Abstract
Recent studies on the plant cell wall assume that hemicellulosic polysaccharides interact closely with cellulose microfibrils through hydrophobic forces. In contrast, hydrogen bonds, which are still emphasized, play a significant role in stabilizing the conformation of the hemicellulose bound on the cellulose surface. However, there is still no consensus on the nature of the interactions between these polysaccharides and on potential interactions of pectins also with cellulose microfibrils. Since the natural plant cell wall is a very complex system, studies of model systems (in vitro) provide information about the interaction between plant polysaccharides. Adsorption studies, which describe the interactions between non-cellulosic polysaccharides and cellulose, are one of these methods. They help to determine the type of these interactions and characterize the adsorption process. This review aims to summarize the knowledge of the interactions between cellulose and representatives of hemicelluloses and pectins, which was mainly provided by adsorption studies.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding the mechanisms of interactions between different polysaccharides in cell walls is critical for the sustainable development of multiple social and economic sectors. The cell wall assembly, i.e. components and their interrelationships, are very important for multiple functions of cell walls in plants: growth, mechanical resistance, plant adaptation to given conditions, and many others (Ka̧czkowski 2003; Park and Cosgrove 2015). Interactions between non-cellulosic polysaccharides and cellulose in the plant cell wall are also of an inestimable value to a multitude of industry sectors, e.g. biofuel production, crop production, paper industry, and packaging manufacturing (Souza et al. 2013; Li et al. 2020; Merino et al. 2019). Furthermore, agricultural wastes containing cellulose, hemicelluloses, and lignin are promising biosorbents characterized by a low cost, a neutral impact on the environment, and above all, high efficiency (Zhao 2011).
The plant cell wall is a complex structure surrounding the cytoplasmic membrane. It is the main safeguard between the external environment and the interior of the cell facilitating the transport of essential substances and serving as a defense against biotic and abiotic stresses (Rui and Dinneny 2020; Tenhaken 2015). Plant cell walls are mainly classified into primary and secondary cell walls differing in their structure and function. The primary cell wall is thinner and more flexible, as it is formed during cell growth and must resist stretching. It consists mainly of cellulose, hemicellulose, pectin, and a small number of proteins (Fig. 1a). The composition of plant cell wall polysaccharides differs among plant types. Homogalacturonan, rhamnogalacturonan type I, and rhamnogalacturonan type II are the typical pectic polysaccharides. The primary cell wall of dicotyledons and gymnosperms is rich in xyloglucan hemicelluloses (30 and 20%, respectively) (Albersheim et al. 2010), while arabinoxylans and β-(1,3)(1,4)-D-glucan (mixed-linkage glucan, MLG) are the main hemicelluloses in the grass family. Compared to the primary cell wall, the secondary cell wall contains less pectin but more cellulose and, additionally, lignin, which gives stiffness and increases the mechanical resistance of the cell (Fig. 1a). Lignin is a branched phenolic polymer based on hydrophobic subunits. Xylan and galactoglucomannan are the dominant secondary cell wall hemicelluloses (Terrett and Dupree 2019). In the case of some fibers, tertiary cell walls are distinguished as well (Gorshkova et al. 2022). The tertiary cell walls are mainly composed of cellulose, and the other predominant polysaccharides are rhamnogalacturonan type I substituted by galactan and fewer mannans (Gorshkova et al. 2022).
Schematic representation of the primary and secondary plant cell wall with visualization of the arrangement of a cellulose microfibril, pectin, hemicellulose, and lignin (a), cellulose molecular structure (b), cellulose crystalline structure (c) (Jarvis 2018, 2023; Moon et al. 2011, Kozlova et al. 2020)
Until recently, it was common to use a plant cell wall model where cellulose microfibrils conferred resistance and mechanical strength, while matrix polysaccharides acted as a charge carrier (Cosgrove 2005). Furthermore, the arrangement of the microfibrils influenced the anisotropy of cell growth, which efficiently took place along fibers (Barbacci et al. 2013). In contrast, a more up-to-date model emphasizing the occurrence of ‘biomechanical hotspots’ seems to be interesting. It reveals that microfibrils are locally connected with a small proportion of xyloglucan and these connections can control the extensibility of the wall (Cosgrove 2014).
The available plant cell wall models have emphasized covalent and non-covalent bonding interactions between plant cell wall polysaccharides (Albersheim et al. 2010). This knowledge was acquired indirectly from the sequential solubilization of each group of polysaccharides in different media (enzymes, chelators, and other chemicals) and from investigations of the interaction between isolated cell wall macromolecules in laboratory conditions. The interaction between cellulose and xyloglucan, i.e. the main representative of hemicelluloses, has been investigated most comprehensively so far. It has been shown that particularly this hemicellulose has a strong affinity for cellulose. It adsorbs on its surface and can even act as a promoter for further binding, for example with pectins, in certain conditions (Zykwinska et al. 2008b). Furthermore, xyloglucan shows the ability not only to interact locally with the surface of cellulose fibrils but also to coat them (Park and Cosgrove 2015). Hence, most of the cell wall models recognize that the xyloglucan-cellulose network is the most important structural element. Currently, pectins are believed to have the ability to interact with cellulose microfibrils mainly through their sidechains: galactose, arabinose, and xylose residues. However, this ability is much weaker than that of hemicelluloses. Therefore, in the commonly used cell wall models, pectins are considered to form an independent matrix-gel network, in which the hemicellulose-cellulose network is embedded (Willats et al. 2001). In contrast, a more recent model of the plant cell wall emphasizes greater contact between cellulose and pectins which is not necessarily stabilized by binding (Cosgrove 2014; Zhang et al. 2021). Additionally, the morphological structure of cellulose microfibrils cannot be neglected in the picture of the interaction between cell wall macromolecules, as it has a significant influence on adsorption efficiency. It is known that cellulose with a more ordered and highly crystalline structure exhibits higher capacity of interaction with cell wall polysaccharides (Gu and Catchmark 2013).
In vivo research of native plant tissues can provide valuable data, but such studies have some limitations due to the complex and multiscale assembly of cell walls. Therefore, in vitro models of plant cell wall analogs based on cellulose microfibrils formed by cellulose-synthesizing bacteria (Komagataeibacter xylinus) have been proposed (Cybulska et al. 2010a, b; Gu and Catchmark 2014). Despite the many advantages of using bacterial cellulose for testing, this method has some drawbacks. For example, after addition of different polysaccharides to the medium in which cellulose-producing bacteria grow, it not possible to control the number of substances incorporated by the bacteria into the cellulose composite. Therefore, to avoid these disadvantages, the adsorption phenomenon where plant cellulose is an adsorbent, while pectin and hemicellulose are polymers that can accumulate on the adsorbent can be used in the research. Investigations of pectin and hemicellulose adsorption on cellulose microfibrils have already been conducted (Myśliwiec et al. 2016; Zykwinska et al. 2005, 2008a, b). The occurrence of interactions between polysaccharides in adsorption experiments can be analyzed using several research methods. They are based on indirect determination of the amount of the adsorbed substance using UV–VIS spectroscopy, chromatography, and other techniques, or direct analysis of the adsorbate concentration in real-time using, for example, quartz crystal microbalance with dissipation (QCM-D) monitoring and surface plasmon resonance spectroscopy (SPR). Experimental results illustrating the quantitative adsorption are used to choose an appropriate adsorption model giving information on the nature of the interaction. Additionally, infrared spectroscopy, Raman spectroscopy, differential scanning calorimetry (DSC), atomic force microscopy (AFM), scanning electron microscopy (SEM), microelectrophoresis, and potentiometric titration are used to support and complete the adsorption data.
It is extremely important to conduct research on a given topic from different perspectives, as in this way, the common parts of conclusions can be highlighted. This review mainly presents experimental research, while it is worth highlighting the relevance of molecular dynamics simulations in this field. For example, the significant role of cellulose as a backbone and, innovatively, a tensile force-transfer network non-covalently linked to hemicelluloses and embedded in a hydrated pectin matrix, was confirmed by coarse-grained molecular dynamics (CGMD) (Zhang et al. 2021).
