Abstract
Cellulose is an excellent starting material for the construction of bioactive polymers. In the present work, we have synthesized quaternized graft copolymers of cellulose and tested their biological properties. Cellulose was grafted with acrylonitrile in a cerium ion catalyzed reaction. High yield of the grafting reaction, 89%, was achieved. Next, the poly (acrylonitrile) chains were aminated using three different amines and finally, the amino functions were quaternized using methyl iodide. In addition to chemical and physical characterization of the polymers, several tests on their bioactive properties have been conducted. The polymers turned out to have good antioxidant properties, as assessed studying how they scavenge ABTS radicals. Anti-inflammatory properties were investigated by a membrane stabilization method. The results showed that the quaternized polymers had anti-inflammatory effects and the one aminated with tris(2-aminoethyl)amine was the most significant compared with indomethacin. The cytotoxicity was evaluated in vitro against HepG2 and WI-38 cell lines. All quaternized polymers showed moderate effects against the cancerous cell line HEPG2. On the other hand, their effect against normal fibroblast WI-38 was weak. The acute toxicity in vivo was evaluated for one of the polymers, for which the LD50 was 6606 mg/kg. The high LD50 indicates the polymer is relatively non-toxic, and will be considered in future for in vivo studies.
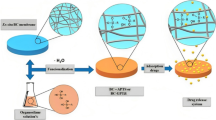
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Novel environmentally friendly and biocompatible materials are needed (Seddiqi et al. 2021). Recently, numerous studies have focused on novel polymeric materials due to their distinguished applications in the biomedical area (Kulshrestha and Mahapatro 2008; Zhu et al. 2022; Pisani et al. 2022; Makvandi et al. 2021; Bolívar-Monsalve et al. 2021). They are easily processed and flexible, have a high strength-to-weight ratio and in the best case, they are recyclable (Chen et al. 2022; Jadoun et al. 2021). Cellulose is a ubiquitous polymer, which serves as an excellent starting material for new compounds (Klemm et al. 2005; Bangar et al. 2022; Abushammala and Mao 2019). Cellulose has already been used in diverse applications as in membranes, pharmaceuticals, coating films and food processing; however, it lacks some of the appealing properties of synthetic polymers. Thus, to widen the scope of cellulose applications it is rational to search for new ways to modify it. Several strategies have been investigated throughout the years, as grafting, cross-linking, esterification, and etherification. Grafting of cellulose with appropriate monomers is a convenient method to add functionalities on the polymer backbone (Kumar 2020). With the choice of the monomer grafted to cellulose, it is possible to modulate copolymer properties, such as hydrophilicity or hydrophobicity, swelling, and thermal stability (Gürdağ et al. 2001). Several reports have been published on the grafting of vinyl monomers onto cellulose (Yu et al. 2016; Gürdağ et al. 1997; Roy et al. 2005).
Acrylonitrile (AN) is a monomer, which allows several ways to further modifying cellulose owing to the active nitrile group. The nitrile groups of polyacrylonitrile (PAN) have been reacted with several reagents such as aliphatic diamines (El-Newehy et al. 2014a, b; Kiani et al. 2011; Kampalanonwat and Supaphol 2010), hydrazine (Chaudhary and Farrell 2014), and hydroxylamine (Yun et al. 2020). Recently, we have synthesized derivatives of PAN and constructed nanocomposites of the polymers and montmorillonite (Kenawy et al. 2022). Due to the importance and ease of PAN modification, studies on the grafting of AN monomer onto cellulose surfaces have been already published (Rahman et al 2021, 2019; Musa et al. 2022; Khullar et al. 2008).
Bhatt et al. (2011) have studied the optimal parameters for the grafting of acrylonitrile onto a cellulose surface. Celluloses used in graft copolymerizations have been extracted from various sources. Interestingly, cellulosic materials derived from waste fiber were grafted with acrylonitrile and then transformed to poly (amidoxime) through the reaction with hydroxylamine. The product was used to decontaminate radioactive wastewater (Wu et al. 2022). In an earlier study, aminated chitosan-g-PAN was synthesized using diethylenetriamine and used to remove heavy metals from aqueous solutions (Dena-Aguilar et al. 2015). El-Khouly et al. (2011) evaluated the antimicrobial activities of Schiff bases of cellulose-g-PAN. Cellulose derivatives with quaternized ammonium units have been actively studied as well. Udoetok et al. (2016) prepared quaternized and cross-linked cellulose by grafting glycidyltrimethylammonium chloride after cross-linking native cellulose with epichlorohydrin. Recently, Lin et al. (2022) quaternized polyethylenimine-cellulose fibers and used those for the recovery of gold, Au(I) from alkaline solutions.
Thus, aminated cellulose derivatives have been described for numerous applications. However, the biomedical applications of cationic cellulose derivatives have not been very much studied yet (Abdelhamid and Mathew 2022; Tortorella et al. 2020). Therefore, we describe the syntheses of new cationic cellulose based graft copolymers and some of their important properties for biomedical use. Microcrystalline cellulose has been used, which is not soluble in organic solvents and thus, the products are microcrystals derivatized at their surfaces. Cellulose has been grafted with poly (acrylonitrile) and then, the copolymer was modified with three different amines. The amino groups were quaternized with iodomethane. Antioxidant activities of the product polymers were screened with the ABTS method. Anti-inflammatory activities were assessed using the membrane stabilization method. In vitro and in vivo cytotoxicities were also evaluated.
