Abstract
Borohydride reduction of dialdehyde cellulose (DAC) is a promising strategy to generate dialcohol cellulose as bio-based alternative to petroleum-based materials. However, the degradation of the polymer backbone according to β-elimination mechanisms limits the practical applications of the reaction. Therefore, we aimed at optimizing the process to suppress degradation reactions by varying reaction time, pH, and reagent stoichiometry. The degree of oxidation (DO) of the DAC intermediates significantly impacts the yields and molecular weights of the isolated dialcohol celluloses, with a “leveling-off” effect at higher DO values. Increasing the amount of sodium borohydride can minimize—but not entirely prevent—chain scissions. Lowering the pH value during reduction slows down the degradation but results in incomplete conversion of the aldehyde functionalities. Our study provides valuable insights into the consequences of side reactions during borohydride reduction of DAC as well as into chemistry and analysis of the dialdehyde cellulose/dialcohol cellulose system.
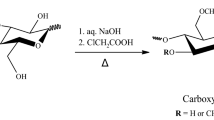
Graphical abstract
About a dilemma in cellulose chemistry: Dialcohol cellulose derived by periodate oxidation and subsequent borohydride reduction of cellulose has received increasing attention in the development of sustainable thermoplastic materials. The present study highlights the challenge of suppressing β-elimination and favoring the reduction pathway to optimize reaction conditions and minimize chain degradation.

Similar content being viewed by others
Introduction
The urgent need for more sustainable products has driven the development of cellulose-based materials, with the intent to replace synthetic polymers made from petroleum. Cellulose is a natural bulk product, has good versatile mechanical properties widely used in paper, fiber and composite products (Ardanuy et al. 2015; Mokhena et al. 2021), and is abundantly available with already high production capacities. However, its non-thermoplastic behavior prevents straightforward reshaping into complex three-dimensional structures (Szcześniak et al. 2008; Li et al. 2022). Partial periodate oxidation to dialdehyde cellulose (DAC) followed by derivatization of the generated aldehyde moieties is known to overcome this issue by introducing the necessary flexibility to the polymer backbone. This strategy generates cellulose-based thermoplastics, e.g., by borohydride reduction (Kasai et al. 2014; Larsson et al. 2014a, b; Lopez Durán et al. 2016; Lei and Feng 2020), Passerini reaction (Esen and Meier 2020), and reductive amination (Simon et al. 2023b). Considering the relatively low price of the reagents and upscalability, borohydride reduction of partially oxidized DAC to generate “dialcohol cellulose” seems to be one of the most promising pathways for large-scale application at first glance (Scheme 1).
Numerous studies have shown that dialcohol cellulose is a thermoplastic with comparable mechanical properties to common plastics. Morooka et al. (1989) prepared transparent dialcohol cellulose films from highly oxidized methylol cellulose (degree of oxidation of 80 % and higher). After that, the development of dialcohol cellulose-based materials was significantly shaped by Wågberg and Larsson who were involved in several related studies. Dialcohol cellulose was investigated in applications for sheet preparation (Larsson et al. 2014b; López Durán et al. 2016; Linvill et al. 2017), in the production of nanocomposite films from cellulose nanofibrils (Larsson et al. 2014a), thermoplastic nanoparticles (Mehandzhiyski et al. 2022), melt-processable composite materials for injection-molded caps (Lo Re et al. 2023), and thermoplastic films from sisal fibers (Lei and Feng 2020). Reduction of DAC by sodium borohydride is a tempting derivatization strategy since the reductant is easy to handle, known to reduce carbonyl groups rapidly under comparably mild conditions, bears no toxicity issues or environmental dangers and is used already on an industrial scale as bleaching agent (borol reduction; Brown 1973; Brown and Krishnamurthy 1979). However, sodium borohydride requires strongly alkaline reaction media to avoid the destruction of the reagent under the formation of hydrogen (comproportionation of hydride with the protons of the aqueous medium). Even at moderately alkaline conditions, it completely degrades within a few hours (t1/2 ~1 h at pH 10; Grzeschik et al. 2020). Both the unwanted side reaction with protons and the targeted reduction eventually generate alkaline by-products and thus further increase the pH during the reaction (Clayden et al. 2012; Grzeschik et al. 2020). At the same time, DAC is known to depolymerize under alkaline conditions, following a β-elimination process that accelerates with increasing alkalinity (Potthast et al. 2009; Koprivica et al. 2016; Hosoya et al. 2018; Ruan et al. 2018; Liu et al. 2020). Consequently, this results in the dilemma that increased alkalinity during the preparation of dialcohol cellulose will favour the intended process, the DAC reduction, by increasing the reductant´s stability, but at the same time will also promote the main competing side reaction which results in considerable molar mass loss (Scheme 2).
Potthast et al. (2009) investigated this issue for slightly oxidized DAC (DO less than 10 %) already in their study in 2009, while most studies afterwards focused on material development aspects and entirely disregarded the possibility of degradation side reactions. Obtained yields and molecular weight distributions were rarely reported. Nonetheless, the question remains whether the chain degradation of DAC can be minimized during borohydride reduction for a wider variety of DO values, especially as higher degrees of oxidation are required to obtain dialcohol cellulose-based materials with advanced mechanical and thermal properties. However, the impact of DO on the depolymerization process during borohydride reduction has remained nearly unstudied. In general, it is advantageous to apply conditions where the reduction proceeds faster than the chain degradation to largely maintain cellulose integrity and to ensure stability of the polymer backbone after reduction (Simon et al. 2023b). Already in 1955, Head (1955) concluded that the rate of chain degradation during borohydride reduction of DAC strongly depends on the pH and proposed an optimized pH of approximately 10 for the preparation of dialcohol cellulose. After that, these conditions were adopted without further optimization attempts, employing either unbuffered media or monobasic sodium phosphate buffer to control the pH increase. The excess of sodium borohydride and the reaction duration was selected arbitrarily without considering stoichiometry or kinetics of the reaction. For both economic and ecological reasons, reaction optimization must be considered in the development of cellulose-based materials and unwanted side reactions must be mitigated to offer truly viable alternatives to fossil fuel-based materials. Unless cellulose degradation during dialcohol cellulose preparation is minimized and the excessive use of sodium borohydride is avoided, the resulting materials will not be genuinely sustainable. In this sense, we were interested in re-evaluating the long-known reaction conditions (Head 1955) by systematically screening the applied parameters using more sophisticated analytical techniques. We examined the pH dependency, influences of stoichiometry, and time-dependency of the borohydride reduction of DAC on the progress of the reaction and cellulose depolymerization, monitored by gel permeation chromatography (GPC) in combination with fluorescence labeling, in order to identify optimized conditions with minimized chain degradation and borohydride usage. The influence of the DO on chain degradation and structure modulation during the oxidation-reduction pathway was investigated by solution-state NMR spectroscopy.