Importance of plant cell wall polysaccharides structure
The greatest influence on the interaction between cellulose and non-cellulosic cell wall polysaccharides is exerted by the structure of these compounds. Cellulose is a homopolymer consisting of D-glucose molecules linked by a β-1,4-glycosidic bond. In nature, cellulose I exists in the crystalline form and is a mixture of two distinct crystalline phases: cellulose Iα and Iβ whose proportion depends on the source of the cellulose. The cell walls of higher plants have greater amounts of cellulose Iβ than Iα, whereas cellulose Iα is abundant mostly in algal cell walls or in cellulose produced by some bacteria as biofilms (Saxena and Brown 2005). Moreover, cellulose Iα and Iβ have the same pattern of hydrogen bonds: O3–H⋅⋅⋅⋅O5 and O2–H⋅⋅⋅⋅O6 intrachains as well as O3–H⋅⋅⋅⋅O6ʹ interchains with parallel cellulose chain alignment (Moon et al. 2011) (Fig. 1b). The difference between the two cellulose I forms lies in their sheet alignment: two chains in each monoclinic unit cell of cellulose Iβ and one chain in the triclinic unit cell of cellulose Iα (Gümüskaya et al. 2003; Festucci-Buselli et al. 2007). The cellulose supramolecular structure is mainly the result of the conformation at C-6. The tg conformation is necessary for the crystalline (ordered) form of cellulose, while the gt and gg conformations are typical for the less ordered forms (Jarvis 2018). This, in turn, influences the structure of the cellulose microfibril; one of the most common models describes the cellulose microfibril as a highly crystalline core surrounded by less ordered regions (Nishiyama 2009; Jarvis 2018). Another model presents microfibrils as crystalline regions disrupted by so-called amorphous cellulose (Moon et al. 2011) (Fig. 1c). It is assumed that the crystalline/amorphous ratio influences the reactivity and water binding capacity of cellulose and the mechanical properties of cellulose-based materials (Jarvis 2023). The structure of cellulose microfibrils necessitates the existence of both hydrophilic and hydrophobic surfaces (Fig. 1c) (Lin et al. 2016). The number of cellulose chains as well as the length and diameter of cellulose microfibrils depend on the plant species (Northcote 1972; Ding and Himmel 2006; Patel 2009; Niimura et al. 2010; Thomas et al. 2013; Jarvis 2023). The physical dimensions of cellulose microfibrils, such as the crystalline structure, also affect the level of adsorption (Salmén 2022). Although cellulose from different sources is chemically identical, its physical forms may differ (Albersheim et al. 2010; Park and Cosgrove 2015).
For example, similar to cellulose, the most abundant hemicellulose in dicots, i.e. xyloglucan, is composed of β-1,4 linked glucose molecules substituted by α-D-xylosyl residues. In most dicots and gymnosperms, xylose units can be decorated by galactose or galactose-fucose units. Xyloglucans undergo only minor galactosylation and contain no fucose units only in the Solanaceae family. In dicots, xyloglucan also contains α-L-arabinosyl residues. Xyloglucan isolated from grasses (Poaceae monocotyledons) contains only small amounts of galactose units. In some plants, the additional monosaccharide residues of xyloglucan can undergo O-acetylation (Albersheim et al. 2010). Xylans are made up of xylosyl units linked by a β-1,4-glycosidic bond with numerous substitutions at C-2 and/or C-3 of the main chain by arabinose, galactose, glucuronic acid, and other monosaccharides, constituting from 10 to 90% of the xylan backbone (Fig. 2) (Albersheim et al. 2010). For example, arabinoxylan, which is the predominant hemicellulose in Poaceae monocotyledons primary cell walls (30–40% of the grass cell wall compared with approx. 5% of the dicotyledon cell wall), contains arabinose residues in an arabinose-to-xylose ratio of 0.6 (Dervilly-Pinel et al. 2004). In turn, xylans are mainly found in secondary cell walls, where they are mostly substituted by glucuronic acid. Xylans in secondary cell walls very frequently undergo O-acetylation (Albersheim et al. 2010). In addition to their role as storage polysaccharides, mannans are present abundantly in secondary cell walls of soft and hardwood and in lower amounts (less than 2 mol%) in primary cell walls. Galactomannans are mannans containing α-1,6-galactose as the sidechain, whereas galactoglucomannans contain additional β-1,4-glucose as the sidechain (Held et al. 2015). Glucomannans and glucuronoxylan are abundant in the secondary cell wall of hardwood (Melton et al. 2009). An important feature of mannans is their O-acetylation (Melton et al. 2009). Finally, β-(1,3)(1,4)-D-glucan (mixed-linkage glucan, MLG) is an unbranched polymer composed of D-glucose molecules connected via a β-1,4-glycosidic bond forming blocks connected with each other by a β-1,3-glycosidic bond. This polysaccharide is especially important during rapid growth (Albersheim et al. 2010).
Homogalacturonans (HG), rhamnogalacturonans I (RGI), and rhamnogalacturonans II (RGII) are the main representatives of pectins. Homogalacturonan (HG), a linear homopolymer composed of D-galacturonic acid, can be methylated and/or acetylated (Costa and Plazanet 2016; Ochoa-Villarreal et al. 2012). Rhamnogalacturonan I (RGI) is a branched polymer of repeating sequences of disaccharides composed of α-1,4-galacturonic acid and α-1,2-rhamnose residues. In addition to being methylated and/or acetylated, it also contains branched structures of arabinans and galactans (Costa and Plazanet 2016; Heredia et al. 1995; Kaczmarska et al. 2022). Rhamnogalacturonan II (RG II), the most complex polysaccharide present in the plant cell wall, is composed of α-1,4-galacturonic acid units which are substituted by branched structures composed of monosaccharides such as xylose, arabinose, fucose, apiose, rhamnose, and galacturonic and glucuronic acids, very often methylated or O-acetylated (Fig. 2) (Albersheim et al. 2010).
Adsorption method in the investigation of interactions between polysaccharides
Interactions between polysaccharides can be characterized using several research methods. One of them is the use of sequential extraction and enzymatic digestion, which gives insight into the possible binding between polysaccharides (Broxterman and Schols 2018). Another method is an in vitro approach using the adsorption technique (Dammak et al. 2015; Gu and Catchmark 2013; Myśliwiec et al. 2016; Villares et al. 2015; Zykwinska et al. 2005, 2008a, b). Interfacial surface studies provide a range of valuable information about the adsorbent and the adsorbate, the type of interactions between them, and the characteristics of the adsorption layer.
Methods for analysis of the level of the adsorption of polysaccharides on cellulose often involve indirect determination of the amount of the adsorbed substance. For this purpose, the concentration of the adsorptive before and after contact with the adsorbent is compared. The concentration can be determined using various analytical methods, e.g. chromatography and UV–VIS spectroscopy. In the general scheme of adsorption studies in xyloglucan/cellulose and pectin/cellulose systems, the xyloglucan or pectin solution is mixed with cellulose for a given time, the filtrate is centrifuged from the sediment, and the total sugar and/or galacturonic acid content in the filtrate is determined colorimetrically. The amount of the adsorbed substance is indirectly determined by subtracting the concentration remaining in the filtrate from the initial concentration of the test compound in relation to the weight of cellulose (Terashima et al. 2004).
Adsorption studies of these systems can also be carried out directly by determination of the adsorbate concentration. In this case, complementary sensor techniques, such as quartz crystal microbalance with dissipation (QCM-D) monitoring and surface plasmon resonance spectroscopy (SPR), are used for real-time monitoring of the thickness and mass of the model cellulose film (Benselfelt et al. 2016; Lin et al. 2018; Jaafar et al. 2019; Yao et al. 2021). The QCM-D technique is based on the piezoelectric properties of the quartz crystal, and the measurement involves determination of changes in the resonant frequency of the oscillating crystal occurring during adsorption (Eronen et al. 2011a, b; Paananen et al. 2004). Changes in the dissipation energy (ΔD) are determined to define the mechanical properties of the adsorbed layer, such as viscoelasticity (Eronen et al. 2011a, b). Briefly, SPR is an optical technique that allows determination of the concentration of analyzed compounds in the vicinity of a gold sensor surface by measuring changes in the refractive index. Laser light falls at a specific angle on the sensor, causing the excitation of surface plasmons (interaction with free gold electrons). A change in the concentration of the compound on the sensor is accompanied by a change in the refractive index, which affects the resonance conditions (Benselfelt et al. 2016; Guo et al. 2021).
The phenomenon of adsorption depends on many factors. The adsorbent and the adsorbate play a key role in this process; therefore, it is important to characterize both. The most common adsorbents are solids classified by their surface porosity and chemical nature; hence, it is useful to determine their specific surface area and surface charge. As far as adsorbates are concerned, their structure, above all the presence and type of functional groups, and their ability to dissociate in the pH conditions of the experiment are certainly crucial (Terashima et al. 2004). Polydispersity and molecular weight are determined in the case of polymers. In addition, the type of basic electrolyte and its ionic strength, the pH of the system, and the temperature may affect the adsorption process (Grzadka and Chibowski 2009). The adsorption of macromolecules is significantly influenced by their conformation, which can be changed as a result of interactions with the electrolyte. This affinity can be modified by changes in temperature, pH, and ionic strength of the solution. An increase in temperature may result in a higher affinity of the macromolecules for the electrolyte and thus the unfolding of coiled polymer chains (Wiśniewska et al. 2013). For example, studies of the adsorption of a non-ionic polymer on a metal oxide in the temperature range of 15–40 °C showed that adsorption increased with increasing temperature (Wiśniewska et al. 2013). This was related to the fact that, at higher temperatures, non-ionic polymers adopted a more favorable conformation, and there was an increase in the linear dimensions of the polymer chains allowing interactions with the adsorbent (Wiśniewska et al. 2013). The adsorption of non-ionic polymers (e.g. hemicellulose) on cellulose has not yet been fully investigated. In these adsorption systems, sodium acetate or phosphate with a pH value in the range of 5.8–6.9 is usually used as a buffer solution, while the temperature is from 20 to 40 °C to keep the study conditions close to those in the plant (Dammak et al. 2015; Gu and Catchmark 2013; Myśliwiec et al. 2016; Villares et al. 2015; Zykwinska et al. 2005; 2008a, b). The adsorption of hemicelluloses on cellulose is characterized by a low heat value (small enthalpy change), which may be indicative of physical adsorption. This type of adsorption mainly occurs at lower temperatures, while desorption may occur at high temperatures. Lopez et al. studied the adsorption of xyloglucan on cellulose, taking into account the effect of temperature on this process. Using isothermal titration calorimetry (ITC), it was demonstrated that higher temperature (in the range of 25–60 °C) increased adsorption. According to the van’t Hoff equation, it was shown to be an endothermic process (Lopez et al. 2010). These assumptions were also confirmed in QCM-D and SPR spectroscopy studies. The increase in temperature contributed to an increase in the accumulation of xyloglucan molecules on the cellulose surface, which displaced water molecules and thus increased the entropy of the system (Benselfelt et al. 2016; Yao et al. 2021). In adsorption research, adsorption energy is the change in free energy (∆G). Its value is not only influenced by the commonly quoted change in enthalpy (∆H) but is also significantly influenced by the change in entropy (∆S) and temperature (T) (Kishani et al. 2021; Wohlert et al. 2022).