Experimental
Materials
Acrylonitrile (AN) was purchased from Sigma-Aldrich (USA) and purified by passing through a column of alumina. Microcrystalline cellulose, extra pure, (average particle size 90 μm) was from Acros Organics (NJ, USA). Tris(2-aminoethyl)amine, triethylenetetramine, 3-dimethylamino propylamine, methyl iodide and solvents were obtained from Acros Organics (NJ, USA) and were used without further purification. Ceric ammonium nitrate (CAN), (≥ 99.99%) (Sigma-Aldrich) and nitric acid (65%, 1.40 g/mL) were supplied from Merck and used as received. Dimethylformamide (DMF) was dried over CaH2 and distilled under reduced pressure.
Instruments
The FT-IR spectra were registered on Tracer-100 spectrometer (Shimadzu, Japan), wavenumber range (4000–500 cm−1) and scanning resolution of 4 cm−1.
X-ray diffraction (XRD) was measured using a Rigaku X-ray diffractometer (Rigaku, Tokyo, Japan) with Cu K (= 1.5418 Å) and 2Ɵ from 5 to 60 with a scanning rate of 0.02°/min. Samples were milled using agate mortar and pestle before loading onto the sample holder. The powder on the the sample holder was gently pressed to obtain a smooth surface using a glass slide before placing in an appropriate XRD slot.
TGA and DTG analyses were performed with Perkin Elmer TGA 4000, (USA) using alumina crucibles containing samples of 5–10 mg and heated from 50 to 800 °C with a rate 30 °C/min in N2 atmosphere (20 mL/min).
SEM images were obtained using JSM-IT100 (JEOL, Japan) operating at 20 kV. Samples were coated with a thin layer of gold using a Bio-Rad ES100 SEN coating machine.
Vacuum oven BINDER Model VD 53 (Germany) was used for drying samples.
Graft copolymerization
Cellulose (1.0 g, 6.0 mmol/ AGU, anhydrous glucose unit) was mixed with 30 mL solution of 0.02 M CAN in 1% nitric acid for half an hour and then, acrylonitrile (24.5 mol/AGU) in 20 mL toluene was added. The mixture was agitated under N2 for 4 h at 40 °C. The grafted cellulose was washed with water and methanol. After drying, the product was extracted in a Soxhlet apparatus with DMF for 48 h to remove the homopolymer.
The percent graft yield (G%) was calculated with Eq. (1) (Shamsuddin et al. 2013):
where Cell F is the final cellulose mass and Cell I is the initial cellulose mass.
Amination of cellulose-graft-polyacrylonitrile (AMCC-g-PAN)
5 g of grafted cellulose (23.24 mmol, based on AGU) was suspended in 50 mL hot DMF, then an appropriate amount of amine (10 times molar excess, 232.4 mmol) was added to the mixture with stirring, and the reaction proceeded for 24 h under N2 at 110 °C. The aminated cellulose-graft-PAN (AMCC-g-PAN) was precipitated in warm water, washed with methanol and dried at 40 °C for 26 h.
The conversion (%) of the cyano groups was calculated using the following equation (Neghlani et al. 2011):
where w0 and w1 are the masses of the grafted cellulose and the aminated one, respectively. M0 is the molecular weight of MCC-g-PAN repeating unit (215 g/mol) and M1 is the amine molecular weight: dimethylaminopropylamine (102 g/mol), tris (2-aminoethyl)amine (146 g/mol), and triethylenetetramine (146 g/mol)
Synthesis of quaternized cellulose-g-polyacrylonitirle (QMCC-g-PAN)
To a pre-heated solution of AMCC-g-PAN (3.0 g) in DMF (15 mL) at 80 °C, 10 mL CH3I (0.16 mol) was added, and temperature was lowered to 30 °C. The resulting mixture was stirred for 23 h at room temperature. The obtained quaternized copolymer (QCell-g-PAN) was precipitated in methanol, filtered, and dried in vacuum for 48 h at 40 °C.
Studies on bioactive properties
Antioxidant activity
The antioxidant activity was assessed using the ABTS method (2,2'-azino-bis(3-ethyl benzothiazoline-6-sulfonic acid). The ABTS reagent was prepared by adding 3 μL MnO2 (25 mg/mL) to 60 μL ABTS mixed with 5 μL phosphate buffer solution (pH 7). The solution was shaken before centrifuging and filtration. To assess the scavenging activity, 20 μL of the studied polymer (1 mg/mL) was added to the ABTS solution in a spectroscopic grade MeOH/buffer (1:1 v/v).
The activity of a polymer to scavenge the ABTS radicals was determined by measuring the absorbance of the ABTS solution at 734 nm, and is given as in Eq. (3):
where A control is absorbance of the ABTS solution. Asample is the absorbance of ABTS after the addition of the polymer. An ascorbic acid (20 μL, 2 mM) solution was applied as a standard antioxidant (positive control).
In vitro anti-inflammatory assay
The anti-inflammatory activity of the polymers was evaluated using membrane stabilization of the human red blood cells (Chopade et al. 2012). Blood was collected from non-smoking healthy male volunteers (aged 23–25 years). 3 mL of blood were centrifuged for 10 min at 3000 rpm to obtain an erythrocyte suspension. The blood pellets were dissolved in a volume of normal saline that was equal to the volume of the supernatant. The pellets were mixed with an isotonic buffer solution (10 mM sodium phosphate buffer, pH 7.4) to obtain a 40% v/v suspension. The phosphate buffer was prepared by adding 0.2 g NaH2PO4, 1.15 g Na2HPO4, and 9 g of NaCl in 1 L of distilled water.
