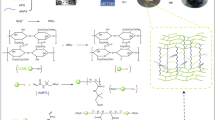
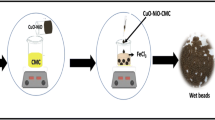
Abstract
Some adsorbent hydrogels made of natural polymeric materials have not yet met the needs for a high level of wastewater purification. In the present study, a novel and environmentally friendly process is described for the production of natural cellulose-based metal oxide nanoparticle hydrogel beads. Recyclable adsorbent components include wastepaper-derived cellulose fiber, metal oxide nanoparticles and polyvinyl alcohol was synthesized, the WCF/PVA MO NP hydrogel beads were synthesized by using cross-linking agents (Ca2+, boric acid and NaCl), and were analyzed by evaluating their ability to remove the acid red 18 (AR18) from aqueous solution. The WCF/PVA MO NP was characterized by FESEM, XRD, EDX, BET, and FTIR. The results revealed that the metal oxide nanoparticles distributed on external and internal channels of the 3D fiber network of WCF with strongly covalent/non-covalent cross-linking bonds would facilitate mass diffusion and enhance the interaction between AR18. The results shown that more than 87.62% of AR18 was removed and adsorption capacity was 88.65 mg·g−1 under optimized conditions ([AR18]: 10 mg·L−1; [adsorbent]: 0.55 g·L−1; pH: 3; Time: 46 min). Results shown that the adsorption kinetics fit the pseudo-second-order model (0.99), and the adsorption isotherms fitted to Langmuir isotherm model (0.99).
Graphical abstract











Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Abe K, Yano H (2011) Formation of hydrogels from cellulose nanofibers. Carbohydr Polym 85:733–737
Abràmoff MD, Magalhães PJ, Ram SJ (2004) Image Processing with ImageJ. Biophotonics Int 11:36–42
Alibak AH, Khodarahmi M, Fayyazsanavi P, Alizadeh SM, Hadi AJ, Aminzadehsarikhanbeglou E (2022) Simulation the adsorption capacity of polyvinyl alcohol/carboxymethyl cellulose based hydrogels towards methylene blue in aqueous solutions using cascade correlation neural network (CCNN) technique. J Clean Prod 337:130509
Alizadeh-Sani M, Tavassoli M, McClements DJ, Hamishehkar H (2021) Multifunctional halochromic packaging materials: Saffron petal anthocyanin loaded-chitosan nanofiber/methyl cellulose matrices. Food Hydrocolloids 111:106237
Araki J (2021) Dye adsorption revisited: application of the cationic dye adsorption method for the quantitative determination of the acidic surface groups of nanocellulose materials. Cellulose 28:7707–7715
Arora C, Soni S, Sahu S, Mittal J, Kumar P, Bajpai P (2019) Iron based metal organic framework for efficient removal of methylene blue dye from industrial waste. J Mol Liq 284:343–352
Ashrafi SD, Rezaei S, Forootanfar H, Mahvi AH, Faramarzi MA (2013) The enzymatic decolorization and detoxification of synthetic dyes by the laccase from a soil-isolated ascomycete, Paraconiothyrium variabile. Int Biodeterior Biodegrad 85:173–181
Athar T (2013) Synthesis and characterization of strontium oxide nanoparticles via wet process. Mater Focus 2:450–453
Atykyan N, Revin V, Shutova V (2020) Raman and FT-IR Spectroscopy investigation the cellulose structural differences from bacteria Gluconacetobacter sucrofermentans during the different regimes of cultivation on a molasses media. AMB Express 10:1–11
Azari A, Nabizadeh R, Nasseri S, Mahvi AH, Mesdaghinia AR (2020) Comprehensive systematic review and meta-analysis of dyes adsorption by carbon-based adsorbent materials: Classification and analysis of last decade studies. Chemosphere 250:126238
