp 37(9):815–827
Wan JQ, Wang Y, Xiao Q (2010) Effects of hemicellulose removal on cellulose fiber structure and recycling characteristics of eucalyptus pulp. Bioresour Technol 101(12):4577–4583. https://doi.org/10.1016/j.biortech.2010.01.026
Wen Y, Yuan Z, Liu X, Qu J, Yang S, Wang A, Wang C, Wei B, Xu J, Ni Y (2019) Preparation and characterization of Lignin-Containing cellulose Nanofibril from Poplar High-Yield Pulp via TEMPO-Mediated oxidation and homogenization. ACS Sustain Chem Eng 7(6):6131–6139. https://doi.org/10.1021/acssuschemeng.8b06355
Xue F, He H, Zhou H, Quan Z, Chen Z, Wu Q, Zhu H, Wang S (2021) Structural design of a cellulose-based hyperbranched adsorbent for the rapid and complete removal of cr(VI) from water. Chem Eng J 417(October 2020):128037. https://doi.org/10.1016/j.cej.2020.128037
Zeng X, Sun J, Yao Y, Sun R, Xu J, Bin, Wong CP (2017) A combination of boron nitride nanotubes and cellulose nanofibers for the preparation of a nanocomposite with high thermal conductivity. ACS Nano 11(5):5167–5178. https://doi.org/10.1021/acsnano.7b02359
Zhang N, Tao P, Lu Y, Nie S (2019) Effect of lignin on the thermal stability of cellulose nanofibrils produced from bagasse pulp. Cellulose 26(13–14):7823–7835. https://doi.org/10.1007/s10570-019-02657-w
Zhao J, Zhang W, Zhang X, Zhang X, Lu C, Deng Y (2013) Extraction of cellulose nanofibrils from dry softwood pulp using high shear homogenization. Carbohydr Polym 97(2):695–702. https://doi.org/10.1016/j.carbpol.2013.05.050
Zhao D, Zhu Y, Cheng W, Xu G, Wang Q, Liu S, Li J, Chen C, Yu H, Hu L (2020) A dynamic gel with reversible and tunable topological networks and performances. Matter 2(2):390–403. https://doi.org/10.1016/j.matt.2019.10.020
Acknowledgments
The author thanks Dr. Raghu Deshpande and Dr. Dimitrios Georgouvelas for assistance with Supermasscolloider. We also acknowledge Dr. Claudia V. Esteves for the help with L&W Fiber Tester and we thank professor Gunnar Henriksson and Martin Lawoko for discussion on lignin structures.
Funding
Open access funding provided by Royal Institute of Technology. HL would like to thank China Scholarship Council (CSC) and the Wood and Pulping Chemistry Research Network (WPCRN) project for financial support of her research work. The authors (BC and OS) acknowledge funding from the Knut and Alice Wallenberg Foundation (KAW) through the Wallenberg Wood Science Center.
Author information
Authors and Affiliations
Contributions
HL: Conceptualization, Methodology, Investigation, Formal analysis, Visualization, Writing Original Draft; BC: Investigation, Formal analysis, Review & writing; AK: Supervision, Methodology, Validation, Review & Writing; VJ: Formal analysis, Review & editing; AM: Review & editing; OS: Conceptualization, Funding acquisition, Validation, Resources, Supervision, Writing, Review & editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, H., Chen, B., Kulachenko, A. et al. A comparative study of lignin-containing microfibrillated cellulose fibers produced from softwood and hardwood pulps. Cellulose 31, 907–926 (2024). https://doi.org/10.1007/s10570-023-05674-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-023-05674-y