Experimental section
Chemicals and reagents
Softwood kraft pulp (a mix of spruce and pine) was provided by UPM-Kymmene Oyj (Lappeenranta, Finland). The hemicellulose content was calculated to be 8.5 % based on the C4 resonance from the 13C solid-state NMR spectrum using the method published by Jusner et al. (2022). The deconvolution data was in good agreement with the results obtained from monosaccharide quantification, according to reported protocols (Sundberg et al. 1996; Becker et al. 2013). The GPC-MALLS statistical moments of the softwood kraft pulp were previously determined: Mn = 55 kDa, Mw = 614 kDa, Mz = 1651 kDa, and Đ = 11.1. The cellulosic substrates used in this study (softwood kraft pulp and cotton linters) were disintegrated before use in deionized water using a commercial kitchen blender. All reagents and chemicals were obtained from commercial suppliers and used without further purification.
Characterization
NMR spectra were recorded on a Bruker NMR AV III 400 spectrometer at an acquisition temperature of 65 °C. Measurements were performed on cotton linters and a series of dialcohol celluloses derived thereof. The materials were characterized by quantitative 1H, diffusion-edited 1H, and multiplicity-edited 1H-13C HSQC experiments. All NMR samples were dissolved according to published procedures in a [P4444][OAc]:DMSO-d6 (1:4 wt%) electrolyte (Cellolyte obtained from Innotope; King et al. 2018; Koso et al. 2020; Fliri et al. 2023). Depending on the molecular weights of the materials concentrations of 2.5 or 5 wt% were chosen. Chemical shifts are reported in parts per million (ppm) relative to the residual proton resonance of DMSO-d6. The obtained spectra are summarized in the Supplementary Information.
GPC was carried out using a size exclusion/multiangle light scattering (SEC-MALLS) system consisting of a MALLS detector (Wyatt Dawn DSP, Wyatt Inc.) coupled with a refractive index detector (Shodex RI-71, Showa Denko K.K.), four Waters SEC columns (Styrage HMW 6E, 7.8 × 300 mm, 15−20 µm), one Agilent guard column (PL gel mixed ALS, 7.8 × 50 mm, 20 µm), and a Bio-Inert 1260 Infinity II pump (Agilent) equipped with an autosampler (HP Series 1100, Agilent). The mobile phase was N,N-dimethylacetamide/lithium chloride (0.9 % w/v; filtered through a 0.02 µm filter), and 100 µL of each sample was injected with a 45-minute run time at a flow rate of 1 mL/min. All GPC samples were first dissolved in N,N-dimethylacetamide/lithium chloride (9 % w/v) following the standard procedure (Potthast et al. 2015) and then filtered through a 0.45 µm syringe filter. The molecular weight distribution (MWD) and the GPC−MALLS statistical moments were calculated based on a refractive index increment of 0.140 mL/g for cellulose in N,N-dimethylacetamide/lithium chloride (0.9 % w/v) at 488 nm. The raw data were processed with Astra 4.7 and GRAMS/AI 7.0 software.
Fourier-transform infrared spectroscopy (FTIR) was performed on a Frontier FTIR spectrophotometer (PerkinElmer, Waltham, MA, USA) in attenuated total reflection (ATR) mode. All measurements included 32 scans per sample at a resolution of 4 cm−1 and a spectral range of 4000 to 650 cm−1.
Potentiometric titrations were carried out using an 877 Titrino plus instrument (Metrohm AG, Herisau, Switzerland) equipped with a 30 mL beaker, a 20 mL dosing unit, and a magnetic stirrer.
Determination of the degree of oxidation (aldehyde content)
The DO of DAC from softwood kraft pulp was determined from their FTIR spectra combined with a partial least squares regression model according to our previous work (Simon et al. 2021, 2022). For the DAC samples derived from cotton linters and isolated dialcohol celluloses, the DO and remaining aldehyde content, respectively, were determined by the oximation with hydroxylamine hydrochloride followed by potentiometric titration of the formed hydrochloric acid according to our adapted protocol (Simon et al. 2023b).
CCOA method: fluorescence labelling of carbonyl groups
To determine the carbonyl groups after reduction, dialcohol celluloses were labeled with carbazole-9-carbonyl-oxy-amine (CCOA) and analyzed by GPC/MALLS-RI-FL according to the standard protocols (Röhrling et al. 2002a, b; Potthast et al. 2003).
Standard procedure for the periodate oxidation
Periodate oxidations were conducted according to our previous work (Simon et al. 2023a). Briefly, softwood kraft pulp or cotton linters were added to a sodium periodate solution. The reaction mixture was stirred in the dark to limit photochemical side reactions. The DO was controlled by varying the temperature, periodate concentration, and reaction time. The resulting dialdehyde celluloses were filtered and thoroughly washed with deionized water. Gel-like DAC samples were additionally treated with a sodium thiosulfate solution to deactivate oxidizing iodine species. All samples were stored in a never-dried state at −20 °C until further characterized or reduced to dialcohol cellulose.
Standard procedure for the reduction with borohydride
The protocol for borohydride reduction in this study resulted from optimization experiments based on various reported procedures. A 100 mL round-bottom flask was charged with sodium borohydride (5 equiv. based on the carbonyl groups in DAC) and 50 mL of an aqueous monobasic sodium phosphate solution, its concentration being set in a way that a pH of 10 at the beginning of the reduction was reached. DAC (400 mg on a dry basis; 1 equiv.) was added, and the mixture was stirred for 140 min at 45 °C. The reaction was stopped by adding acetic acid until hydrogen formation ceased. Dialcohol celluloses prepared from highly oxidized DAC became (partially) soluble in water. These samples were precipitated in methanol and isolated by centrifugation instead of filtration. The crude product was washed thoroughly with deionized water and ethanol. Water-soluble dialcohol celluloses were washed with cold methanol. All samples were stored in a never-dried state at −20 °C for further analytical characterization. To analyze the chemical structure of the isolated dialcohol celluloses in a more straightforward manner, cotton linters was used as the starting material in a separate batch instead of the more complex softwood kraft pulp to avoid peak overlapping in the NMR spectra and solubility issues in the NMR electrolyte due to the presence of hemicelluloses and the high molecular weight, respectively.