Hypotonicity induced hemolysis
The impact of the quaternized polymers on the hypotonicity induced hemolysis of the red blood cells was assessed using the method described by Anosike et al. (2012) Briefly, aqueous saline solutions of the quaternized polymers were prepared (100, 200, 400, 600, 800 and 1000 μg/mL), and these were then used as hypotonic solutions. Isotonic solutions (prepared as described above) contained similar concentrations of the polymers. 0.1 mL of erythrocyte suspension was added to each of the hypotonic and isotonic sample solution (5 mL) and shaken gently. The samples were incubated at 37 °C and centrifuged. The hemoglobin absorbance (OD) of the supernatant was measured photometrically at 540 nm. Indomethacin 200 (μg/mL) was employed as a control.
The inhibition of hemolysis, expressed in percentage, is given as follows:
where OD1 is absorbance of the test sample in isotonic solution, OD2 is absorbance of the test sample in hypotonic solution, OD3 is absorbance of the control sample in hypotonic solution.
Cytotoxicity by MTT Assay (in vitro)
A MTT assay was used to investigate the inhibitory effect of various quaternized polymers on the cell proliferation in human lung fibroblast (WI38) and hepatocellular carcinoma (HEPG-2) cell lines (Denizot and Lang 1986). This colorimetric assay is based on the conversion of the yellow tetrazolium bromide (MTT) to a purple formazan derivative by mitochondrial succinate dehydrogenase in viable cells. The cell lines were cultured in RPMI-1640 medium with 10% fetal bovine serum. Antibiotics added were 100 units/ml penicillin and 100 µg/ml streptomycin at 37 °C in a 5% CO2 incubator. The cell lines were seeded in a 96-well plate at a density of 1.0 × 104 cells/well at 37 °C for 48 h under 5% CO2. After incubation the cells were treated with different concentrations of polymers and incubated for 24 h. After 24 h of drug treatment, 20 µL of MTT solution (5 mg/mL) was added and incubated for 4 h. 100 µL of dimethyl sulfoxide (DMSO) was added into each well to dissolve the purple formazan formed. The colorimetric assay was measured and absorbance at 570 nm was recorded using a plate reader (EXL 800, USA). The relative cell viability in percentage was calculated as (A570 of treated samples/A570 of untreated sample) × 100.
Acute toxicity study (LD50)
An in vivo study was performed to define the lethal dose (LD50) and establish the safety dose of one of the quaternized polymers, the ammonium salt of grafted cellulose containing tris amine (polymer VI). This information is essential for the further hypolipidemic evaluations in the future. Twenty adult albino mice (20–25 g) were used in the oral toxicity investigation, and they were maintained for 7 days in groups of four per cage in a perfect laboratory setting (Oecd 2001). The ethical protocols were approved by Tanta University, Egypt (IACUC-SCI-TU-0299). All animals were treated orally once and different doses (1000, 3000, 6000, 12,000 mg/kg) were administered, while a control animal received distilled H2O. Following the injection of the dose, animal behaviors were monitored for 12 h, and 24 h later, mortality was reported.
Statistical analysis
In order to evaluate the data, SPSS version 15 was used. The mean ± SD is used to express all values. One-way ANOVA was used to examine the data, and Duncan's new multiple ranges were used to determine how much the means differed. Statistics were judged significant at P < 0.05.
Results and discussion
Acrylonitrile was grafted onto microcrystalline cellulose (MCC) with ceric ion initiated polymerization, see Scheme 1. The yields of the reactions are given in percentages as shown in Table 1, each yield is an average of three experiments (SD = standard deviation). The percent graft yield (G%) was 89% when using 24.5 mol/AGU acrylonitrile monomer, 0.02 M CAN initiator at 40 °C for 4 h. The grafted cellulose was modified through reactions with three different amines, dimethylaminopropylamine, tris(2-aminoethyl)amine and triethylenetetramine. Unreacted amines were removed by washing the products with hot water and methanol. The aminated grafted cellulose was formed as a yellow precipitate as referred to an earlier study (El-Khouly et al. 2011) and it was dried at 40 °C for 26 h before calculation of the conversion (%). The amination reaction using tris (2-aminoethyl) amine yielded the highest degree of conversion of cyano groups (36.7%) followed by triethylenetetramine (29.4%) and dimethylaminopropylamine (18.7%). The degree of amination was not high, obviously because of the low solubility of the aminated grafted cellulose. The products are ideal for our future work, however, because the aim has been to prepare hydrophobic cationic celluloses, which may act as bile acid sequestrants for lowering cholesterol (Nichifor et al. 1998; Zhu et al. 2015). This is why we needed to determine the lethal dose (acute toxicity) and the other bioactive properties of the cationic celluloses.
Because the aim was to produce cationic polymers, the aminated graft copolymers were treated with methyl iodide to create quaternary ammonium substituents, see Scheme 2. Owing to the limited solubility of the modified polymers, an excess of methyl iodide was used (10 molar excess) to assure complete quaternization.