Recent studies address this issue and report that the increase in entropy caused by the displacement of solvent/water molecules by the adsorbate from the cellulose surface is the main driving force behind the adsorption of hemicelluloses on cellulose (Benselfelt et al. 2016; Kishani et al. 2021).
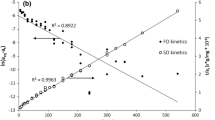
The kinetics and equilibrium of the process are investigated in adsorption experiments (Fig. 3). The kinetics of the process gives information about the dynamics and the time required to achieve equilibrium in the system. The kinetic process is usually modeled by empirical or semi-empirical equations of the first order (FO) and second order (SO) (Myśliwiec et al. 2016). The FO kinetic model assumes that the adsorption rate is directly proportional to the difference in the concentration versus time. In turn, the SO model assumes that the adsorption rate is proportional to the available active sites on the adsorbent and is dependent on the amount of the adsorbate on the surface of the adsorbent. The adsorption kinetics is the measure of the adsorption speed and depends on the number of particles colliding with the adsorbate surface per second. It provides information about the mechanism of the adsorbate surface coating, which is important for biomolecules that have the possibility of rearrangement. In the case of slow adsorption processes, the biomolecules have time to rearrange and assume a flat conformation on the adsorbate surface, while loops and tails are rather formed in the case of fast processes.
After determination of the time after which the equilibrium in the adsorption system is achieved, quantitative results are used to match an appropriate model of adsorption (Fig. 3a). Literature data show that the Langmuir and Freundlich adsorption isotherms are the best isotherms to describe interactions between non-cellulosic polysaccharides and cellulose. The Langmuir isotherm is the basic adsorption equation regarded as the starting equation for more elaborate studies. It is mainly used to describe chemisorption with the following assumptions: the presence of a certain number of active sites, the formation of an adsorption monolayer, the adsorbate molecules do not move on the adsorbent surface, and the lateral interactions between the adsorbate molecules are neglected. Previous studies of the adsorption of hemicelluloses/pectins on cellulose were only based on the Langmuir isotherm, but with consideration of heterogeneity effects, lateral interactions, and multilayer effects (Gu and Catchmark 2013; Dammak et al. 2015; Hayashi et al. 1994b; Kabel et al. 2007; Villares et al. 2015; Zykwinska et al. 2008b). The general Langmuir isotherm is given by the formula:
where b is the adsorption constant of the process, qm is the maximum adsorption (adsorption capacity), qe is the amount of adsorbed non-cellulosic polysaccharides per mg of cellulose, and Ce stands for the equilibrium concentration.
In contrast to the Langmuir isotherm, the Freundlich isotherm, which describes the multi-site adsorption isotherm for rough and energetically heterogeneous surfaces (Kabel et al. 2007; Zykwinska et al. 2008b), is given by the formula:
where b and n are the adsorption constants of the process, qe is the amount of adsorbed non-cellulosic polysaccharides per mg of cellulose, and Ce stands for the equilibrium concentration. Myśliwiec et al. (2016) showed that the adsorption of xyloglucan on Avicel cellulose is described by the Fowler–Guggenheim isotherm:
where b is the adsorption constant of the process, qm is the maximum adsorption (adsorption capacity), qe is the amount of adsorbed non-cellulosic polysaccharides per mg of cellulose, Ce stands for the equilibrium concentration, and α is a constant that describes interactions between molecules in the adsorbed layer. This isotherm is a generalized form of the Langmuir equation isotherm and gives evidence that lateral interactions play an important role in adsorption.
The typical theoretical models of adsorption are presented in Fig. 3a.
Adsorption of hemicelluloses on cellulose
As mentioned above, with their diverse structure, hemicelluloses are an interesting group of plant cell wall polysaccharides. Hemicelluloses have been shown to interact with cellulose within the cell wall to form a network. It is believed that xyloglucan in dicots and arabinoxylan in grasses have the greatest affinity for interactions with cellulose (Albersheim et al. 2010). Interestingly, native hemicelluloses and celluloses from the same plant have a higher affinity for each other and can influence cellulose aggregation during morphogenesis (Chambat et al. 2005). However, most studies on the interaction of hemicellulose with cellulose were based on Avicel or bacterial cellulose (Kabel et al. 2007; Kiemle et al. 2014; Lima et al. 2004) (SM Table 1).
The interaction of the main representative of hemicelluloses, i.e. xyloglucan, with cellulose microfibrils affects the mechanical properties of the primary cell wall, creating more stretchy structures essential for tissue growth (Whitney et al. 1999). It is known that xyloglucan interacts via hydrogen bonds with the surface of cellulose microfibrils and can crosslink two adjacent cellulose microfibrils to form a xyloglucan-cellulose network. Interactions also take place between the hemicellulose backbone and cellulose, while the side branches may even hinder the molecule from reaching its favorable conformation. Pauly et al. (1999) showed that up to 64% of all xyloglucans were associated with cellulose. In contrast, solid-state nuclear magnetic resonance (ss-NMR) spectroscopy studies showed only a few xyloglucan-cellulose linkages (Dick-Pérez et al. 2011). Park and Cosgrove (2015) also demonstrated a limited amount of xyloglucan in contact with cellulose, introducing the concept of biomechanical hotspots and revising the commonly used tethered network model of the plant cell wall. Small amounts of xyloglucan were shown to be present at the point of contact between two cellulose microfibrils. Importantly, coarse-grained molecular dynamics (CGMD) simulations showed that tensile forces were not transmitted through the non-cellulosic polysaccharides of the matrix, but precisely through the connection points between the cellulose microfibrils (Zhang et al. 2021).
The literature reports that there can be ionic interactions, hydrophobic forces, van der Waals dispersion forces, and hydrogen bonds between hemicelluloses and cellulose (Heinonen et al. 2022; Wohlert et al. 2022; Zykwinska et al. 2008a,b). In the case of adsorption on cellulose, the presence of available functional groups, e.g. hydroxyl groups, on its hydrophilic surface was highlighted by many researchers. It is assumed that intrachain O3–H⋅⋅⋅⋅O5 bonds are stable and do not take part in the creation of hydrogen bonds; hence, only some hydroxyl groups can serve as donors. However, recent studies have shown that these assumptions are farfetched and hydrogen bonds are not the main factor responsible for the interactions between xyloglucan and cellulose (Wohlert et al. 2022). The significance of solvent (water) molecules has been underlined. Molecular dynamics studies have shown that, at room temperature, an increase in entropy is the main driver of the adsorption process due to the release of water molecules from the interfacial surface (Kishani et al. 2021). From a thermodynamic point of view (Eq. 1), hydrogen bonds between hemicellulose and cellulose do not play a major role in adsorption. The formation of weak hydrogen bonds results in a small enthalpy change (Kishani et al. 2021), and this cannot be considered the main driving force of adsorption. On the other hand, it is worth emphasizing that hydrogen bonds influence the stabilization of the conformation of the adsorbed polymer chain (Heinonen et al. 2022; Simmons et al. 2016). The molecular dynamic simulation has also revealed that the interaction with cellulose occurs over a short distance, and adsorption on the hydrophobic surface of the microfibril is preferable, indicating the importance of hydrophobic forces (Oehme et al. 2015; Park and Cosgrove 2015).