“Level-off” DP experiments
DAC samples (160 mg, on a dry basis; 1 equiv.) with varying DO were treated with 20 mL of a 0.15 M carbonate/bicarbonate buffer (pH 10) without adding sodium borohydride to investigate the influence of alkali-induced chain-degradation independent of borohydride reduction. The reaction mixtures were shaken according to the standard procedure for 140 min at 45 °C before the reaction was terminated with acetic acid and the residue filtered off and washed with deionized water and ethanol. The depolymerized DAC samples and the dialcohol celluloses were analyzed with GPC/MALLS-RI.
Time-dependency of the borohydride reduction
An additional study was performed to better understand the rate of the borohydride reduction of DAC. 40%-oxidized DAC was reduced according to the above standard procedure. The borohydride reduction of DAC was terminated after different reaction times of 6, 30, 60, 140 min, and 24 h. The remaining aldehyde content was determined by CCOA labeling followed by GPC/MALLS-RI-FL.
pH-dependency experiments
To investigate the influence of the pH on the two competitive processes of chain degradation and aldehyde conversion, DAC samples with varying degrees of oxidation (DO of 10, 18, and 26 %) were reduced according to the above standard procedure, but different buffer solutions were used: 0.5 M boric acid-borax buffer (pH 7.4), 0.5 M boric acid-borax buffer (pH 8.1), 0.1 M potassium phosphate buffer (pH 8.1); 1 M tris buffer (pH 8.6), 0.1 M sodium carbonate/bicarbonate buffer (pH 9.3), 0.1 M sodium carbonate/bicarbonate buffer (pH 9.9). The isolated dialcohol celluloses were analyzed by fluorescence labeling followed by GPC/MALLS-RI-FL.
Experiments with excess sodium borohydride
To minimize chain-degradation, 39%-oxidized DAC was reduced according to the standard procedure, but with a large excess (5, 20, and 50 equiv.; 358.60 mg, 1.47 g, and 3.69 g, respectively) of sodium borohydride in 50 mL of a 0.15 M sodium carbonate/bicarbonate buffer to ensure a similar pH of 10 in all cases. The isolated dialcohol celluloses were analyzed by GPC/MALLS-RI.
Results and discussion
NMR analysis of chain integrity in dialcohol celluloses
Applications of dialcohol celluloses rely on starting materials that are partially oxidized DAC. Other than their simplifying name suggests, dialcohol celluloses are actually copolymers consisting of the “original” β-1,4-linked D-glucopyranose units and ring-opened structures which contain the former C-2 and C-3 as primary alcohols moieties—not secondary ones as in cellulose. The term “dialcohol cellulose”, although widely accepted and used in the pertinent literature (and also in this account), is thus evidently far from being correct nomenclature (3-[1-formyl-1-alkoxy-methoxy]-4-hydroxy-2-alkoxybutyraldehyde moieties as building blocks with “alkoxy” denoting the two attached polymer chains). The periodate oxidation/borohydride reduction sequence thus converts the biopolymer cellulose into the bio-derived copolymer dialcohol cellulose. The arrangement of the copolymers´ monomeric units in the polymer chain will significantly impact the materials´ properties. In the case of dialcohol cellulose, the arrangement of the units is predominantly governed by the supramolecular progress of the periodate oxidation resulting in the DAC intermediate. For this, various models exist, with some research groups advocating a core–shell modification (Fumagalli et al. 2013; Larsson et al. 2014b; Leguy et al. 2019; Mehandzhiyski et al. 2022), while others suggest a cluster-like modification (Kim et al. 2000; Nypelö et al. 2021). Previous studies have shown that periodate oxidation occurs randomly in the case of low-DO DAC (Potthast et al. 2009). Recently, also “peeling” of DAC chains during oxidation was discussed, which would result in further randomization of the reaction at higher DO values (Nypelö et al. 2021). The different proposed models would result in copolymers with different monomer patterns. Disregarding degradation side reactions, the core–shell model should result in a binary mixture of largely unmodified cellulose and low-glucopyranose dialcohol cellulose, the cluster-like modification should result in a block copolymer and random progress of the reaction would yield a statistical copolymer. Analytical methods such as solid-state NMR and FTIR—commonly used to characterize dialcohol cellulose (Kasai et al. 2014; López Durán et al. 2016; Lei and Feng 2020)—lack the required resolution to determine if oxidized glucopyranose units are statistically, blockwise or randomly distributed. We addressed this problem by conducting a solution-state NMR study of a series of dialcohol celluloses generated from DAC with DO values ranging from 4 to 88 %. So far, solution-state NMR spectra of dialcohol celluloses were only reported for strongly derivatized materials, which showed solubility in the used NMR solvents (Maekawa and Koshijima 1991; Siller et al. 2015). To overcome the solubility issues and open the method for a wider range of degrees of modification, a protocol relying on tetra-n-butylphosphonium acetate [P4444][OAc]:DMSO-d6 (1:4 wt%) mixtures as dissolving medium was applied (King et al. 2018; Koso et al. 2020; Fliri et al. 2023). Cotton linters were used as a model substrate to avoid peak superposition by hemicelluloses, as previously observed in diamine celluloses prepared from softwood kraft pulp (Simon et al. 2023b). The model substrate was first oxidized with sodium periodate to varying degrees of oxidation and then reduced with five molar equivalents of sodium borohydride following the standard procedure described in the experimental section. Quantitative 1H, diffusion-edited 1H, and multiplicity-edited 1H-13C HSQC spectra were recorded for all prepared samples. Expectedly, the complex structure of the copolymers resulted in massively overlapping spectra, furthermore complicated by the broadening of the peaks owing to the high molecular weight of the polymers. Thus, the assignment of the whole spectra of the different dialcohol celluloses from the one-dimensional experiments was not possible (see the exemplary whole-range diffusion-edited 1H spectrum in Fig. 1A). The HSQC experiments revealed four overlapping spin systems, which were identified by four separate peaks in the acetal region (C1-H moieties) of the spectra (see Supplementary Information, Chap. 2). They result from the conversion of glucopyranose units to the dialcohol cellulose moieties and their distribution in the polymer chain. The chemical environment of the C1-H moiety is influenced by the adjacent unit, resulting in magnetic inequivalence. As the peaks in the acetal region did not significantly overlap, it was possible to evaluate the connectivity based on the diffusion-edited 1H NMR spectra (Fig. 1B).