The product polymers are listed in Table 1. From now on, the Roman numerals are used.
Physiochemical characterization of the functionalized microcrystalline cellulose
The FT-IR spectra of cellulose-g-PAN (I) and its modifications are shown in Fig. 1. As a result of the grafting with AN, the intensity of the hydroxyl band of cellulose around 3367 cm−1 decreases, and a sharp cyano band appears at 2247 cm−1. It is worth noting that the other bands related to MCC keep unchanged; 2921, 1638, 1060 cm−1, originating from the C–H stretching, the bending mode of absorbed water, and the C–O–C vibration. The observations are in accordance with those by Rahman et al. (2019).
The spectra of the aminated MCC-g-PANs (II–IV) exhibit several noteworthy changes. A broad peak is seen at 3329 cm−1, indicating the N–H stretching. The reduction in the intensity of the absorption band of the nitrile group (–CN) stretching at 2247 cm−1 reveals the introduction of amino groups into the polymers. Not all of the CN groups were involved in the amination. A new absorption band at 1372 cm−1 attributed to N–H stretching indicates the occurrence of primary amine groups (Kenawy et al. 2022; Lin et al. 2022). A new peak at 1452 cm−1 is assigned to N–CH3 in the spectra of quaternized MCC-g-PAN (V–VII) (Wei et al. 2019).
The TGA curves of MCC and its modifications (I–VII) are shown in Fig. 2. The temperature at 5 and 50% weight loss (T−5 wt%, T−50 wt%), temperature at which the maximum decomposition took place (TDTG), and the total weight loss of the polymers are given in Table 2. The decomposition processes of pure cellulose (MCC) and the grafted one (MCC-g-PAN) are quite different. The cellulose thermogram shows a one-step degradation in the temperature range 330–380 °C lwith a 92% mass loss. This can be attributed to processes such as depolymerization and decomposition of the glycosidic bonds (C–O–C) (Kumar et al. 2019). On the other hand, grafted MCC shows two main steps of degradation, first within 320–397 °C with a mass loss of 48% and then at 400–668 °C with a mass loss 47%.
TGA of aminated cellulose (II–IV) shows initial degradation earlier than grafted cellulose I due to the incorporation of hydrophilic amino groups. At temperatures from 50 to 150 °C, moisture and small particles are removed. The second step at around 390–430 °C causes 50% mass loss due to the degradation of the amine groups (El-Newehy et al. 2014a, b).
TGA of aminated (II–IV) and quaternized celluloses (V–VII) show similar patterns of mass losses (Nichifor et al. 1998). The total mass losses of all the polymers are quite similar, except for the polymer (VI), tris-QMCC-g-PAN, which has a residue of 10% as shown in Table 2. This may be attributed to the high content of amine comparing to the other aminated grafted celluloses.
The XRD results of MCC-g-PAN and its quaternary ammonium salts are shown in Fig. 3. The characteristic peaks of MCC-g-PAN are detected at 2θ 16.7, 22.6 and 34.3°, which agree with an earlier study (Rahman et al. 2020). It is obvious that the graft polymerization does not change the crystal structure of cellulose. Apparently, PAN fits in the cellulose lattice. Quaternization affects considerably the crystallinity of the polymers. For the quaternized polymer V containing propyl amine moieties, sharp diffraction peaks can be observed at 2θ 17, 22.3° and 34.5°. On the other hand, quaternization with tris (2-aminoethyl) amine (VI) disrupts the crystalline order of cellulose and results in an amorphous structure. The diffraction peak of teta-QMCC-g-PAN VII at 22.5° decreases in intensity whereas the peak intensity at 17° increases compared to the diffraction pattern of grafted MCC.
Morphological analysis
The changes in microcrystalline cellulose structure due to grafting, amination, and quaternization were studied by SEM and representative micrographs are shown in Figs. 5 and 6. MCC-g-PAN surfaces are irregular with some rod-like aggregates (Fig. 4a) as already observed earlier (Panaitescu et al. 2008; Karim et al. 2014). The MCC surface was not totally grafted with PAN and this is why the morphological characteristics of MCC are still evident in the grafted copolymer. The surface of the grafted cellulose is irregular but relatively smooth compared to the other derivatives, as can be seen in the enlarged image (Fig. 4b). After the modification with amines, SEM reveals completely different surface morphologies confirming the amination. The surface of II (propyl-MCC-g-PAN) is composed of aggregate bundles with small bumps. The surface of III (tris-MCC-g-PAN) is unsmooth and flaky (Fig. 4d), and the surface of IV (teta-MCC-g-PAN) is highly flaky, porous and lumpish (Fig. 4e, f). The surfaces of the quaternized celluloses are very rough compared with the aminated ones (Fig. 5). The surface of the polymer (V) can be described as wrinkled (Fig. 5g), that of polymer VI as rocky (Fig. 5h) and the surface of VII is strongly rugged and irregular (Fig. 5i). In general, the observations show that the microstructures of MCC changed after each chemical modification.
Bioactive properties
In vitro antioxidant activity
The antioxidant capacities of the quaternized polymers were assessed using the ABTS technique (Metwally et al. 2012). As is shown in Fig. 6, the scavenging of ABTS radicals increases steadily with increasing the polymer content of the sample, though the values are lower than those for ascorbic acid. From this data, IC50 values were calculated, which give the concentrations of different polymers needed to scavenge 50% of the free radicals present. High IC50 values imply inactive antioxidants. The radical-scavenging activity of the polymer V (QMCC-g-PAN) with a propyl amine moiety is the highest, 54 μg/mL, whereas VI and VII have IC50 values 68 and 78 μg/mL, respectively, see Fig. 7.