Bazrafshan E, Alipour MR, Mahvi AH (2016) Textile wastewater treatment by application of combined chemical coagulation, electrocoagulation, and adsorption processes. Desalin Water Treat 57:9203–9215
Bazrafshan E, Mostafapour FK, Hosseini AR, Raksh Khorshid A, Mahvi AH (2013) Decolorisation of reactive red 120 dye by using single-walled carbon nanotubes in aqueous solutions. J Chem 2013
Beh JH, Lim TH, Lew JH, Lai JC (2020) Cellulose nanofibril-based aerogel derived from sago pith waste and its application on methylene blue removal. Int J Biol Macromol 1:836–845
Chaleshtori AN, Meghadddam FM, Sadeghi M, Rahimi R, Hemati S, Ahmadi A (2017) Removal of Acid Red 18 (Azo-Dye) from aqueous solution by adsorption onto activated charcoal prepared from almond shell. J Environ Sci Manag 20:9–16
Chaugule AA, Mane VS, Bandal HA, Kim H, Kumbhar AS (2019) Ionic liquid-derived Co3O4-N/S-doped carbon catalysts for the enhanced water oxidation. ACS Sustain Chem Eng 7:14889–14898
Chen P, Liu X, Jin R, Nie W, Zhou Y (2017) Dye adsorption and photo-induced recycling of hydroxypropyl cellulose/molybdenum disulfide composite hydrogels. Carbohydr Polym 167:36–43
Chen X, Chen C, Zhu J (2019) Facile preparation of cellulose–attapulgite nanocomposite hydrogel for dye adsorption. Iran Polym J 28:347–359
Czepirski L, Balys MR, Komorowska-Czepirska E (2000) Some generalization of Langmuir adsorption isotherm. Internet J Chem 3:1099–8292
Dai H, Huang Y, Huang H (2018) Eco-friendly polyvinyl alcohol/carboxymethyl cellulose hydrogels reinforced with graphene oxide and bentonite for enhanced adsorption of methylene blue. Carbohydr Polym 185:1–11
Dalvand A, Nabizadeh R, Ganjali MR, Khoobi M, Nazmara S, Mahvi AH (2016) Modeling of Reactive Blue 19 azo dye removal from colored textile wastewater using L-arginine-functionalized Fe3O4 nanoparticles: optimization, reusability, kinetic and equilibrium studies. J Magn Magn Mater 404:179–189
Dehghani MH, Sanaei D, Ali I, Bhatnagar A (2016) Removal of chromium (VI) from aqueous solution using treated waste newspaper as a low-cost adsorbent: kinetic modeling and isotherm studies. J Mol Liq 215:671–679
Dubinin M (1960) The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem Rev 60:235–241
Ebadi A, Mohammadzadeh JSS, Khudiev A (2009) What is the correct form of BET isotherm for modeling liquid phase adsorption? Adsorption 15:65–73
Edzwald J, Association AWW (2011) Water quality & treatment: a handbook on drinking water. McGraw-Hill Education, New York
French AD (2014) Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 21:885–896
French AD (2017) Glucose, not cellobiose, is the repeating unit of cellulose and why that is important. Cellulose 24:4605–4609
Freundlich H (1906) Over the adsorption in solution. J Phys Chem 57:1100–1107
Gholami-Borujeni F, Mahvi AH, Naseri S, Faramarzi MA, Nabizadeh R, Alimohammadi M (2011a) Application of immobilized horseradish peroxidase for removal and detoxification of azo dye from aqueous solution. Res J Chem Environ 15:217–222
Gholami-Borujeni F, Mahvi AH, Nasseri S, Faramarzi MA, Nabizadeh R, Alimohammadi M (2011b) Enzymatic treatment and detoxification of acid orange 7 from textile wastewater. Appl Biochem Biotechnol 165:1274–1284
Hamidi F, Dehghani MH, Kasraee M, Salari M, Shiri L, Mahvi AH (2022) Acid red 18 removal from aqueous solution by nanocrystalline granular ferric hydroxide (GFH); Optimization by response surface methodology & genetic-algorithm. Sci Rep 12:1–15