NMR study of a series of dialcohol celluloses with increasing DO: A Diffusion-edited 1H NMR spectrum ([P4444][OAc] : DMSO-d6, 1:4 wt%; 400 MHz; 65 °C) of dialcohol cellulose obtained from 32%-oxidized cellulose (cotton linters); B Cascade plot of NMR spectra focusing on the C1-H moieties (4.1 to 5.1 ppm) of dialcohol celluloses with varying degrees of oxidative modification. The four boxes display the four C1-H moieties with different chemical (and magnetic) environments. The samples were obtained from partially oxidized cellulose (DAC from cotton linters, with their initial DO being indicated) by sodium borohydride reduction, and the spectrum of unmodified cotton linters is shown for comparison; C C1-H moiety ratio (in %) extracted from the integration of the corresponding peaks in the quantitative 1H NMR spectra versus the degree of oxidation. The concentrations of the cellulosic materials varied between 2.5 and 5 wt% depending on the molecular weight of the samples
The signal at 4.40 ppm is characteristic of unmodified cellulose (King et al. 2018)—it was the only visible C1-H resonance for the cotton linters starting material. For highly modified dialcohol cellulose (from DAC with DO of 88 %), the signal at 4.40 ppm disappeared completely, while the adjacent dialcohol cellulose units gave a characteristic signal at 4.74 ppm, as observed in previous studies (Siller et al. 2015). In the samples where both monomeric units were present, two additional peaks appeared, one at 4.28 ppm for the C1-H of glucopyranose units adjacent to dialcohol units and one at 4.94 ppm for the “former” C1-H of dialcohol celluloses neighboring glucopyranose units (Fig. 1B).The four different CH-1 moieties seemed to follow a build-up and decay regime along the gradient of increasing DO, see the diffusion-edited 1H spectra in Fig. 1B. Note that the diffusion-edited 1H spectra do not use a quantitative pulse sequence and therefore do not provide strictly quantitative information. For quantitative evaluation, the relative peak intensities for the different signals were extracted from the quantitative 1H experiments. Owing to significant peak superposition with the water signal and an irregular baseline, peak deconvolution and fitting was necessary for the integration of the C1-H signals (the deconvoluted quantitative 1H NMR spectra are summarized in the Supplementary Information, Chap. 3). The plot of the relative peak intensities confirmed the previous qualitative observations (Fig. 1C). The relative intensity of the peak assigned to dialcohol cellulose moieties neighboring glucopyranose units (DiAlc-Cel, 4.94 ppm) increased until a DAC-DO of 32 % before it returned to zero in the final examined sample (DO = 88 %). In contrast, the resonances at 4.40 ppm (Cel-Cel) and 4.74 ppm (DiAlc-DiAlc) steadily decreased or increased, respectively, with increasing DO. Interestingly, the summarized integrals of the dialcohol cellulose moieties in all investigated samples were higher than the theoretical values for complete conversion of all DAC moieties. This points towards a preferred removal of oligosaccharide fractions during the reaction and the subsequent work-up procedure. It was especially pronounced in the sample generated from an 88%-oxidized DAC, where no cellulose resonances were visible although 12 % glucopyranose units were determined in the DAC intermediate. However, a quantitative interpretation of the results should be approached with caution, given the discrepancies among different analytical techniques for determining DO values (Leguy et al. 2019; Simon et al. 2022).
The presence of an “intermediate peak” with a buildup and decay behavior also allows conclusions as to the progress of the initial periodate oxidation. The modification seems to proceed randomly in the early stages of the oxidation with the resonance of the dialcohol cellulose moiety adjacent to a glucopyranose (4.94 ppm) being the most intensive until a DO of approximately 15 %. After that, the integral from the C1-H resonance assigned to two adjacent dialcohol cellulose units (4.74 ppm) constantly increased while the signal from the unmodified cellulose backbone decreased at a similar rate. Therefore, the modification pattern seems to become more cluster-like in this stage. Modified and unmodified domains exist in parallel, with unmodified domains getting depleted with increasing DO. This finding agrees with the GPC study of Potthast et al. (2009), demonstrating random attack by periodate for pulp with small DO (lower than 10 %). A cluster-like modification was also suggested by the uneven distribution patterns observed in gold labeling experiments (Kim et al. 2000). In contrast, the results of the NMR study do not align with a progress of the oxidation according to the core–shell model, which has often been reported in the literature (Fumagalli et al. 2013; Larsson et al. 2014b; Leguy et al. 2019; Mehandzhiyski et al. 2022). According to this model, the different layers of the cellulose crystallites should react consecutively (layer-by-layer). Consequently, the “intermediate peak” at 4.74 ppm should exhibit a build-up and decay behavior independently for every layer. As this has not been observed experimentally, a layer-by-layer oxidation seems rather unlikely. Nonetheless, there is solid experimental evidence for DAC exhibiting a core–shell arrangement with highly oxidized moieties predominantly on the surface. In the light of the present results, it seems more likely that this is a consequence of supramolecular rearrangement of highly oxidized and, thus, more water-soluble DAC fractions following a recently proposed peeling mechanism (Kim et al. 2000; Nypelö et al. 2021), rather than of a layer-by-layer oxidation mechanism.
Influence of the degree of oxidation on chain degradation during borohydride reduction
The effect of chain degradation owing to β-elimination side reactions on the resulting molecular weight distributions was studied. A softwood kraft pulp, as an industrially more relevant cellulose substrate, was oxidized with sodium periodate to DAC with varying degrees of oxidation (DO ranging from 8 to 74 %). The generated DAC samples were reduced to dialcohol cellulose with sodium borohydride and investigated by GPC. The presence of significant amounts of residual aldehydes remaining after reduction was excluded by oximation analysis (potentiometric titration of the generated hydrochloric acid after reaction of the aldehyde groups with hydroxylamine hydrochloride). The average molecular weight decreased exponentially as the DO of the DAC intermediates increased and “leveled off” at a DO of 18 % and an average molecular weight (Mw) of 62 kDa (Fig. 2). This is significantly smaller than the molecular weight obtained for a dialcohol cellulose with an initial DO of 8 % (Mw = 482 kDa) and the unmodified softwood kraft pulp (Mw = 614 kDa). The GPC data indicated that β-elimination processes competed vigorously with the borohydride reduction, leading to severe chain degradation. This effect was also evident from the isolated yields. Dialcohol cellulose with an initial DO of 8 % was isolated in good yield (90 %), while the yield leveled off at around 60 % for dialcohol celluloses with initial degrees of oxidation of 28 % and higher (Table S3).