Membrane stabilization assay
The in vitro anti-inflammatory activity was evaluated using the membrane stabilization technique (Adnan et al. 2019; Mir et al. 2022). Among all the tested polymeric ammonium salts, polymer VI (QMCC-g-PAN) containing tris amine moiety significantly inhibits (p ≤ 0.05) the rupturing of the erythrocyte membranes caused by the hypotonicity with a value of 74% at a dosage of 1000 μg/mL (see Tables 3,4,5). In general, the prevention of hemolysis was dosage dependent, increasing with rising concentrations of all the polymers as shown in Fig. 8. As comparison, the corresponding value for indomethacin has been reported to be 71.43% at 200 μg/mL (Anosike et al. 2012).
Cytotoxicity
The cytotoxicity tests were conducted using a MTT assay (Kenawy et al. 2023) and two cell lines, a non-cancerous one (WI-38) to indicate the safety of the polymers and a cancerous cell line HePG2. As is shown in Table 6 the preliminary screening indicated that the polymers V–VII have low cytotoxicities on normal cells (WI-38), i.e. they are nontoxic. Interestingly, the polymer VII containing teta amine gave IC50 value as high as 82.00. The cytotoxicities against HePG2 were moderate. Doxorubicin (DOX) has been used as a reference.
Acute toxicity
Of the three ammonium salts of the grafted cellulose, polymer VI (tris QMMC-g-PAN) was selected for the determination of its lethal dose. In this polymer, the amino (and correspondingly the ammonium) content is the highest. This polymer will be used in future as a bile acid sequestrant to control hypercholesterolemia (Lopez-Jaramillo et al. 2015; Wang et al. 2016). The toxicological study with different doses administered orally in mice was conducted by the Miller–Tainter method (Randhawa 2009; Kasolo et al. 2011). The percentage of mortality at each dose was converted to probit (Table 7) using Finney’s method (Finney 1952). The percentage dead 0 and 100 were corrected before the determination of probits as follows. For 0% dead: 100 (0.25/n) and for 100% dead: 100 (n − 0.25/n), where n = 4 mice (Raj et al. 2013). The probit values were plotted against log dose and then the dose corresponding to probit 5, (50% deaths) was obtained (Fig. 9). The results reveal the median lethal dose, LD50 is 6606 mg/kg. Thus, according to the Hodge and Sterner toxicity scale (Ahmed 2015), the polymer can be considered nontoxic, the LD50 being in the range 5000–15,000 mg/kg.
Conclusions
Cellulose has been successfully grafted with poly(acrylonitrile), PAN, and the grafts have been aminated and quaternized. The toxicity, antioxidant, and anti-inflammatory properties of the bioactive polymers have been evaluated.
The grafting yield of PAN onto cellulose surface was 89%. The PAN chains were reacted with three amines, dimethylaminopropyl amine, tris(2-aminoethyl) amine and triethylenetetramine. The reaction with tris(2-aminoethyl) amine gave the highest degree of conversion of cyano groups (37%). Next, the amino functions were quaternized with methyl iodide. Owing to the insolubility of the product polymers the degree of quaternization is not known; however, an excess of CH3I was used to complete the reaction.
The quaternized polymers V–VII have high antioxidant capacity, decreasing in the order V > VII > VI. IC50 values for the polymers varied in the range 54–78 μg/mL.
The polymers showed promising anti-inflammatory effects. VI showed the highest % of hemolysis inhibition (74%), followed by VII and V showing 56% and 46%, respectively.
The quaternized polymers had only a weak effect on the viability of normal cells WI38, and their effect on the viability on cancerous cells in the line HEPG-2 was moderate. The acute in vivo toxicity of the polymer VI was shown to be low, the LD50 of 6600 mg/kg means the polymer may be considered as non-toxic one. This polymer will be used in further in vivo studies.
In general, the polymeric quaternary ammonium salts (QAS) showed promising biological activities due to the presence of the fixed positive charge which easily binds with the constituents of the culture medium and leads to a decrease of the cytotoxicity towards eukaryotic cells. Further, QAS have been extensively studied and applied to a variety of antimicrobial-relevant areas since they disrupt the cell membranes through the electrostatic interaction between negatively charged membranes and cationic ammonium salts.
Data availability
The authors declare that all the data generated or analyzed during this study are available within the article.