The Langmuir and Freundlich models have been applied in investigations of xyloglucan adsorption onto cellulose microfibrils. Langmuir suggests the creation of a single layer of hemicellulose on cellulose microfibrils, and the Freundlich model assumes several different types of binding. The best description of the interaction in this case is provided by the Freundlich model suggesting the formation of multilayers. In contrast, the Fowler–Gugenheim isotherm used in previous investigations of xyloglucan-cellulose adsorption confirmed the role of lateral interactions and showed that chain–chain interactions as well as xyloglucan reconformation may have limited the adsorption kinetics (Myśliwiec et al. 2016). The Freundlich and Langmuir models are unable to include lateral interactions in the adsorbed layer. Bootten et al. (2004) showed that the xyloglucan backbone is only partially rigid and not only forms a crosslink between two microfibrils but can also crosslink other non-cellulosic polysaccharides. Moreover, the xyloglucan-cellulose interaction depends on the sidechains and molecular weight of xyloglucan (Hayashi et al. 1994a; Hayashi and Kaida 2011; Lima et al. 2004). The galactosylation and fucosylation of xyloglucan also influence the interaction with cellulose microfibrils (Lima and Buckeridge 2001). Fucosylated xyloglucan has been reported to interact with cellulose better than xyloglucan without fucose. However, as shown by Chambat et al. (2005), L-fucose substitution of xyloglucan does not influence adsorption or may even hinder the interaction with cellulose. On the other hand, the galactosylation pattern also influences the interaction with cellulose, i.e. unevenly distributed galactan sidechains exhibit better binding, while the even distribution results in a twisted structure of xyloglucan and the worst binding to cellulose microfibrils. Longer sidechains increase the strength of the interaction but decrease its efficiency, and each xyloglucan molecule can form not more than 4 H–bonds (Hanus and Mazeau 2006). Using the Langmuir model, Gu and Catchmark (2013) showed that the adsorption of xyloglucan onto cellulose was irreversible (Oehme et al. 2015). Most studies of adsorption of xyloglucan on cellulose were carried out with the use of tamarind seed xyloglucan. This compound serves as a reserve polysaccharide, and its structure differs from that of cell wall xyloglucan. Moreover, the botanical source of xyloglucan has an impact on its structure (Park and Cosgrove 2015). For example, apple xyloglucan was composed of unbranched linear glucan motifs, typical XXXG motifs, and fucosylated (XXFG) and galactosylated (XXLG, XLXG, and XLLG) parts (Chen et al. 2022; Zhao et al. 2014). The greatest binding affinity was exhibited by the fucosylated and galactosylated xyloglucan fractions. The influence of the different molecular weights of apple xyloglucan additionally modified by enzyme action on adsorption onto cellulose nanofibers was investigated by Chen et al. (2022). The lower molecular weight of the apple xyloglucan was associated with better adsorption on cellulose nanofibers. Moreover, again it was confirmed that XG adsorbs in an extended conformation (trains) at a low XG to cellulose ratio, while tails and loops are formed at a high XG concentration and a saturated cellulose surface.
The research conducted by Zykwinska et al. (2005) also showed that the adsorption of xyloglucan on native plant cellulose is two times higher than on microcrystalline Avicel cellulose, which may prove that the crystallinity of cellulose can influence the interaction between cellulose and hemicelluloses. The influence of the morphological structure of cellulose on the efficiency of xyloglucan adsorption was investigated (Benselfelt et al. 2016; Gu and Catchmark 2013; Kiemle et al. 2014). For example, the interaction of two forms of cellulose: highly crystalline cellulose nanowhiskers (CNW) and amorphous PASCNW (phosphoric acid swollen cellulose nanowhiskers) with xyloglucan was analyzed (Gu and Catchmark 2013). Xyloglucan adsorption on CNW was about two times higher than on PASCNW. These results proved that the degree of cellulose crystallinity significantly influences adsorption. The binding constant of xyloglucan with highly crystalline and highly amorphous cellulose was also tested and was higher in the case of crystalline nanowhiskers than amorphous PASCNW. Generally, the surface area, porosity, and degree of order i.e. crystallinity, may affect the interaction of cellulose with hemicelluloses (Gu and Catchmark 2013). Also, an interesting conclusion was made, i.e. the binding interactions of hemicelluloses depend on the biological origin of cellulose. Adsorption is also influenced by the orientation of particles accumulated at the interphase surface. The ionic strength of the buffer in the xyloglucan-cellulose CNW adsorption system does not affect the efficiency of the process. This is probably related to the neutral charge of hemicelluloses (Gu and Catchmark 2013). Recently, xyloglucan has been successfully adsorbed on nanocellulose (Villares et al. 2015). The adsorption of xyloglucan (XG), galactoglucomannan (GGM), and arabinoxylans (AX) in aqueous solutions on the nanofibrillated cellulose (NFC) film covering the quartz sensor of QCM-D was compared. All these hemicelluloses were adsorbed, but the amount of GGM adsorbed and the scattering energy ΔD values were lower than in the case of XG; this indicates a less viscoelastic structure of GGM, which formed a more rigid layer. The arabinoxylans showed the lowest affinity for cellulose and exhibited the highest ΔD values, compared to other hemicelluloses, which indicates the presence of a loose adsorption layer with more water molecules than in the other systems (Eronen et al. 2011b).
The concentration of xyloglucan in the adsorption system influences the adsorption process. As shown by Dammak et al. (2015), the adsorption process at the hemicellulose concentration below 3.5 µg/l led to chain rearrangement on the cellulose surface, whereas saturation of the surface with formation of strings and loops was observed in systems with hemicellulose concentrations above 3.5 µg/L. In systems with lower concentrations, xyloglucan and cellulose can form multilayer sandwich-type structures.
Similar to xyloglucans, xylans can interact with cellulose, but their interactions are weaker and take place only on the surface of cellulose microfibrils (Gu and Catchmark 2013; Hayashi 1989). Xylans are smaller macromolecules and, in contrast to xyloglucan, cannot form trains and loops and the most preferable conformation in terms of the cellulose axis is parallel (Falcoz-Vigne et al. 2017) and antiparallel (Heinonen et al. 2022). Analyses of NMR spectra have shown that xyloglucan molecules adsorbed onto cellulose have a twofold conformation, which is similar to that of cellulose. During adsorption, xyloglucan molecules change their conformation from the threefold structure and match the twofold conformation of crystalline cellulose (Falcoz-Vigne et al. 2017; Heinonen et al. 2022; Jaafar et al. 2019). Furthermore, an increase in the xylan concentration above the amount that saturates the specific surface area of cellulose induces multilayer adsorption. It has been shown that only xylan molecules in the first adsorption layer have a twofold structure (Falcoz-Vigne et al. 2017). During adsorption, xylan forms hydrogen bonds with cellulose but, as in the case of xyloglucan, this is not the driving force behind this process, which takes place on the hydrophobic surface of cellulose. Several studies confirm that, due to their conformational fit, xylans can be a crystalline extension of cellulose. As xylans can contain different substituents, Jaafar et al. (2019) have investigated the effect of xylan acetylation on the adsorption on cellulose. Using QCM-D and molecular dynamics simulations, they showed that the adsorption layer of acetylated xylan was more rigid and more densely packed than that of deacetylated xylan, which is more hydrated and has a looser structure. The acetylation process influenced the xylan conformation, i.e. acetylated xylan had a twofold structure. In turn, deacetylated xylan had a twofold conformation only in the first adsorption layer in close contact with cellulose, while the other xylan molecules had a threefold conformation (Jaafar et al. 2019).
Similar to xyloglucan, arabinoxylans with fewer sidechains have been found to interact with cellulose microfibrils (Heredia et al. 1995; Lampugnani et al. 2018; Ochoa-Villarreal et al. 2012). In contrast to xyloglucans, arabinoxylans may also contain glucosyluronic acid residues giving acidic properties, which in turn enable arabinoxylans to interact with other polysaccharides (Albersheim et al. 2010). In the case of adsorption of arabinoxylan isolated from rye, oat, and wheat onto cellulose microfibrils, the higher molecular weight and the higher substitution degree were associated with its lower affinity for cellulose (Eronen et al. 2011b).
Mixed-linkage (1,3)(1,4)-β-D-Glucan (MLG), like xyloglucan, also adsorbs on cellulose. Studies show differences in the interactions of this linear polymer with microcrystalline cellulose (Avicel) and regenerated non-microfibrillar amorphous cellulose (RC). Based on the binding isotherms, it has been found that there is irreversible adsorption of MLG on Avicel, which initially (for approx. 10 h) increases rapidly and then runs slowly, which may be related to the more difficult diffusion of adsorbate particles in microcrystalline cellulose pores. The experiment was carried out at various temperatures: 22 °C, 40 °C, 60 °C, and 80 °C, and its results proved that the adsorption efficiency increased significantly with the temperature increase. The presence of additional insoluble hemicelluloses did not affect the interaction of MLG with microcrystalline cellulose (Kiemle et al. 2014). Studies conducted with the use of quartz crystal microbalance with dissipation (QCM-D) monitoring have shown that MLG adsorbs irreversibly on the RC surface forming a hydrogel layer with a thickness depending on the concentration of MLG in the system (Kiemle et al. 2014). Also, this technique was used in analyses of spruce galactoglucomannan (GGM) adsorption onto hardwood cellulose nanofibrils; in this system, hemicellulose adsorbed irreversibly quite well in a lower amount than in the case of xyloglucan (Eronen et al. 2011b).