Han T-T, Bai H-L, Liu Y-Y, Ma J-F (2018) Synthesis of nanoporous cobalt/carbon materials by a carbonized zeolitic imidazolate framework-9 and adsorption of dyes. New J Chem 42:717–724
Hashemkhani M, Rezvani Ghalhari M, Bashardoust P, Hosseini SS, Mesdaghinia A, Mahvi AH (2022) Fluoride removal from aqueous solution via environmentally friendly adsorbent derived from seashell. Sci Rep 12:9655
Heibati B, Rodriguez-Couto S, Al-Ghouti MA, Asif M, Tyagi I, Agarwal S, Gupta VK (2015a) Kinetics and thermodynamics of enhanced adsorption of the dye AR 18 using activated carbons prepared from walnut and poplar woods. J Mol Liq 208:99–105
Jiang M, Niu N, Chen L (2022) A template synthesized strategy on bentonite-doped lignin hydrogel spheres for organic dyes removal. Sep Purif Technol 285:120376
Jin L, Li W, Xu Q, Sun Q (2015) Amino-functionalized nanocrystalline cellulose as an adsorbent for anionic dyes. Cellulose 22:2443–2456
Joshi G, Naithani S, Varshney V, Bisht SS, Rana V, Gupta P (2015) Synthesis and characterization of carboxymethyl cellulose from office waste paper: a greener approach towards waste management. J Waste Manag 38:33–40
Kord Mostafapour F, Zolghadr R, Khodadadi Saloot M, Mahvi AH, Balarak D, Safari E (2022) Removal of Acid blue 113 from aqueous medium using a novel magnetic adsorbent derived from activated carbon fiber. Int J Environ Anal Chem 102:1–16
Kumar KY, Muralidhara H, Nayaka YA, Balasubramanyam J, Hanumanthappa H (2013) Low-cost synthesis of metal oxide nanoparticles and their application in adsorption of commercial dye and heavy metal ion in aqueous solution. Powder Technol 246:125–136
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Li J, Yang Z-l, Ding T, Song Y-J, Li H-C, Li D-q, Chen S, Xu F (2022a) The role of surface functional groups of pectin and pectin-based materials on the adsorption of heavy metal ions and dyes. Carbohydr Polym 276:118789
Li K, Li X, Wang D, Yang B, Liu Y, Wang H, Shen Y (2022b) Preparation of cross-linking PVA copolymer modified by DAAM/ADH and application in paper surface sizing. Cellulose 29:6845–6863
Lin F, You Y, Yang X, Jiang X, Lu Q, Wang T, Huang B, Lu B (2017) Microwave-assisted facile synthesis of TEMPO-oxidized cellulose beads with high adsorption capacity for organic dyes. Cellulose 24:5025–5040
Liu Z, Zhang F, Liu T, Peng N, Gai C (2016) Removal of azo dye by a highly graphitized and heteroatom doped carbon derived from fish waste: adsorption equilibrium and kinetics. J Environ Manage 182:446–454
Lyu W, Li J, Zheng L, Liu H, Chen J, Zhang W, Liao Y (2021) Fabrication of 3D compressible polyaniline/cellulose nanofiber aerogel for highly efficient removal of organic pollutants and its environmental-friendly regeneration by peroxydisulfate process. Chem Eng J 414:128931
Mahvi AH, Dalvand A (2020) Kinetic and equilibrium studies on the adsorption of Direct Red 23 dye from aqueous solution using montmorillonite nanoclay. Water Qual Res J 55:132–144
Maiti M, Sarkar M, Maiti S, Malik MA, Xu S (2020) Modification of geopolymer with size controlled TiO2 nanoparticle for enhanced durability and catalytic dye degradation under UV light. J Clean Prod 255:120183
Mirzadeh S-S, Khezri S-M, Rezaei S, Forootanfar H, Mahvi AH, Faramarzi MA (2014) Decolorization of two synthetic dyes using the purified laccase of Paraconiothyrium variabile immobilized on porous silica beads. J Environ Health Sci Eng 12:1–9