Plot of the weight-averaged molecular weights (Mw) of dialcohol celluloses against the degrees of oxidation of the initial dialdehyde celluloses (green dots). The Mw data was fitted exponentially (green line) with a goodness-of-fit (R2) of 97.4. Unmodified pulp and the weight-averaged molecular weights of some dialdehyde cellulose samples treated under the same conditions (pH, T) without adding sodium borohydride (β-elimination only) are shown for comparison (purple dots)
For comparison, similarly prepared DAC samples were treated with a carbonate/bicarbonate buffer at pH 10 without adding sodium borohydride to investigate β-elimination independently (Fig. 2). The molecular weight was found to “level off” around 20 kDa but dropped already to 57 kDa for 8 %-oxidized softwood kraft pulp—in contrast to 482 kDa when reduced. It is important to note that the amounts of isolated DAC fragments after degradation were very small when treating DAC samples with degrees of oxidation of 40 % or higher, thus preventing a meaningful calculation of the yields. This is explained by forming low molecular weight organic degradation products and water-soluble oligomers. For instance, the C2-unit containing the “former” C1–C2 is released as glyoxal upon β-elimination, immediately forming hydrates and oligomers. Lui et al. (2020) reported DAC degradation in strongly alkaline conditions, predominantly resulting in the formation of cellulose nanocrystals and formiate.
The molecular weight distribution (MWD) changed significantly with increasing DO (Fig. 3). Dialcohol celluloses with an initial DO of 20 % or higher showed little to no separate peaks in the hemicellulose region. The MWD curves lost their usual, bimodal shape and became monomodal (Fig. 3A). In the β-elimination series, the MWD curves narrowed as the DO increased and deviated strongly from the conventional bimodal shape already at the lowest level of modification (initial DO of 8 %). However, the MWD curves indicated a fraction with very low molecular weight, possibly due to oligomers from β-elimination or degraded hemicelluloses (Fig. 3B). These low molecular weight degradation products were not present in the samples reduced with borohydride (Fig. 3A). They were either not formed or were further reduced and “washed out” during the work-up procedure and, therefore, not evident in the molecular weight distributions.
Molecular weight distributions: A Dialcohol celluloses synthesized by sodium borohydride reduction (45 °C, 140 min, pH 10) from DAC with varying degrees of oxidation (softwood kraft pulp); B DAC-fragments from the degradation of DAC samples (45 °C, 140 min, pH 10, no borohydride) with varying degrees of oxidation (softwood kraft pulp). Only samples up to a degree of oxidation at which the molecular weight distribution remains constant (“level-off” DP) are shown
The GPC data showed that borohydride reduction limited β-elimination by reducing some aldehydes before they could enter the competitive degradation process. However, a significant portion of polymer chains were still degraded to low molecular weight oligomers that were too small to be isolated, even by precipitation in methanol (e.g., the glyoxal oligomers mentioned above). It is important to note that precipitation in methanol was necessary to isolate any polymeric fractions from the reduction of highly oxidized DACs. However, such an isolation protocol would be difficult to implement on an industrial scale. The extent of degradation and the resulting significant decrease in yield that otherwise occur suggest that the currently applied conditions are only acceptable for generation of dialcohol celluloses from DAC with degrees of oxidation of approximately 15 % and below. Above this threshold, much additional optimization is required to further curb β-elimination and to render dialcohol celluloses economically and ecologically competitive to their fossil-based alternatives.
Time-dependence of the borohydride reduction
The reaction time for the reduction of DAC with sodium borohydride, as reported, was one (López Durán et al. 2016), two (Morooka et al. 1989; Mehandzhiyski et al. 2022), three (Kasai et al. 2014), four (Larsson et al. 2014a; Lei and Feng 2020), and 48 h (Potthast et al. 2009). To determine the time required for complete conversion, 40%-oxidized softwood kraft pulp was reduced with five equivalents of sodium borohydride according to the standard procedure, and the reaction was terminated with acetic acid after various reaction times. The aldehyde conversion was monitored using fluorescence labeling (CCOA method) to detect remaining aldehyde groups after the borohydride reduction (Fig. 4).
Time-dependent conversion of the aldehyde groups of 40%-oxidized DAC from softwood kraft pulp upon borohydride reduction to the corresponding dialcohol celluloses. The number of remaining aldehydes was calculated from the GPC-fluorescence signal after labeling with carbazole-9-carbonyl-oxy-amine (see CCOA method in the experimental section)
Conversion of the aldehyde moieties occurred rapidly within the first 6 min of the reaction, after which only 98 µmol/g aldehyde groups remained, starting from a DO of 40 % which corresponds to 5507 µmol/g. Thus, 98 % of the initial aldehydes were already converted. The remaining 2 % reacted within two more hours (99.9 % conversion after 140 min). Fluorescence labeling allowed for comparison of the number of aldehydes before and after the reaction. It was thus impossible to distinguish between consumption due to reduction or the strongly competing β-elimination side reactions (Fig. 2). Nevertheless, both β-elimination and borohydride reduction proceeded almost instantly, and the conversion was largely complete after a timespan of only a few minutes. The molecular weights of the isolated dialcohol celluloses only slightly decreased upon conversion of the few residual aldehyde groups and then remained constant (Figure S37), demonstrating the stability of the dialcohol celluloses´ polymer backbone under alkaline conditions.
Influence of pH on chain degradation and aldehyde conversion
A viable strategy to disfavor β-elimination in comparison to borohydride reduction might be to perform the reaction under less alkaline conditions to hold the alkali-induced chain degradation at bay. Although sodium borohydride becomes less stable under less alkaline conditions (Grzeschik et al. 2020), our GPC data (Fig. 4) showed that DAC was converted almost instantly also at pH values lower than 10, making this approach worth exploring. Therefore, a 10%-oxidized softwood kraft pulp was reduced using our standard procedure (5 equiv. of sodium borohydride, 45 °C and 140 min) in different buffer systems, at a pH ranging from 7.9 to 9.9, and the isolated dialcohol celluloses were analyzed using fluorescence labeling and GPC (Fig. 5 and Table S6).