Glucomannan has a disaccharide repeating unit with different ratios of mannose to glucose depending on the plant species (Melton et al. 2009). It is involved in interactions with both hydrophobic and hydrophilic surfaces of cellulose (Yu et al. 2018). Moreover, a lower degree of mannan O-acetylation was related to higher binding affinity for hemicellulose on cellulose (Melton et al. 2009). It was even concluded that, similar to xyloglucan and xylan, glucomannans can act as crosslinking agents between cellulose microfibrils. Previously, it has also been suggested that mixed-linked glucomannan is tightly bound to cellulose in low-arabinoxylan cell walls and can interact with arabinoxylan (Smith-Moritz et al. 2015).
Generally, the conclusions drawn from the aforementioned studies indicate that the adsorption of hemicelluloses depends on their molecular weight (Mw): the higher the Mw value, the lower the adsorption (Lima et al. 2004). The low-Mw hemicelluloses probably have a flat conformation on the cellulose surface, leading to the blocking of its active sites (Fig. 4a, b). Also, the concentration of hemicelluloses in the solution triggers different adsorption mechanisms—hemicelluloses in low-concentrated solutions have a flat arrangement on the cellulose surface (Fig. 4c), while the train and loop conformation of hemicelluloses on the cellulose surface is observed in high-concentrated solutions (Fig. 4d). The substitution with neutral sugars, such as xylose, fucose, or galactose, cannot be neglected either; however, a recent study has shown greater affinity of unbranched glycosylated and fucosylated xyloglucan for cellulose, and these xyloglucan motifs represent a minor part of apple XG (Chen et al. 2022). The Table summarizing the amounts of adsorbed hemicelluloses taking into account the adsorption conditions and cellulose sources is presented as Supplementary Material.
Adsorption of pectins on cellulose
The interaction of pectins with cellulose microfibrils in in vitro adsorption systems is not obvious. It probably takes place between sidechains consisting of neutral sugars, such as galactose, arabinose, and xylose (Gu and Catchmark 2013). For example, the influence of pectic arabinan and galactan sidechains on the ability of these compounds to adsorb on cellulose has been extensively studied due to their importance for xyloglucan-poor cell walls (Zykwinska et al. 2007). Also, the degree of pectin methylation does not directly affect these interactions (Patel 2009). The effect of the degree of esterification and side branching was investigated during the synthesis of cellulose in a calcium ion-free system (Lin et al. 2016). It has been shown that homogalacturonan adsorbs on cellulose but in a very small amount, significantly lower than that of pectins containing neutral sugar sidechains, mainly galactose and arabinose. On the other hand, Zykwinska et al. (2007) showed that only 8% of arabinan-rich pectins can bind to cellulose. The degree of esterification (DE) of pectin is not a key factor; however, pectins with a lower DE can bind cellulose more efficiently, but these differences are very small. This is probably related to the presence of negatively charged (–COO–) groups of galacturonic acid in low-methylated pectins. The presence of divalent Ca2+ and Mg2+ cations in the system is also important, as they can form bonds with the above-mentioned group between two homogalacturonan chains, resulting in the creation of a network with different properties (Gu and Catchmark 2013; Paul et al. 2012; Zykwinska et al. 2007).
Pectins adsorb reversibly on cellulose, and interactions between this adsorbate and the adsorbent are weak and limited only to the cellulose surface (Lin et al. 2016; Zykwinska et al. 2007). Arabinan and galactan sidechains are usually too short to be entrapped in cellulose microfibrils and to tether two adjacent cellulose microfibrils. On the other hand, experiments with strong alkali extraction showed reasonable amounts of arabinan-rich polysaccharides in the extracts, which may suggest that arabinan-rich pectins can bind to cellulose (Zykwinska et al. 2007). Further information can be provided by monosaccharide analysis of cellulose residues after strong alkali treatment. It is postulated that the presence of unextracted polysaccharides in the final cellulose residue is a result of strong interactions between these polysaccharides and cellulose. The analysis of the monosaccharide composition of the polysaccharides indicates mostly RG I rich in galactan sidechains. As concluded by Broxterman and Schols (2018), RG I consisting of galactose and arabinose sidechains is covalently linked to cellulose in the final residue of the extracted carrot cell wall. Similar studies of cellulose-retained polysaccharides involved wood and flax bast fibers (Gorshkova et al. 2015; Gurjanov et al. 2008). In the gelatinous layer of tension wood, the presence of RG I and β-(1-4)-galactan was detected, which evidenced their entrapment between cellulose microfibrils (Gorshkova et al. 2015). In the case of flax bast fibers, the cellulose-retained polysaccharide was identified as galactan (Gurjanov et al. 2008).
Research also shows the influence of the morphological structure of cellulose on the pectin adsorption efficiency, similar to that described above for xyloglucan. The Langmuir and Freundlich adsorption models were the best to describe the interaction between pectin and cellulose. However, the Freundlich model, revealing the importance of heterogeneity of the cellulose surface and multilayer formation, was chosen as better fitted (Zykwinska et al. 2008b). The adsorption of apple pectins took place on high-crystalline CNW cellulose, whereas the values obtained for high-amorphous PASCNW were below the detection limit. In turn, the degree of xyloglucan adsorption was much greater than that of pectins in the same conditions, probably because pectins interact with cellulose primarily through sidechains. Their main chain consisting of galacturonic acid showed no significant interactions (Gu and Catchmark 2013). The importance of arabinan-rich pectins in the cell wall of drought-resistant plants has been highlighted (Moore et al. 2008). Probably, during water deficit, arabinan-rich pectins prevent the formation of tight pectin junctions, e.g. egg-box or hydrogen bonding, between cellulose and xyloglucan, which enables the cell wall to maintain its flexibility. The Table summarizing the amounts of adsorbed pectins taking into account adsorption conditions and cellulose sources is presented as Supplementary Material (SM Table 1).
No significant interactions between cellulose and pectin have been demonstrated using in vitro binding assays. However, solid-state NMR spectroscopy has shown that there is strong contact between these compounds, which may be related to the crowding of these macromolecules in the cell wall rather than formation of bonds or van der Waals interactions. Therefore, it is most likely that pectins become trapped between the microfibrils of cellulose already during plant cell wall biosynthesis and probably have a role in the formation of the cell wall structure in the case of hemicellulose deficit (Wang et al. 2015; Phyo et al. 2017). As shown by Ng et al. (2014), RGI in the apple cell wall contains both free and cellulose microfibril-bound arabinan and galactan sidechains. It was also assumed that branched arabinan did not bind to cellulose, while the NMR result showed two states of the linear forms of arabinan: less mobile attached to cellulose and with higher mobility attached to RGI (Ng et al. 2014; Phyo et al. 2017). Also, it was shown that the rigid parts of pectic polysaccharides (HG and RGI) were more prone to interact with cellulose (Phyo et al. 2017). Further NMR studies showed that HG was less crosslinked in the proximity to cellulose, while shorter chains of HG interacted with cellulose less efficiently. These results are of great importance for understanding the mechanism of growing cells—the limited pectin-cellulose interaction facilitates cell wall loosening and expansion (Phyo et al. 2017). Finally, the general conclusion from the NMR study, which is in contradiction to in vitro studies, is that the pectin backbone rather than neutral arabinan and galactan sidechains is involved in the pectin-cellulose interaction (Phyo et al. 2017).
Adsorption of hemicellulose and pectins on cellulose
The most important factors in the formation of the plant cell wall are differences in interactions between cellulose and pectins in the presence of hemicelluloses in the adsorption system. The interaction of xyloglucan not only with cellulose but also with acidic pectins puts hemicellulose in the central place of control of cell wall extensibility and cell enlargement and mechanical properties of tissues (Chanliaud et al. 2002). On the other hand, coating cellulose by xyloglucan enforces a minimal distance between microfibrils, thereby preventing their aggregation (Thimm et al. 2002). Zykwinska et al. (2005) showed that there is always competition between non-cellulosic polysaccharides in binding to cellulose. Xyloglucan, for example, binds more strongly to cellulose than pectins or pectic domains because there is better complementarity between the surface of xyloglucan and cellulose and the structure of xyloglucan is favorable in the interaction with cellulose. Nevertheless, pectic polysaccharides can bind to cellulose when there is an insufficient amount of hemicelluloses (Bootten et al. 2004). For example, Zykwinska et al. (2008a) showed adsorption of arabinan-rich pectin at a low concentration of xyloglucan. Also, covalent bonding of xyloglucan with acidic pectins has been shown (Thompson and Fry 2000). Pectins can interact with each other too. Arabinans, galactans, and arabinogalactans have been shown to form covalent bonds with rhamnogalacturonan I (RG I) (Heredia et al. 1995). RG I and RG II can also form bonds, e.g. a glycosidic bond with HG; however, the exact position of this bond is not known (Willats et al. 2001). It has also been reported that an increase in the concentration of pectins in the adsorption system leads to their greater adsorption on cellulose even in the presence of xyloglucan (Zykwinska et al. 2008b). The competitive binding between xyloglucan and pectin was stressed (Zykwinska et al. 2007, 2008b).