References
Abdelhamid HN, Mathew AP (2022) Cellulose-based nanomaterials advance biomedicine: a review. Int J Mol Sci 23:5405. https://doi.org/10.3390/ijms23105405
Abushammala H, Mao JA (2019) review of the surface modification of cellulose and nanocellulose using aliphatic and aromatic mono-and di-isocyanates. Molecules 24:2782. https://doi.org/10.3390/molecules24152782
Adnan AZ, Armin FI, Sudji IR, Novida MD, Roesma DI, Ali HA, Fauzana AN (2019) In vitro anti-inflammatory activity test of tinocrisposide and freeze-dried aqueous extract of Tinospora crispa stems on human red blood cell by increasing membrane stability experiment. Asian J Pharm Clin Res 12:125–129
Ahmed M (2015) Acute toxicity (lethal dose 50 calculation) of herbal drug somina in rats and mice. Pharmacol Pharm 6:185. https://doi.org/10.4236/pp.2015.63019
Anosike CA, Obidoa O, Ezeanyika LU (2012) Membrane stabilization as a mechanism of the anti-inflammatory activity of methanol extract of garden egg (Solanum aethiopicum). DARU J Pharm Sci 20:1–7. https://doi.org/10.1186/2008-2231-20-76
Bangar SP, Harussani MM, Ilyas RA, Ashogbon AO, Singh A, Trif M, Jafari SM (2022) Surface modifications of cellulose nanocrystals: processes, properties, and applications. Food Hydrocoll 130:107689. https://doi.org/10.1016/j.foodhyd.2022.107689
Bhatt N, Gupta PK, Naithani S (2011) Ceric-induced grafting of acrylonitrile onto alpha cellulose isolated from lantana camara. Cellul Chem Technol 45:321
Bolívar-Monsalve EJ, Alvarez MM, Hosseini S, Espinosa-Hernandez MA, Ceballos-González CF, Sanchez-Dominguez M, Ryon Shin S, Cecen B, Hassan S, Di Maio E, Trujillo-de Santiago G (2021) Engineering bioactive synthetic polymers for biomedical applications: a review with emphasis on tissue engineering and controlled release. Mater Adv 2:4447–4478. https://doi.org/10.1039/D1MA00092F
Chaudhary BK, Farrell J (2014) Preparation and characterization of homopolymer polyacrylonitrile-based fibrous sorbents for arsenic removal. Environ Eng Sci 31:593–601. https://doi.org/10.1089/ees.2014.0169
Chen WH, Chen QW, Chen Q, Cui C, Duan S, Kang Y, Chen X (2022) Biomedical polymers: synthesis, properties, and applications. Sci China Chem 65:1010–1075. https://doi.org/10.1007/s11426-022-1243-5
Chopade AR, Somade PM, Sayyad FJ (2012) Membrane stabilizing activity and protein denaturation: a possible mechanism of action for the anti-inflammatory activity of Phyllanthus amarus. J Krishna Inst Medical Sci Univ 1:67–72
Dena-Aguilar JA, Jauregui-Rincon J, Bonilla-Petriciolet A, Romero-Garcia J (2015) Synthesis and characterization of aminated copolymers of polyacrylonitrile-graft-chitosan and their application for the removal of heavy metals from aqueous solution. J Chil Chem Soc 60:2876–2880. https://doi.org/10.4067/S0717-97072015000200003
Denizot F, Lang R (1986) Rapid colorimetric assay for cell growth and survival: modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods 89:271–277. https://doi.org/10.1016/0022-1759(86)90368-6
El-Khouly AS, Kenawy E, Safaan AA, Takahashi Y, Hafiz YA, Sonomoto K, Zendo T (2011) Synthesis, characterization and antimicrobial activity of modified cellulose-graft-polyacrylonitrile with some aromatic aldehyde derivatives. Carbohydr polym 83:346–353. https://doi.org/10.1016/j.carbpol.2010.07.047
El-Newehy MH, Alamri A, Al-Deyab SS (2014a) Optimization of amine-terminated polyacrylonitrile synthesis and characterization. Arabian J Chem 7:235–241. https://doi.org/10.1016/j.arabjc.2012.04.041
El-Newehy MH, El-Hamshary H, Al-Deyab SS, Abdel-Megeed A (2014b) Synthesis of quaternized amine-terminated polyacrylonitrile and their antimicrobial assessment. J Macromol Sci Part A 51:527–537. https://doi.org/10.1080/10601325.2014.906270
Finney DJ (1952) Probit analysis: a statistical treatment of the sigmoid response curve. Cambridge University Press, Cambridge
Gürdağ G, Yaşar M, Gürkaynak MA (1997) Graft copolymerization of acrylic acid on cellulose: reaction kinetics of copolymerization. J Appl Polym Sci 66:929–934. https://doi.org/10.1002/(SICI)1097-4628(19971031)66:5%3c929::AID-APP13%3e3.0.CO;2-I
Gürdağ G, Güçlü G, Özgümüş S (2001) Graft copolymerization of acrylic acid onto cellulose: effects of pretreatments and crosslinking agent. J Appl Polym Sci 80:2267–2272. https://doi.org/10.1002/app.1331
Jadoun S, Riaz U, Budhiraja V (2021) Biodegradable conducting polymeric materials for biomedical applications: a review. Med Devices Sens 4:e10141. https://doi.org/10.1002/mds3.10141