The MWD curves of the dialcohol celluloses were clearly shifted towards lower values with increasing pH (Fig. 5A). Both shifting and narrowing of the MWD curves with increasing pH can be explained by increased β-elimination and degradation of low-molecular-weight fractions (i.e., hemicelluloses). The dispersity decreased from 3.4 to 2.0 when conducting the reaction at pH 7.9 or 9.9, respectively. However, fluorescence labeling of the remaining carbonyl groups revealed that the number of aldehydes decreased in parallel with the decreasing weight average molecular weight and increasing pH (Fig. 5B). For the sample at a pH of 7.9, a residual amount of carbonyl functionalities of 477 µmol/g was determined, suggesting about one-third of the generated oxidized glucopyranose units in the DAC precursor remained in the polymer backbone of the dialcohol cellulose. While β-elimination was slowed down with decreasing pH, also the conversion of aldehyde groups was diminished. Thus, lowering the pH did not achieve the desired effect of complete conversion at a minimized extent of elimination side reactions. Instead, a partially reduced dialcohol cellulose with considerable amounts of residual aldehyde moieties was obtained. Thus, also the potential for crosslinking, typical of DAC, is also retained, which influences the material’s properties and stability and might even be beneficial for certain applications (e.g., for cellulose-based foams). The pH study was reproduced with 18%- and 25%-oxidized softwood kraft pulp with similar outcomes (Tables S7/8 and Figures S39/40). Starting with a higher DO, the fluorescence signal from the labeled remaining aldehydes was too intense for reliable quantitation with the chosen experimental setup.
Influence of excess sodium borohydride on the degradation
Because lowering the pH during borohydride reduction did not effectively reduce chain degradation without also significantly decreasing the reduction efficiency, we thus tried in the last part of our study to promote the reduction by increasing the concentration of sodium borohydride. DAC from softwood kraft pulp (DO of 39 %) was reduced at 45 °C with 5, 20, and 50 molar equivalents of sodium borohydride. The reaction was buffered at pH 10 with aqueous carbonate/bicarbonate to ensure pH constancy. It is important to note that 50 molar equivalents of sodium borohydride equal an excess of 200 carbonyl equivalents since sodium borohydride can theoretically reduce four aldehyde groups. However, in most cases, including borohydride reduction of DAC, the actual efficiency is lower (Clayden et al. 2012). Additionally, β-elimination results in the formation of short-chain aldehydes, which are also be further reduced and might even be more reactive than the polymeric material. This causes additional consumption of sodium borohydride in addition to its decomposition in water. The shift of the MWD curves (Fig. 6) towards higher molecular weights with increasing borohydride concentration proved better maintenance of chain integrity.
The weight average molecular weight (Mw) of the isolated dialcohol celluloses when using 20 or 50 equivalents of reductant was significantly higher than with five equivalents of sodium borohydride according to the standard procedure (Table 1). A slight decrease in molecular weight is acceptable as the molecular weight of isolated dialcohol celluloses is still high enough to not affect their thermal and mechanical properties negatively. However, the material loss during reduction remains substantial, in addition to the use of enormous amounts of sodium borohydride. The yields of the isolated dialcohol celluloses using 20 and 50 equivalents were 10 % higher than in the experiment with five equivalents, reaching 80 %. This finding agrees well with previously reported yields for borohydride reduction of DAC (Larsson et al. 2014a; Lei and Feng 2020). To compete with petroleum-based materials, the yield needs to be further improved. While using large amounts of sodium borohydride proved effective in suppressing β-elimination to some extent, thereby increasing yield and Mw of the isolated material, the losses were still significant. Furthermore, such large excess of reductant as 20 or 50 equivalents would hardly be acceptable in the light of sustainable materials development.
Conclusions
Dialcohol celluloses were prepared through periodate oxidation of cellulose followed by borohydride reduction. The underlying chemistry was (re-)investigated by several screening experiments applying state-of-the-art analytical techniques. Solution-state NMR spectroscopy revealed that the different monomeric units in partially modified dialcohol celluloses were distributed randomly until a DO of approximately 15 %, and cluster-like at higher DO. The molecular weights of the isolated dialcohol celluloses decreased exponentially with increasing DO of the DAC intermediates and “levelled-off” at around one-tenth of the initial molecular weight of softwood kraft pulp. This demonstrated that borohydride reduction of DAC and β-elimination always occured simultaneously, the latter having significant negative effects on isolable yields and chain integrity. The kinetics of the reaction were shown to be very fast, with a conversion of 98 % of the aldehydes in DAC within the first 6 min.
Attempts to suppress the β-elimination mechanism were only partially successful. Lowering the pH during reduction slowed down both competitive processes to approximately the same extent. While the decrease in the MWDs was less pronounced when conducting the reduction at lower pH, the isolated dialcohol celluloses still contained residual aldehyde functionalities. Instead, increasing the amount of sodium borohydride led to an improved conversion of the aldehydes to the corresponding alcohols and was able to suppress the degradation. The yields were improved and reached 80 % when starting with medium-oxidized DAC (DO of 39 %), and the isolated materials showed higher molecular weights. Complete conversion of carbonyl groups occurred within the first hour, making prolonged reaction times unnecessary.
Sodium borohydride reduction of DAC is attractive because it rapidly reduces aldehydes under mild conditions with a cost-efficient and readily available reductant. For dialcohol celluloses prepared from slightly oxidized DAC (DO of 15 % and below), the chain degradation and loss in yield were in an acceptable range. However, for modification levels higher than approximately 15 %, this approach will not result in genuinely sustainable materials owing to significant material losses during preparation, and a high excess of reductant needed.