Mondal AK, Xu D, Wu S, Zou Q, Lin W, Huang F, Ni Y (2022) High lignin containing hydrogels with excellent conducting, self-healing, antibacterial, dye adsorbing, sensing, moist-induced power generating and supercapacitance properties. Int J Biol Macromol 207:48–61
Noorimotlagh Z, Mirzaee SA, Martinez SS, Alavi S, Ahmadi M, Jaafarzadeh N (2019) Adsorption of textile dye in activated carbons prepared from DVD and CD wastes modified with multi-wall carbon nanotubes: equilibrium isotherms, kinetics and thermodynamic study. Chem Eng Res Des 141:290–301
Peng N, Hu D, Zeng J, Li Y, Liang L, Chang C (2016) Superabsorbent cellulose–clay nanocomposite hydrogels for highly efficient removal of dye in water. ACS Sustain Chem Eng 4:7217–7224
Rahmani M, Kaykhaii M, Sasani M (2018) Application of Taguchi L16 design method for comparative study of ability of 3A zeolite in removal of Rhodamine B and Malachite green from environmental water samples. Spectrochim Acta A Mol Biomol Spectrosc 188:164–169
Rejek M, Grzechulska-Damszel J, Schmidt B (2021) Synthesis, characterization, and evaluation of degussa P25/chitosan composites for the photocatalytic removal of sertraline and acid Red 18 from water. J Polym Environ 29: 1–8
Ruthven DM (1984) Principles of adsorption and adsorption processes. John Wiley & Sons, Hoboken
Sani MA, Maleki M, Eghbaljoo-Gharehgheshlaghi H, Khezerlou A, Mohammadian E, Liu Q, Jafari SM (2022) Titanium dioxide nanoparticles as multifunctional surface-active materials for smart/active nanocomposite packaging films. Adv Coll Interface Sci 300:102593
Saratale RG, Sivapathan SS, Jung WJ, Kim HY, Saratale GD, Kim DS (2016) Preparation of activated carbons from peach stone by H4P2O7 activation and its application for the removal of Acid Red 18 and dye containing wastewater. J Environ Sci Health A 51:164–177
Shadkam R, Naderi M, Ghazitabar A, Akbari S (2021) Adsorption performance of reduced graphene-oxide/cellulose nano-crystal hybrid aerogels reinforced with waste-paper extracted cellulose-fibers for the removal of toluene pollution. Mater Today Commun 28:102610
Shirmardi M, Mesdaghinia A, Mahvi AH, Nasseri S, Nabizadeh R (2012) Kinetics and equilibrium studies on adsorption of acid red 18 (Azo-Dye) using multiwall carbon nanotubes (MWCNTs) from aqueous solution. E-J Chem 9:541909
Sillanpää M, Mahvi AH, Balarak D, Khatibi AD (2021) Adsorption of Acid orange 7 dyes from aqueous solution using Polypyrrole/nanosilica composite: experimental and modelling. Int J Environ Anal Chem 101: 1–18
Siripongpreda T, Somchob B, Rodthongkum N, Hoven VP (2021) Bacterial cellulose-based re-swellable hydrogel: facile preparation and its potential application as colorimetric sensor of sweat pH and glucose. Carbohydr Polym 256:117506
Sotomayor FJ, Cychosz KA, Thommes M (2018) Characterization of micro/mesoporous materials by physisorption: concepts and case studies. Acc Mater Surf Res 3:34–50
Spagnoli AA, Giannakoudakis DA, Bashkova S (2017) Adsorption of methylene blue on cashew nut shell based carbons activated with zinc chloride: the role of surface and structural parameters. J Mol Liq 229:465–471
Temkin M (1940) Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochim URSS 12:327–356
Thombare N, Mahto A, Singh D, Chowdhury AR, Ansari MF (2023) Comparative FTIR characterization of various natural gums: a criterion for their identification. J Polym Environ 31: 1–9
Vargas AM, Cazetta AL, Martins AC, Moraes JC, Garcia EE, Gauze GF, Costa WF, Almeida VC (2012) Kinetic and equilibrium studies: adsorption of food dyes Acid Yellow 6, Acid Yellow 23, and Acid Red 18 on activated carbon from flamboyant pods. Chem Eng J 181:243–250