Hemicelluloses probably form a layer on the surface of cellulose that has the potential to interact with pectins (Kiemle et al. 2014). The presence of soluble hemicelluloses and neutral pectins influences the interactions between (1,3)(1,4)-β-D-Glucan (MLG) and microcrystalline cellulose (Avicel), which may be either supportive or inhibitory. The order in which polymers are placed in the adsorption system is of great importance. Cellulose that is first bonded by neutral pectins, i.e. arabinan, and galactan, significantly reduces MLG adsorption. On the other hand, the initial binding of MLG with Avicel has a positive effect on pectin adsorption and increases the binding of arabinan, galactan, and cellulose. Xyloglucan adsorbs very well on cellulose and thus inhibits the interaction between MLG and Avicel to the greatest extent. The affinity of xyloglucan for cellulose is so high that the earlier binding of the adsorbent to MLG only slightly reduces its adsorption. Soluble arabinoxylan also inhibits the interaction between MLG and Avicel more effectively than arabinan and galactan, but to a lesser extent than xyloglucan.
Conclusions and future prospects
Experimental studies of the adsorption of non-cellulosic polysaccharides on microfibrillar cellulose can provide many valuable insights into the interactions between these macromolecular compounds. Adsorption studies test the nature of the interactions between hemicelluloses/pectins and microfibrillar cellulose. Adsorption kinetics gives information about the process of accumulation of the adsorbate on the adsorbent. The adsorption equilibrium shows the quantitative maximum adsorption, thereby providing information about a polymer with the highest affinity for cellulose. These results allow further consideration of the interactions, taking into account specific trends, e.g. a favorable polymer conformation, the presence of specific functional groups, and specific structural units. This information is vital for designing new biomaterials.
The adsorption between cellulose and xyloglucan, i.e. the main representative of hemicelluloses, has been most extensively studied so far. This hemicellulose in particular has been shown to have a strong affinity for cellulose. It adsorbs on its surface and can even act as a promoter for further binding, for example with pectins, in certain conditions. Furthermore, xyloglucan shows the ability not only to interact locally with the surface of cellulose fibrils but also to coat them. In adsorption studies, no significant interactions between pectins and cellulose have been demonstrated. If they do occur, their neutral sugars, e.g. arabinose, galactose, and xylose, are believed to be mainly responsible for this phenomenon. The morphological structure of cellulose microfibrils cannot be neglected in the picture of the interaction between cell wall macromolecules, as it has a significant influence on the adsorption efficiency. The more ordered and highly crystalline structure is associated with higher interaction capacity. Interactions between cellulose and hemicelluloses are thought to influence the aggregation of fibrils and even the formation of crystalline cellulose microfibrils (Zhang et al. 2021).
Model adsorption studies represent in vitro experimental investigations. Polymer solutions are diluted and it is not possible to select the exact concentration of polymers that is present in the plant cell wall, which certainly varies. Another drawback is that the exact quantitative ratio of hemicelluloses/pectins to cellulose that can be found in nature is unknown. Similarly, the entire cell wall system cannot be accurately mapped in studies of the interactions between its major components. Presumably, these interactions are influenced by the medium with a specific pH value, the temperature, the climate in the plant habitat, certain enzymatic reactions, the growth stage, and many others. The contribution of hydrogen bonds, hydrophobic interactions, or van der Waals forces is not the only source of stabilization of the contact between hemicelluloses/pectins and cellulose. For example, coarse-grained molecular dynamics (CGMD) and NMR studies have revealed that pectins are in close contact with cellulose, which is determined not only by these interactions but also by the packing/crowding of these polymers between the cellulose microfibrils. Additionally, studies of cellulose residues after strong alkali extraction give evidence that some hemicelluloses and pectins are probably trapped within cellulose microfibrils.
The adsorption studies of the interaction between cellulose and hemicelluloses/pectins are of great interest from a biological point of view, as they help to improve the models of the plant cell wall. Importantly, they also have applications in industry, especially in the field of biosorption, which is in line with green chemistry. Furthermore, there are still questions waiting to be answered, e.g. about the impact of the degrees of methylation and acetylation of non-cellulosic polysaccharides on the adsorption of these components on cellulose.
Abbreviations
- AFM:
-
Atomic force microscopy
- AX:
-
Arabinoxylan
- CGMD:
-
Coarse-grained molecular dynamics
- CNW:
-
Cellulose nanowhiskers
- DE:
-
Degree of esterification
- DSC:
-
Differential scanning calorimetry
- GGM:
-
Galactoglucomannan
- HG:
-
Homogalacturonan
- MLG:
-
Mixed-linkage glucan
- NFC:
-
Nanofibrillar cellulose
- PASCNW:
-
Phosphoric acid swollen cellulose nanowhiskers
- QCM-D:
-
Quartz crystal microbalance with dissipation monitoring
- RC:
-
Regenerated cellulose
- RGI:
-
Rhamnogalacturonan I
- RGII:
-
Rhamnogalacturonan II
- SEM:
-
Scanning electron microscopy
- SPR:
-
Surface plasmon resonance
- SS-NMR:
-
Solid-state nuclear magnetic resonance
- XG:
-
Xyloglucan
References
Albersheim P, Darvill A, Roberts K, Sederoff R, Staehelin A (2010) Plant cell walls: from chemistry to biology, 1st edn. Garland Science, New York
Barbacci A, Lahaye M, Magnenet V (2013) Another brick in the cell wall: biosynthesis dependent growth model. PLoS ONE. https://doi.org/10.1371/journal.pone.0074400
Benselfelt T, Cranston ED, Ondaral S, Johansson E, Brumer H, Rutland MW, Wågberg L (2016) Adsorption of xyloglucan onto cellulose surfaces of different morphologies: an entropy-driven process. Biomacromol. https://doi.org/10.1021/acs.biomac.6b00561
Bootten TJ, Harris PJ, Melton LD, Newman RH (2004) Solid-state 13C-NMR spectroscopy shows that the xyloglucans in the primary cell walls of mung bean (Vigna radiata L.) occur in different domains: a new model for xyloglucan-cellulose interactions in the cell wall. J Exp Bot. https://doi.org/10.1093/jxb/erh065
Broxterman SE, Schols HA (2018) Characterisation of pectin-xylan complexes in tomato primary plant cell walls. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2018.06.003
Chambat G, Karmous M, Costes M, Picard M, Joseleau JP (2005) Variation of xyloglucan substitution pattern affects the sorption on celluloses with different degrees of crystallinity. Cellulose. https://doi.org/10.1007/s10570-004-1040-z
Chanliaud E, Burrows KM, Jeronimidis G, Gidley MJ (2002) Mechanical properties of primary plant cell wall analogues. Planta. https://doi.org/10.1007/s00425-002-0783-8
Chen M, Cathala B, Lahaye M (2022) Adsorption of apple xyloglucan on cellulose nanofiber depends on molecular weight, concentration and building blocks. Carbohydr Polym 296:119994
Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol. https://doi.org/10.1038/nrm1746
Cosgrove DJ (2014) Re-constructing our models of cellulose and primary cell wall assembly. Curr Opin Plant Biol. https://doi.org/10.1016/j.pbi.2014.11.001
Costa G, Plazanet I (2016) Plant cell wall, a challenge for its characterisation. Adv Biol Chem. https://doi.org/10.4236/abc.2016.63008
Cybulska J, Konstankiewicz K, Zdunek A, Skrzypiec K (2010a) Nanostructure of natural and model cell wall materials. Int Agrophys 24:107–114
Cybulska J, Vanstreels E, Ho QT, Courtin CM, Craeyveld V, Nicolaï B, Zdunek A, Konstankiewicz K (2010b) Mechanical characteristics of artificial cell walls. J Food Eng. https://doi.org/10.1016/j.jfoodeng.2009.08.001
Dammak A, Quémener B, Bonnin E, Alvarado C, Bouchet B, Villares A, Moreau C, Cathala B (2015) Exploring architecture of xyloglucan cellulose nanocrystal complexes through enzyme susceptibility at different adsorption regimes. Biomacromol. https://doi.org/10.1021/bm5016317
Dervilly-Pinel G, Tran V, Saulnier L (2004) Investigation of the distribution of arabinose residues on the xylan backbone of water-soluble arabinoxylans from wheat flour. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2003.09.004