Generally, dialcohol cellulose has promising properties as a potential bioderived and biodegradable thermoplastic of the future. However, the observed losses in yield and molecular weight during preparation, owing to β-elimination side reactions, need to be considered for large-scale applications. With the presented study we intended to highlight this important but often overlooked dilemma. Furthermore, we have shown possibilities to influence the materials properties by the reaction conditions used. It was possible to prepare dialcohol celluloses, albeit with remaining aldehyde functionalities, by using less alkaline buffer systems and to suppress degradation side reactions by applying superstoichiometric amounts of the reductant. Clearly, more work is needed to overcome issues with degradation side reactions and associated loss in yield. Our continuing contributions towards this goal will be reported in due course.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Ardanuy M, Claramunt J, Toledo Filho RD (2015) Cellulosic fiber reinforced cement-based composites: a review of recent research. Constr Build Mater 79:115–128. https://doi.org/10.1016/j.conbuildmat.2015.01.035
Becker M, Zweckmair T, Forneck A, Rosenau T, Potthast A, Liebner F (2013) Evaluation of different derivatisation approaches for gas chromatographic–mass spectrometric analysis of carbohydrates in complex matrices of biological and synthetic origin. J Chromatogr A 1281:115–126. https://doi.org/10.1016/j.chroma.2013.01.053
Brown HC (1973) Boranes in organic chemistry based upon the Roger Adams award address to the Division of Organic Chemistry of the American Chemical Society in Ann Arbor, Michigan, June 15, 1971. In: Stone FGA, West R (eds) Advances in organometallic chemistry. Academic Press, pp 1–20
Brown HC, Krishnamurthy S (1979) Forty years of hydride reductions. Tetrahedron 35:567–607. https://doi.org/10.1016/0040-4020(79)87003-9
Clayden J, Greeves N, Warren SG (2012) Organic chemistry. In: Organic chemistry, 2nd ed. Oxford University Press, pp 130–132
Esen E, Meier MAR (2020) Sustainable functionalization of 2,3-dialdehyde cellulose via the Passerini three-component reaction. ACS Sustain Chem Eng 8:15755–15760. https://doi.org/10.1021/acssuschemeng.0c06153
Fliri L, Heise K, Koso T, Todorov AR, del Cerro DE, Hietala S, Fiskari J, Kilpeläinen I, Hummel M, King AWT (2023) Solution-state nuclear magnetic resonance spectroscopy of crystalline cellulosic materials using a direct dissolution ionic liquid electrolyte. Nat Protoc Accept. https://doi.org/10.1038/s41596-023-00832-9
Fumagalli M, Ouhab D, Boisseau SM, Heux L (2013) Versatile gas-phase reactions for surface to bulk esterification of cellulose microfibrils aerogels. Biomacromolecules 14:3246–3255. https://doi.org/10.1021/bm400864z
Grzeschik R, Schäfer D, Holtum T, Küpper S, Hoffman A, Schlücker S (2020) On the overlooked critical role of the pH value on the kinetics of the 4-nitrophenol NaBH4-reduction catalyzed by noble-metal nanoparticles (Pt, Pd, and Au). J Phys Chem C 124:2939–2944. https://doi.org/10.1021/acs.jpcc.9b07114
Head FSH (1955) 25—The reduction of the aldehyde groups in periodate oxycelluloses by sodium borohydride. J Text Inst Trans 46:T400–T406. https://doi.org/10.1080/19447027.1955.10750328
Hosoya T, Bacher M, Potthast A, Elder T, Rosenau T (2018) Insights into degradation pathways of oxidized anhydroglucose units in cellulose by β-alkoxy-elimination: a combined theoretical and experimental approach. Cellulose 25:3797–3814. https://doi.org/10.1007/s10570-018-1835-y
Jusner P, Bacher M, Simon J, Bausch F, Khaliliyan H, Schiehser S, Sumerskii I, Schwaiger E, Potthast A, Rosenau T (2022) Analyzing the effects of thermal stress on insulator papers by solid-state 13 C NMR spectroscopy. Cellulose 29:1081–1095. https://doi.org/10.1007/s10570-021-04338-z
Kasai W, Morooka T, Ek M (2014) Mechanical properties of films made from dialcohol cellulose prepared by homogeneous periodate oxidation. Cellulose 21:769–776. https://doi.org/10.1007/s10570-013-0153-7
Kim U-J, Kuga S, Wada M, Okano T, Kondo T (2000) Periodate oxidation of crystalline cellulose. Biomacromolecules 1:488–492. https://doi.org/10.1021/bm0000337
King AWT, Mäkelä V, Kedzior SA, Laaksonen T, Partl GJ, Heikkinen S, Koskela H, Heikkinen HA, Holding AJ, Cranston ED, Kilpeläinen I (2018) Liquid-state NMR analysis of nanocelluloses. Biomacromolecules 19:2708–2720. https://doi.org/10.1021/acs.biomac.8b00295
Koprivica S, Siller M, Hosoya T, Roggenstein W, Rosenau T, Potthast A (2016) Regeneration of aqueous periodate solution by ozone treatment: a sustainable approach for dialdehyde cellulose production. Chemsuschem 9(8):825–833
Koso T, Rico del Cerro D, Heikkinen S, Nypelö T, Buffiere J, Perea-Buceta JE, Potthast A, Rosenau T, Heikkinen H, Maaheimo H, Isogai A, Kilpeläinen I, King AWT (2020) 2D assignment and quantitative analysis of cellulose and oxidized celluloses using solution-state NMR spectroscopy. Cellulose 27:7929–7953. https://doi.org/10.1007/s10570-020-03317-0
Larsson PA, Berglund LA, Wågberg L (2014a) Ductile all-cellulose nanocomposite films fabricated from core–shell structured cellulose nanofibrils. Biomacromolecules 15:2218–2223. https://doi.org/10.1021/bm500360c
Larsson PA, Berglund LA, Wågberg L (2014b) Highly ductile fibres and sheets by core–shell structuring of the cellulose nanofibrils. Cellulose 21:323–333. https://doi.org/10.1007/s10570-013-0099-9
Leguy J, Nishiyama Y, Jean B, Heux L (2019) Ultrastructural characterization of the core–shell structure of a wide range of periodate-oxidized cellulose from different native sources by solid-state 13C CP-MAS NMR. ACS Sustain Chem Eng 7:412–420. https://doi.org/10.1021/acssuschemeng.8b03772
Lei B, Feng Y (2020) Sustainable thermoplastic bio-based materials from sisal fibers. J Clean Prod 265:121631. https://doi.org/10.1016/j.jclepro.2020.121631
Li C, Wu J, Shi H, Xia Z, Sahoo JK, Yeo J, Kaplan DL (2022) Fiber-based biopolymer processing as a route toward sustainability. Adv Mater 34:2105196. https://doi.org/10.1002/adma.202105196
Linvill E, Larsson PA, Östlund S (2017) Advanced three-dimensional paper structures: mechanical characterization and forming of sheets made from modified cellulose fibers. Mater Des 128:231–240. https://doi.org/10.1016/j.matdes.2017.05.002
Liu P, Pang B, Dechert S, Zhang XC, Andreas LB, Fischer S, Meyer F, Zhang K (2020) Structure selectivity of alkaline periodate oxidation on lignocellulose for facile isolation of cellulose nanocrystals. Angew Chem Int Ed 59:3218–3225. https://doi.org/10.1002/anie.201912053