Wang C-H, Hsu H-C, Hu J-H (2014) High-energy asymmetric supercapacitor based on petal-shaped MnO2 nanosheet and carbon nanotube-embedded polyacrylonitrile-based carbon nanofiber working at 2 V in aqueous neutral electrolyte. J Power Sources 249:1–8
Wang Z, Qiu T, Guo L, Ye J, He L, Li X (2019) The building of molecularly imprinted single hole hollow particles: a miniemulsion polymerization approach. Chem Eng J 357:348–357
Wang W, Hu J, Zhang R, Yan C, Cui L, Zhu J (2021) A pH-responsive carboxymethyl cellulose/chitosan hydrogel for adsorption and desorption of anionic and cationic dyes. Cellulose 28:897–909
Wang X, Fan X, Xie H, Li X, Hao C (2022) Polyacrylic acid/carboxymethyl cellulose/activated carbon composite hydrogel for removal of heavy metal ion and cationic dye. Cellulose 29:483–501
Wang X, Deng B, Yu L, Cui E, Xiang Z, Lu W (2020) Degradation of azo dyes Congo red by MnBi alloy powders: performance, kinetics and mechanism. Mater Chem Phys 251: 123096
Weber TW, Chakravorti RK (1974) Pore and solid diffusion models for fixed-bed adsorbers. AlChE J 20:228–238
Wong S, Yac’cob NAN, Ngadi N, Hassan O, Inuwa IM (2018) From pollutant to solution of wastewater pollution: synthesis of activated carbon from textile sludge for dye adsorption. Chin J Chem Eng 26:870–878
Wu S, Sun A, Zhai F, Wang J, Xu W, Zhang Q, Volinsky AA (2011) Fe3O4 magnetic nanoparticles synthesis from tailings by ultrasonic chemical co-precipitation. Mater Lett 65:1882–1884
Xie Y, Guo F, Mao J, Huang J, Chen Z, Jiang Y, Lai Y (2021) Freestanding MoS2@carbonized cellulose aerogel derived from waste cotton for sustainable and highly efficient particulate matter capturing. Sep Purif Technol 254:117571
Zare K, Sadegh H, Shahryari-Ghoshekandi R, Maazinejad B, Ali V, Tyagi I, Agarwal S, Gupta VK (2015) Enhanced removal of toxic Congo red dye using multi walled carbon nanotubes: kinetic, equilibrium studies and its comparison with other adsorbents. J Mol Liq 212:266–271
Zhang W-X, Lai L, Mei P, Li Y, Li Y-H, Liu Y (2018) Enhanced removal efficiency of acid red 18 from aqueous solution using wheat bran modified by multiple quaternary ammonium salts. Chem Phys Lett 710:193–201
Acknowledgments
The authors would like to gratefully acknowledge the financial support provided by the Tehran University of Medical Sciences.
Funding
Research reported in this publication was supported by Elite Researcher Grant Committee under award number [46988] from Tehran University of Medical Sciences (TUMS), Tehran, Iran.
Author information
Authors and Affiliations
Contributions
MRG: Conceptualization, Methodology, Validation, Writing—original draft, Writing—review and editing. DS: Conceptualization, Methodology, review and editing, Writing—original draft. RN: Methodology, Conceptualization, Visualization, Validation. AHM: Conceptualization, Methodology, review and editing, Writing—original draft, Supervision, Project administration.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Ethical approval
IR.TUMS.SPH.REC.1399.014.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghalhari, M.R., Sanaei, D., Nabizadeh, R. et al. Cellulose-based hydrogel beads derived from wastepapers: application for organic dye adsorption. Cellulose 30, 9669–9691 (2023). https://doi.org/10.1007/s10570-023-05467-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-023-05467-3