Dick-Pérez M, Zhang Y, Hayes J et al (2011) Structure and interactions of plant cell-wall polysaccharides by two- and three-dimensional magic-angle-spinning solid-state NMR. Biochemistry. https://doi.org/10.1021/bi101795q
Ding SY, Himmel ME (2006) The maize primary cell wall microfibril: a new model derived from direct visualization. J Agric Food Chem. https://doi.org/10.1021/jf051851z
Eronen P, Junka K, Laine J, Österberg M (2011a) Interaction between water-soluble polysaccharides and native nanofibrillar cellulose thin films. BioResources 6:4200
Eronen P, Österberg M, Heikkinen S, Tenkanen M, Laine J (2011b) Interactions of structurally different hemicelluloses with nanofibrillar cellulose. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2011.06.031
Falcoz-Vigne L, Ogawa Y, Molina-Boisseau S et al (2017) Quantification of a tightly adsorbed monolayer of xylan on cellulose surface. Cellulose. https://doi.org/10.1007/s10570-017-1401-z
Festucci-Buselli RA, Otoni WC, Joshi CP (2007) Structure, organization, and functions of cellulose synthase complexes in higher plants. Braz J Plant Physiol 19:1–13
Gorshkova T, Mokshina N, Chernova T, Ibragimova N, Salnikov V, Mikshina P, Tryfona T, Banasiak A, Immerzeel P, Dupree P, Mellerowicz EJ (2015) Aspen tension wood fibers contain β-(1→4)-galactans and acidic arabinogalactans retained by cellulose microfibrils in gelatinous walls. Plant Physiol 169(3):2048–2063. https://doi.org/10.1104/pp.15.00690
Gorshkova T, Petrova A, Mikshina P (2022) Review: tertiary cell wall of plant fibers as a source of inspiration in material design. Carbohydr Polym 295:119849. https://doi.org/10.1016/j.carbpol.2022.119849
Grzadka E, Chibowski S (2009) Influence of a kind of electrolyte and its ionic strength on the adsorption and zeta potential of the system: polyacrylic acid/MnO2/electrolyte solution. Physicochem Probl Miner Process 43:31–42
Gu J, Catchmark JM (2013) The impact of cellulose structure on binding interactions with hemicellulose and pectin. Cellulose 20:1613–1627. https://doi.org/10.1007/s10570-013-9965-8
Gu J, Catchmark JM (2014) Roles of xyloglucan and pectin on the mechanical properties of bacterial cellulose composite films. Cellulose. https://doi.org/10.1007/s10570-013-0115-0
Gümüskaya E, Usta M, Kirci H (2003) The effects of various pulping conditions on crystalline structure of cellulose in cotton linters. Polym Degrad Stab. https://doi.org/10.1016/S0141-3910(03)00157-5
Guo J, Zhang X, Tian J et al (2021) Evaluating the refractive index, thickness and porosity of ultrathin cellulose nanocrystal films with different polymorphs by SPR technique. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2021.10.158
Gurjanov OP, Ibragimova NN, Gnezdilov OI, Gorshkova TA (2008) Polysaccharides, tightly bound to cellulose in cell wall of flax bast fibre: isolation and identification. Carbohydr Polym 72(4):719–729. https://doi.org/10.1016/j.carbpol.2007.10.017
Hanus J, Mazeau K (2006) The xyloglucan-cellulose assembly at the atomic scale. Biopolymers. https://doi.org/10.1002/bip.20460
Hayashi T (1989) Xyloglucans in the primary cell wall. Annu Rev Plant Physiol Plant Mol Biol. https://doi.org/10.1146/annurev.pp.40.060189.001035
Hayashi T, Kaida R (2011) Functions of xyloglucan in plant cells. Mol Plant. https://doi.org/10.1093/mp/ssq063
Hayashi T, Ogawa K, Mitsuishi Y (1994a) Characterization of the adsorption of xyloglucan to cellulose. Plant Cell Physiol. https://doi.org/10.1093/oxfordjournals.pcp.a078714
Hayashi T, Takeda T, Ogawa K, Mitsuishi Y (1994b) Effects of the degree of polymerization on the binding of xyloglucans to cellulose. Plant Cell Physiol. https://doi.org/10.1093/oxfordjournals.pcp.a078674
Heinonen E, Henriksson G, Lindström ME et al (2022) Xylan adsorption on cellulose: preferred alignment and local surface immobilizing effect. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2022.119221
Held MA, Jiang N, Basu D, Showalter AM, Faik A (2015) Plant cell wall polysaccharides: structure and biosynthesis. Polysaccharides. https://doi.org/10.1007/978-3-319-16298-0_73
Heredia A, Jiménez A, Guillén R (1995) Composition of plant cell walls. Z Lebensm Unters Forsch. https://doi.org/10.1007/BF01192903
Jaafar Z, Mazeau K, Boissière A, le Gall S, Villares A, Vigouroux J, Beury N, Moreau C, Lahaye M, Cathala B (2019) Meaning of xylan acetylation on xylan-cellulose interactions: a quartz crystal microbalance with dissipation (QCM-D) and molecular dynamic study. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2019.115315
Jarvis MC (2018) Structure of native cellulose microfibrils, the starting point for nanocellulose manufacture. Philos Trans R Soc A Math Phys Eng Sci 376:20170045
Jarvis MC (2023) Hydrogen bonding and other non-covalent interactions at the surfaces of cellulose microfibrils. Cellulose. https://doi.org/10.1007/s10570-022-04954-3
Kabel MA, van den Borne H, Vincken JP, Voragen AGJ, Schols HA (2007) Structural differences of xylans affect their interaction with cellulose. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2006.09.006
Ka̧czkowski MA (2003) Structure, function and metabolism of plant cell wall. Acta Physiol Plant. https://doi.org/10.1007/s11738-003-0010-7
Kaczmarska A, Pieczywek PM, Cybulska J, Zdunek A (2022) Structure and functionality of Rhamnogalacturonan I in the cell wall and in solution: a review. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2021.118909
Kiemle SN, Zhang X, Esker AR et al (2014) Role of (1,3)(1,4)-β-glucan in cell walls: interaction with cellulose. Biomacromol. https://doi.org/10.1021/bm5001247
Kishani S, Benselfelt T, Wågberg L, Wohlert J (2021) Entropy drives the adsorption of xyloglucan to cellulose surfaces—a molecular dynamics study. J Colloid Interface Sci. https://doi.org/10.1016/j.jcis.2020.12.113
Kozlova LV, Nazipova AR, Gorshkov OV et al (2020) Elongating maize root: zone-specific combinations of polysaccharides from type I and type II primary cell walls. Sci Rep 10:10956. https://doi.org/10.1038/s41598-020-67782-0
Lampugnani ER, Khan GA, Somssich M, Persson S (2018) Building a plant cell wall at a glance. J Cell Sci. https://doi.org/10.1242/jcs.207373
Li Q, Wang S, Jin X et al (2020) The application of polysaccharides and their derivatives in pigment, barrier, and functional paper coatings. Polymers. https://doi.org/10.3390/POLYM12081837
Lima DU, Buckeridge MS (2001) Interaction between cellulose and storage xyloglucans: the influence of the degree of galactosylation. Carbohydr Polym. https://doi.org/10.1016/S0144-8617(00)00297-6
Lima DU, Loh W, Buckeridge MS (2004) Xyloglucan-cellulose interaction depends on the sidechains and molecular weight of xyloglucan. Plant Physiol Biochem. https://doi.org/10.1016/j.plaphy.2004.03.003
Lin D, Lopez-Sanchez P, Gidley MJ (2016) Interactions of pectins with cellulose during its synthesis in the absence of calcium. Food Hydrocoll. https://doi.org/10.1016/j.foodhyd.2015.06.004
Lin D, Lopez-Sanchez P, Selway N, Gidley MJ (2018) Viscoelastic properties of pectin/cellulose composites studied by QCM-D and oscillatory shear rheology. Food Hydrocoll. https://doi.org/10.1016/j.foodhyd.2017.12.019
Lopez M, Bizot H, Chambat G et al (2010) Enthalpic studies of xyloglucan-cellulose interactions. Biomacromol. https://doi.org/10.1021/bm1002762
Melton LD, Smith BG, Ibrahim R, Schröder R (2009) Mannans in primary and secondary plant cell walls. N Z J Sci 39:153–160
Merino D, Casalongué C, Alvarez VA (2019) Polysaccharides as eco-nanomaterials for agricultural applications. Food Hydrocoll. https://doi.org/10.1016/j.foodhyd.2015.06.004
Moon RJ, Martini A, Nairn J et al (2011) Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev. https://doi.org/10.1039/c0cs00108b
Moore JP, Farrant JM, Driouich A (2008) A role for pectin-associated Arabians in maintaining the flexibility of the plant cell wall during water deficit stress. Plant Signal Behav. https://doi.org/10.4161/psb.3.2.4959
Myśliwiec D, Chylińska M, Szymańska-Chargot M, Chibowski S, Zdunek A (2016) Revision of adsorption models of xyloglucan on microcrystalline cellulose. Cellulose. https://doi.org/10.1007/s10570-016-0995-x
Ng JKT, Zujovic ZD, Smith BG et al (2014) Solid-state 13C NMR study of the mobility of polysaccharides in the cell walls of two apple cultivars of different firmness. Carbohydr Res. https://doi.org/10.1016/j.carres.2013.12.019
Niimura H, Yokoyama T, Kimura S et al (2010) AFM observation of ultrathin microfibrils in fruit tissues. Cellulose. https://doi.org/10.1007/s10570-009-9361-6