López Durán V, Larsson PA, Wågberg L (2016) On the relationship between fibre composition and material properties following periodate oxidation and borohydride reduction of lignocellulosic fibres. Cellulose 23:3495–3510. https://doi.org/10.1007/s10570-016-1061-4
Maekawa E, Koshijima T (1991) Preparation and structural consideration of nitrogen-containing derivatives obtained from dialdehyde celluloses. J Appl Polym Sci 42:169–178. https://doi.org/10.1002/app.1991.070420120
Mehandzhiyski AY, Engel E, Larsson PA, Re GL, Zozoulenko IV (2022) Microscopic insight into the structure–processing–property relationships of core–shell structured dialcohol cellulose nanoparticles. ACS Appl Biomater 5:4793–4802. https://doi.org/10.1021/acsabm.2c00505
Mokhena TC, Sadiku ER, Mochane MJ, Ray SS, John MJ, Mtibe A (2021) Mechanical properties of cellulose nanofibril papers and their bionanocomposites: a review. Carbohydr Polym 273:118507. https://doi.org/10.1016/j.carbpol.2021.118507
Morooka T, Norimoto M, Yamada T (1989) Periodate oxidation of cellulose by homogeneous reaction. J Appl Polym Sci 38:849–858. https://doi.org/10.1002/app.1989.070380508
Nypelö T, Berke B, Spirk S, Sirviö JA (2021) Review: periodate oxidation of wood polysaccharides—modulation of hierarchies. Carbohydr Polym 252:117105. https://doi.org/10.1016/j.carbpol.2020.117105
Potthast A, Röhrling J, Rosenau T, Borgards A, Sixta H, Kosma P (2003) A novel method for the determination of carbonyl groups in cellulosics by fluorescence labeling. 3. Monitoring oxidative processes. Biomacromolecules 4:743–749. https://doi.org/10.1021/bm020029q
Potthast A, Schiehser S, Rosenau T, Kostic M (2009) Oxidative modifications of cellulose in the periodate system-reduction and beta-elimination reactions. Holzforschung 63:12–17. https://doi.org/10.1515/HF.2009.108
Potthast A, Rosenau T, Henniges U, Schiehser S, Kosma P, Saake B, Lebioda S, Radosta S, Vorwerg W, Wetzel H, Koschella A, Heinze T, Strobin G, Sixta H, Strlic M, Isogai A (2015) Comparison testing of methods for gel permeation chromatography of cellulose: coming closer to a standard protocol. Cellulose 22:1591–1613. https://doi.org/10.1007/s10570-015-0586-2
Re GL, Engel ER, Björn L, Sicairos MG, Liebi M, Wahlberg J, Jonasson K, Larson PE (2023) Melt processable cellulose fibres engineered for replacing oil-based thermoplastics. Chem Eng J 458:141372. https://doi.org/10.1016/j.cej.2023.141372
Röhrling J, Potthast A, Rosenau T, Lange T, Borgards A, Sixta H, Kosma P (2002a) A novel method for the determination of carbonyl groups in cellulosics by fluorescence labeling. 2. Validation and applications. Biomacromolecules 3:969–975. https://doi.org/10.1021/bm020030p
Röhrling J, Potthast A, Rosenau T, Lange T, Ebner G, Sixta H, Kosma P (2002b) A novel method for the determination of carbonyl groups in cellulosics by fluorescence labeling. 1. Method development. Biomacromolecules 3:959–968. https://doi.org/10.1021/bm020029q
Ruan C-Q, Strømme M, Lindh J (2018) Preparation of porous 2,3-dialdehyde cellulose beads crosslinked with chitosan and their application in adsorption of Congo red dye. Carbohydr Polym 181:200–207. https://doi.org/10.1016/j.carbpol.2017.10.072
Siller M, Amer H, Bacher M, Rosenau T, Potthast A (2015) Effects of periodate oxidation on cellulose polymorphs. Cellulose 22:2245–2261. https://doi.org/10.1007/s10570-015-0648-5
Simon J, Tsetsgee O, Iqbal NA, Sapkota J, Ristolainen M, Rosenau T, Potthast A (2021) Fourier transform and near infrared dataset of dialdehyde celluloses used to determine the degree of oxidation with chemometric analysis. Data Brief 40:107757. https://doi.org/10.1016/j.dib.2021.107757
Simon J, Tsetsgee O, Iqbal NA, Sapkota J, Ristolainen M, Rosenau T, Potthast A (2022) A fast method to measure the degree of oxidation of dialdehyde celluloses using multivariate calibration and infrared spectroscopy. Carbohydr Polym 278:118887. https://doi.org/10.1016/j.carbpol.2021.118887
Simon J, Fliri L, Drexler F, Bacher M, Sapkota J, Ristolainen M, Hummel M, Potthast A, Rosenau T (2023a) Debugging periodate oxidation of cellulose: why following the common protocol of quenching excess periodate with glycol is a bad idea. Carbohydr Polym 310:120691. https://doi.org/10.1016/j.carbpol.2023.120691
Simon J, Fliri L, Sapkota J, Ristolainen M, Miller S, Hummel M, Rosenau T, Potthast A (2023b) Reductive amination of dialdehyde cellulose: access to renewable thermoplastics. Biomacromolecules 24:166–177. https://doi.org/10.1021/acs.biomac.2c1022
Sundberg A, Sundberg K, Lillandt C, Holmhom B (1996) Determination of hemicelluloses and pectins in wood and pulp fibres by acid methanolysis and gas chromatography. Nord Pulp Paper Res J 11:216–219. https://doi.org/10.3183/npprj-1996-11-04-p216-219
Szcześniak L, Rachocki A, Tritt-Goc J (2008) Glass transition temperature and thermal decomposition of cellulose powder. Cellulose 15:445–451. https://doi.org/10.1007/s10570-007-9192-2
Acknowledgments
We kindly thank Business Finland and UPM-Kymmene Oyj (Finland) for their financial support. The BOKU doctoral school ABC&M and the Austrian Biorefinery Center Tulln (ABCT) are gratefully acknowledged for their support. We also thank Sonja Schiehser for her help with GPC measurements.
Funding
Open access funding provided by University of Natural Resources and Life Sciences Vienna (BOKU). Open access funding provided by University of Natural Resources and Life Sciences, Vienna (BOKU). The financial support by Business Finland, UPMKymmene Oyj (Finland), the Austrian Biorefinery Center Tulln (ABCT), and the doctoral school “Advanced Biorefineries: Chemistry & Materials” (ABC&M) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
JS, LF, JS, MR, AP, and TR contributed to the study conception and design. Material preparation was performed by JS and FF. Data collection, analysis, and interpretation were performed by JS and LF. The original draft of the manuscript was written by JS, including visualization. Review and editing by LF, MH, AP, and TR. Supervision by JS, MR, MH, AP, and TR. Project administration and funding acquisition by AP and TR. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agreed to the publication in the submitted form.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Simon, J., Fliri, L., Fröhlich, F. et al. Insights into the borohydride reduction of dialdehyde cellulose: the dilemma of competing reduction and β-elimination reactions. Cellulose 30, 8205–8220 (2023). https://doi.org/10.1007/s10570-023-05350-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-023-05350-1