Kampalanonwat P, Supaphol P (2010) Preparation and adsorption behavior of aminated electrospun polyacrylonitrile nanofiber mats for heavy metal ion removal. ACS Appl Mater Interfaces 2:3619–3627. https://doi.org/10.1021/am1008024
Karim MZ, Chowdhury ZZ, Abd Hamid SB, Ali ME (2014) Statistical optimization for acid hydrolysis of microcrystalline cellulose and its physiochemical characterization by using metal ion catalyst. Materials 7:6982–6999. https://doi.org/10.3390/ma7106982
Kasolo JN, Bimenya GS, Ojok L, Ogwal-Okeng J (2011) Phytochemicals and acute toxicity of Moringa oleifera roots in mice. Pharmacogn Phytother 3:38
Kenawy ER, Tenhu H, Khattab SA, Eldeeb AA, Azaam MM (2022) Highly efficient adsorbent material for removal of methylene blue dye based on functionalized polyacrylonitrile. Eur Polym J 169:111138. https://doi.org/10.1016/j.eurpolymj.2022.111138
Kenawy ERS, Kamoun EA, Ghaly ZS, Shokr ABM, El-Meligy MA, Mahmoud YAG (2023) Novel physically cross-linked curcumin-loaded PVA/Aloe vera hydrogel membranes for acceleration of topical wound healing: in vitro and in vivo experiments. Arab J Sci Eng 48:497–514. https://doi.org/10.1007/s13369-022-07283-6
Khullar R, Varshney VK, Naithani S, Soni PL (2008) Grafting of acrylonitrile onto cellulosic material derived from bamboo (Dendrocalamus strictus). Express Polym Lett 2:12–18. https://doi.org/10.3144/expresspolymlett.2008.3
Kiani GR, Sheikhloie H, Arsalani N (2011) Heavy metal ion removal from aqueous solutions by functionalized polyacrylonitrile. Desalination 269:266–270. https://doi.org/10.1016/j.desal.2010.11.012
Klemm D, Heublein B, Fink HP, Bohn A (2005) Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Int Ed 44:3358–3393. https://doi.org/10.1002/anie.200460587
Kulshrestha AS, Mahapatro A (2008) Polymers for biomedical applications. Am Chem Soc. https://doi.org/10.1021/bk-2008-0977.pr001
Kumar S (2020) Grafting of Poly(acrylonitrile) on Cellulose to synthesize and characterize potential functional polymers for development of metal ion sorbents. IRJASH 2:23–27. https://doi.org/10.47392/IRJASH.2020.194
Kumar R, Sharma RK, Singh AP (2019) Synthesis and characterization of cellulose based graft copolymers with binary vinyl monomers for efficient removal of cationic dyes and Pb(II) ions. J Polym Res 26:1–20. https://doi.org/10.1007/s10965-019-1790-9
Lin X, Tran DT, Song MH, Yun YS (2022) Development of quaternized polyethylenimine-cellulose fibers for fast recovery of Au (CN)2- in alkaline wastewater: Kinetics, isotherm, and thermodynamic study. J Hazard Mater 422:126940. https://doi.org/10.1016/j.jhazmat.2021.126940
Lopez-Jaramillo FJ, Giron-Gonzalez MD, Salto-Gonzalez R, Hernandez-Mateo F, Santoyo-Gonzalez F (2015) In vitro and in vivo evaluation of novel cross-linked saccharide based polymers as bile acid sequestrants. Molecules 20:3716–3729. https://doi.org/10.3390/molecules20033716
Makvandi P, Iftekhar S, Pizzetti F, Zarepour A, Zare EN, Ashrafizadeh M, Rossi F (2021) Functionalization of polymers and nanomaterials for water treatment, food packaging, textile and biomedical applications: a review. Environ Chem Lett 19:583–611. https://doi.org/10.1007/s10311-020-01089-4
Metwally MA, Gouda MA, Harmal AN, Khalil AM (2012) Synthesis, antitumor, cytotoxic and antioxidant evaluation of some new pyrazolotriazines attached to antipyrine moiety. Eur J Med Chem 56:254–262. https://doi.org/10.1016/j.ejmech.2012.08.034
Mir PA, Mahajan S, Verma A, Kumar N, Arora M, Nagpal N (2022) In-vitro antioxidant and anti-inflammatory potential of ficus infectoria fruits. Pharmacogn Res. https://doi.org/10.5530/pres.14.2.22
Musa A, Ifo MI, Salisu A, Yamani AM (2022) Synthesis of poly-amidoxime resin from grafted millet husk cellulose for adsorption of Congo red. Bayero J Pure Appl Sci 13:163–172
Neghlani PK, Rafizadeh M, Taromi FA (2011) Preparation of aminated-polyacrylonitrile nanofiber membranes for the adsorption of metal ions: comparison with microfibers. J Hazard Mater 186:182–189. https://doi.org/10.1016/j.jhazmat.2010.10.121
Nichifor M, Cristea D, Mocanu G, Carpov A (1998) Aminated polysaccharides as bile acid sorbents: in vitro study. J Biomater Sci Polym Ed 9:519–534. https://doi.org/10.1163/156856298X00019
OECD O (2001) Guidelines for testing of chemicals, acute oral toxicity-fixed dose procedure. Organ Econ Coop Dev.