Nishiyama Y (2009) Structure and properties of the cellulose microfibril. J Wood Sci 55:241
Northcote DH (1972) Chemistry of the plant cell wall. Annu Rev Plant Physiol. https://doi.org/10.1146/annurev.pp.23.060172.000553
Ochoa-Villarreal M, Aispuro-Hernndez E, Vargas-Arispuro I, Ngel M (2012) Plant cell wall polymers: function, structure and biological activity of their derivatives. Polymerization. https://doi.org/10.5772/46094
Oehme DP, Doblin MS, Wagner J, Bacic A, Downton MT, Gidley MJ (2015) Gaining insight into cell wall cellulose macrofibril organisation by simulating microfibril adsorption. Cellulose. https://doi.org/10.1007/s10570-015-0778-9
Paananen A, Österberg M, Rutland M, Tammelin T, Saarinen T, Tappura K, Stenius P (2004) Interaction between cellulose and xylan: an atomic force microscope and quartz crystal microbalance study. In: ACS Symposium Series. https://doi.org/10.1021/bk-2004-0864.ch018
Park YB, Cosgrove DJ (2015) Xyloglucan and its interactions with other components of the growing cell wall. Plant Cell Physiol. https://doi.org/10.1093/pcp/pcu204
Patel PH (2009) Charaterization of a crosslink between xyloglucan and rhamnogalacturonan from cotton cell walls. Oklahoma State University
Paul UC, Manian AP, Široká B, Duelli H, Bechtold T (2012) Sorption of anionic polysaccharides by cellulose. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2011.08.049
Pauly M, Albersheim P, Darvill A, York WS (1999) Molecular domains of the cellulose/xyloglucan network in the cell walls of higher plants. Plant J. https://doi.org/10.1046/j.1365-313X.1999.00630.x
Phyo P, Wang T, Xiao C et al (2017) Effects of pectin molecular weight changes on the structure, dynamics, and polysaccharide interactions of primary cell walls of Arabidopsis thaliana: insights from solid-state NMR. Biomacromol. https://doi.org/10.1021/acs.biomac.7b00888
Rui Y, Dinneny JR (2020) A wall with integrity: surveillance and maintenance of the plant cell wall under stress. New Phytol. https://doi.org/10.1111/nph.16166
Salmén L (2022) On the organization of hemicelluloses in the wood cell wall. Cellulose 29:1349–1355
Saxena IM, Brown RM (2005) Cellulose biosynthesis: current views and evolving concepts. Ann Bot 96:9–21
Simmons TJ, Mortimer JC, Bernardinelli OD et al (2016) Folding of xylan onto cellulose fibrils in plant cell walls revealed by solid-state NMR. Nat Commun. https://doi.org/10.1038/ncomms13902
Smith-Moritz AM, Hao Z, Fernández-Niño SG, Fangel JU, Verhertbruggen Y, Holman HY, Willats WGT, Ronald PC, Hv S, Heazlewood JL, Vega-Sánchez ME (2015) Structural characterization of a mixed-linkage glucan deficient mutant reveals alteration in cellulose microfibril orientation in rice coleoptile mesophyll cell walls. Front Plant Sci. https://doi.org/10.3389/fpls.2015.00628
Souza AP, Leite DCC, Pattathil S, Hahn MG, Buckeridge MS (2013) Composition and structure of sugarcane cell wall polysaccharides: implications for second-generation bioethanol production. Bioenergy Res. https://doi.org/10.1007/s12155-012-9268-1
Tenhaken R (2015) Cell wall remodeling under abiotic stress. Front Plant Sci. https://doi.org/10.3389/fpls.2014.00771
Terashima M, Fukushima M, Tanaka S (2004) Influence of pH on the surface activity of humic acid: micelle-like aggregate formation and interfacial adsorption. Colloids Surf A Physicochem Eng Asp. https://doi.org/10.1016/j.colsurfa.2004.08.028
Terrett OM, Dupree P (2019) Covalent interactions between lignin and hemicelluloses in plant secondary cell walls. Curr Opin Biotechnol 56:97–104
Thimm JC, Burritt DJ, Sims IM, Newman RH, Ducker WA, Melton LD (2002) Celery (Apium graveolens) parenchyma cell walls: cell walls with minimal xyloglucan. Physiol Plant. https://doi.org/10.1034/j.1399-3054.2002.1160205.x
Thomas LH, Trevor Forsyth V, Šturcová A et al (2013) Structure of cellulose microfibrils in primary cell walls from collenchyma. Plant Physiol. https://doi.org/10.1104/pp.112.206359
Thompson JE, Stephen CF (2000) Evidence for covalent linkage between xyloglucan and acidic pectins in suspension-cultured rose cells. Planta. https://doi.org/10.1007/s004250000287
Villares A, Moreau C, Dammak A, Capron I, Cathala B (2015) Kinetic aspects of the adsorption of xyloglucan onto cellulose nanocrystals. Soft Matter. https://doi.org/10.1039/c5sm01413a
Wang T, Park YB, Cosgrove DJ, Hong M (2015) Cellulose-pectin spatial contacts are Inherent to never-dried Arabidopsis primary cell walls: evidence from solid-state nuclear magnetic resonance. Plant Physiol. https://doi.org/10.1104/pp.15.00665
Whitney SEC, Gothard MGE, Mitchell JT, Gidley MJ (1999) Roles of cellulose and xyloglucan in determining the mechanical properties of primary plant cell walls. Plant Physiol. https://doi.org/10.1104/pp.121.2.657
Willats WGT, Mccartney L, Mackie W, Knox JP (2001) Pectin: cell biology and prospects for functional analysis. Plant Mol Biol. https://doi.org/10.1023/A:1010662911148
Wiśniewska M, Grzadka E, Mendrek B (2013) Influence of the solid type on the adsorption mechanism of nonionic polymers in the metal oxide/water solution system-temperature effect. Powder Technol. https://doi.org/10.1016/j.powtec.2013.06.024
Wohlert M, Benselfelt T, Wågberg L, Furó I, Berglund LA, Wohlert J (2022) Cellulose and the role of hydrogen bonds: not in charge of everything. Cellulose. https://doi.org/10.1007/s10570-021-04325-4
Yao M, Liang C, Yao S et al (2021) Kinetics and thermodynamics of hemicellulose adsorption onto nanofibril cellulose surfaces by QCM-D. ACS Omega. https://doi.org/10.1021/acsomega.1c04391
Yu L, Lyczakowski JJ, Pereira CS, Kotake T, Yu X, Li A, Mogelsvang S, Skaf MS, Dupree P (2018) The patterned structure of galactoglucomannan suggests it may bind to cellulose in seed mucilage. Plant Physiol. https://doi.org/10.1104/pp.18.00709
Zhang Y, Yu J, Wang X, Durachko DM, Zhang S, Cosgrove DJ (2021) Molecular insights into the complex mechanics of plant epidermal cell walls. Science 72(6543):706–711. https://doi.org/10.1126/science.abf2824
Zhao G (2011) Sorption of heavy metal ions from aqueous solutions: a review. The Open Colloid Sci J. https://doi.org/10.2174/1876530001104010019
Zhao Z, Crespi VH, Kubicki JD et al (2014) Molecular dynamics simulation study of xyloglucan adsorption on cellulose surfaces: effects of surface hydrophobicity and side-chain variation. Cellulose. https://doi.org/10.1007/s10570-013-0041-1
Zykwinska A, Ralet MCJ, Garnier CD, Thibault JFJ (2005) Evidence for in vitro binding of pectin side chains to cellulose. Plant Physiol. https://doi.org/10.1104/pp.105.065912
Zykwinska A, Thibault JF, Ralet MC (2007) Organization of pectic arabinan and galactan side chains in association with cellulose microfibrils in primary cell walls and related models envisaged. J Exp Bot. https://doi.org/10.1093/jxb/erm037
Zykwinska A, Thibault JF, Ralet MC (2008a) Modelling of xyloglucan, pectins and pectic side chains binding onto cellulose microfibrils. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2008.01.011
Zykwinska A, Thibault JF, Ralet MC (2008b) Competitive binding of pectin and xyloglucan with primary cell wall cellulose. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2008.05.004
Acknowledgments
This research was funded in whole or in part by National Science Center-Poland projects no. NCN OPUS UMO-2018/29/B/NZ9/00141 and nr NCN PRELUDIUM BIS 2 2020/39/O/NZ9/00241. For the purpose of Open Access, the author has applied a CC-BY public copyright license to any Author Accepted Manuscript (AAM) version arising from this submission.
Funding
This research was funded in whole or in part by National Science Center-Poland projects no. NCN OPUS UMO-2018/29/B/NZ9/00141 and no. NCN PRELUDIUM BIS 2 2020/39/O/NZ9/00241. For the purpose of Open Access, the author has applied a CC-BY public copyright license to any Author Accepted Manuscript (AAM) version arising from this submission.
Author information
Authors and Affiliations
Contributions
PP wrote the original manuscript and corrected the manuscript. MSC obtained financial support, conceived and wrote the original manuscript, revised and corrected the manuscript. AZ reviewed and corrected the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pękala, P., Szymańska-Chargot, M. & Zdunek, A. Interactions between non-cellulosic plant cell wall polysaccharides and cellulose emerging from adsorption studies. Cellulose 30, 9221–9239 (2023). https://doi.org/10.1007/s10570-023-05442-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-023-05442-y