Panaitescu DM, Vuluga DM, Paven H, Iorga MD, Ghiurea M, Matasaru I, Nechita P (2008) Properties of polymer composites with cellulose microfibrils. Mol Cryst Liq Cryst 484:86–452. https://doi.org/10.1080/15421400801903502
Pisani S, Genta I, Modena T, Dorati R, Benazzo M, Conti B (2022) Shape-memory polymers hallmarks and their biomedical applications in the form of nanofibers. Int J Mol Sci 23:1290. https://doi.org/10.3390/ijms23031290
Rahman ML, Sarjadi MS, Arshad SE, Yusoff MM, Sarkar SM, Musta B (2019) Kenaf cellulose-based poly (amidoxime) ligand for adsorption of rare earth ions. Rare Met 38:259–269. https://doi.org/10.1007/s12598-018-1061-7
Rahman ML, Fui CJ, Ting TX, Sarjadi MS, Arshad SE, Musta B (2020) Polymer ligands derived from jute fiber for heavy metal removal from electroplating wastewater. Polymers 12:2521. https://doi.org/10.3390/polym12112521
Rahman ML, Wong ZJ, Sarjadi MS, Soloi S, Arshad SE, Bidin K, Musta B (2021) Heavy metals removal from electroplating wastewater by waste fiber-based poly (Amidoxime) ligand. Water 13:1260. https://doi.org/10.3390/w13091260
Raj J, Chandra M, Dogra TD, Pahuja M, Raina A (2013) Determination of median lethal dose of combination of endosulfan and cypermethrin in wistar rat. Toxicol Int 20:1. https://doi.org/10.4103/0971-6580.111531
Randhawa MA (2009) Calculation of LD50 values from the method of Miller and Tainter, 1944. J Ayub Med Coll Abbottabad 21:184–185
Roy D, Guthrie JT, Perrier S (2005) Graft polymerization: grafting poly (styrene) from cellulose via reversible addition−fragmentation chain transfer (RAFT) polymerization. Macromolecules 38:10363–10372. https://doi.org/10.1021/ma0515026
Seddiqi H, Oliaei E, Honarkar H, Jin J, Geonzon LC, Bacabac RG, Klein-Nulend J (2021) Cellulose and its derivatives: towards biomedical applications. Cellulose 28:1893–1931. https://doi.org/10.1007/s10570-020-03674-w
Shamsuddin MR, Fauzee SN, Anuar FH, Abdullah I, Othaman R (2013) Modification of cellulose by polymethyl methacrylate grafting for membrane applications. J Teknol 65:47–53
Tortorella S, Buratti VV, Maturi M, Sambri L, Franchini MC, Locatelli E (2020) Surface-modified nanocellulose for application in biomedical engineering and nanomedicine: A review. Int J Nanomed 15:9909
Udoetok IA, Wilson LD, Headley JV (2016) Quaternized cellulose hydrogels as sorbent materials and pickering emulsion stabilizing agents. Materials 9:645. https://doi.org/10.3390/ma9080645
Wang X, Jing S, Qiu X, Zhao S, Liu Y, Tan Y (2016) Novel bile acid sequestrant: a biodegradable hydrogel based on amphiphilic allylamine copolymer. Chem Eng J 304:493–502. https://doi.org/10.1016/j.cej.2016.06.104
Wei L, Li Q, Chen Y, Zhang J, Mi Y, Dong F, Lei C, Guo Z (2019) Enhanced antioxidant and antifungal activity of chitosan derivatives bearing 6-O-imidazole-based quaternary ammonium salts. Carbohyd polym 206:493–503. https://doi.org/10.1016/j.carbpol.2018.11.022
Wu MB, Liu SC, Fei JY, Ye H, Ma LL (2022) Natural cellulose-based microspheres decorated with amidoxime groups for decontamination of radioactive wastewater. Appl Surf Sci. https://doi.org/10.1016/j.apsusc.2022.153659
Yu T, Liu S, Xu M, Peng J, Li J, Zhai M (2016) Synthesis of novel aminated cellulose microsphere adsorbent for efficient Cr(VI) removal. Radiat Phys Chem 125:94–101. https://doi.org/10.1016/j.radphyschem.2016.03.019
Yun J, Wang Y, Liu Z, Li Y, Yang H, Xu ZL (2020) High efficient dye removal with hydrolyzed ethanolamine-Polyacrylonitrile UF membrane: rejection of anionic dye and selective adsorption of cationic dye. Chemosphere 259:127390. https://doi.org/10.1016/j.chemosphere.2020.127390
Zhu X, Wen Y, Cheng D, Li C, An X, Ni Y (2015) Cationic amphiphilic microfibrillated cellulose (MFC) for potential use for bile acid sorption. Carbohydr Polym 132:598–605. https://doi.org/10.1016/j.carbpol.2015.06.063
Zhu Y, Xu P, Zhang X, Wu D (2022) Emerging porous organic polymers for biomedical applications. Chem Soc Rev 51:1377–1414. https://doi.org/10.1039/D1CS00871D
Acknowledgments
The authors thank Ahmed Abbas, Department of Pharmacognosy, Faculty of Pharmacy, Mansoura University, Egypt, for his technical assistance in assessing biological properties of the polymers. Samar thanks the Egyptian government for providing the necessary to complete this research work.
Funding
Open Access funding provided by University of Helsinki (including Helsinki University Central Hospital).
Author information
Authors and Affiliations
Contributions
EK: supervision, investigation and review. SK: methodology and analyzed the data, writing original draft preparation. HT: supervision, investigation, writing—reviewing and editing and data curation. MA: supervision and investigation. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All experiments were conducted in accordance with the Guidelines of World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects and approved by the ethics committee at Faculty of Science, Tanta University, Egypt (IACUC-SCI-TU-0299).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kenawy, ER., Khattab, S.A., Tenhu, H. et al. Ammonium salts of microcrystalline cellulose-g-poly (acrylonitrile): toxicity, antioxidant and anti-inflammatory properties. Cellulose 30, 11665–11680 (2023). https://doi.org/10.1007/s10570-023-05582-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-023-05582-1