Abstract
A round robin on GPC of a wide range of different pulp samples was conducted among leading groups in cellulose analysis. The aim was to survey the status quo of the methods available to date. The pulp samples covered not only fully-bleached dissolving pulps but also bleached paper pulps and one unbleached sample. The methods applied were current state-of-the-art GPC with RI, MALLS, and viscosimetry detectors. Different dissolution protocols were compared as well. Following from the obtained results, more standardized protocols were proposed for approaches with different equipment (RI or MALLS/RI) and solvent systems (direct dissolution or derivatization). Major influencing factors, such as derivatization compared to direct solution, calibration versus light scattering and in-between lab variation, were discussed.












Similar content being viewed by others
Notes
Mw, Eq. 2, in order to better reflect the meaning of this statistical parameter it could be called weighted-average, as the average is a weighted average.
References
Arndt KF, Müller G (1996) Polymercharakterisierung. Carl Hanser, München, Wien Austin, PL (1977). US patent, 4,059,457
Barth HG, Boyes BE, Jackson C (1998) Size exclusion chromatography and related separation techniques. Anal Chem 70(12):251–278
Berggren R, Berthold F, Sjöholm E, Lindström M (2003) Improved methods for evaluating the molar mass distributions of cellulose in kraft pulp. J Appl Polym Sci 88(5):1170–1179
Bikova T, Treimanis A (2002) Problems of the MMD analysis of cellulose by SEC using DMA/LiCl: a review. Carbohydr Polym 48(1):23–28
Burchard W (1999). Solution properties of branched macromolecules. Advan Polym Sci (Branched Polymers II) 143:113–194
Carolan G, Catley BJ, McDougal FJ (1983) The location of tetrasaccharide units in pullulan. Carbohydr Res 114(2):237–243
Catley BJ, Ramsay A, Servis C (1986) Observations on the structure of the fungal extracellular polysaccharide, pullulan. Carbohydr Res 153(1):79–86
Chrapava S, Touraud D, Rosenau T, Potthast A, Kunz W (2003) The investigation of the influence of water and temperature on the LiCl/DMAc/cellulose system. Phys Chem Chemi phy 5(9):1842–1847
Christensen BE, Ulset A-S, Beer MU, Knuckles BE, Williams DL, Fishman ML, Chau HK, Wood PJ (2001) Macromolecular characterization of three barley β-glucan standards by size-exclusion chromatography combined with light scattering and viscometry: an inter-laboratory study. Carbohydr Polym 45(1):11–22
Dawsey TR, McCormick CL (1990) The lithium chloride/dimethylacetamide solvent for cellulose: a literature review. J Macromol Sci Part C Polym Rev 30(3&4):405–440
Dupont AL, Harrison G (2004) Conformation and dn/dc determination of cellulose in N,N-dimethylacetamide containing lithium chloride. Carbohydr Polym 58(3):233–243
El Seoud OA, Koschella A, Fidale LC, Dorn S, Heinze T (2007) Applications of ionic liquids in carbohydrate chemistry: a window of opportunities. Biomacromolecules 8(9):2629–2647
Engel P, Hein L, Spiess AC (2012) Derivatization-free gel permeation chromatography elucidates enzymatic cellulose hydrolysis. Biotechnol Biofuels 5(1):8
Fukaya Y, Tsukamoto A, Kuroda K, Ohno H (2011) High performance “ionic liquid” chromatography. Chem Commun 47(7):1994–1996
Gallot-Grubisic Z, Rempp P, Benoit H (1967) Universal calibration for gel permeation chromatography. J Polym Sci Polym Let Ed 5(9):753–759
Ganster J, Fink HP (1999). Physical constants of Cellulose. In: Brandrup J, Immergut EH, Grulke EH (eds) Polymer Handbook. 4th edn. John Wiley and Sons: New York, Chichester, Weinheim, Brisbane, Singapore, Toronto pp 135–157
Hall DM, Horne JR (1973) Preparation of cellulose triacetate and cellulose tricarbanilate by nondegradative methods. J Appl Polym Sci 17(12):3729–3734
Hasani M, Henniges U, Idström A, Nordstierna L, Westman G, Rosenau T, Potthast A (2013) Nano-cellulosic materials: the impact of water on their dissolution in DMAc/LiCl. Carbohydr Polym 98(2):1565–1572
Henniges U, Kloser E, Patel A, Potthast A, Kosma P, Fischer M, Fischer K, Rosenau T (2007) Studies on DMSO-containing carbanilation mixtures: chemistry, oxidations and cellulose integrity. Cellulose 14(5):497–511
Henniges U, Kostic M, Borgards A, Rosenau T, Potthast A (2011) Dissolution behavior of different celluloses. Biomacromolecules 12(4):871–879
Henniges U, Vejdovszky P, Siller M, Jeong MJ, Rosenau T, Potthast A (2011) Finally dissolved! activation procedures to dissolve cellulose in DMAc/LiCl prior to size exclusion chromatography analysis: a review. Curr Chromatogr 1(1):52–68
Kes M, Christensen BE (2013) A re-investigation of the Mark–Houwink–Sakurada parameters for cellulose in Cuen: a study based on size-exclusion chromatography combined with multi-angle light scattering and viscometry. J Chromatogr A 1281:32–37
Klemm D, Philipp B, Heinze T, Heinze U, Wagenknecht W (1998) Comprehensive cellulose chemistry, vol 2. Wiley, Weinheim
Kuroda K, Fukaya Y, Ohno H (2013) Direct HPILC analysis of cellulose depolymerisation in ionic liquids. Anal Methods 5(13):3172–3176
Leathers TD (2004) Chapter 1: Pullulan. In: biopolymers, vol. 6, Polysaccharides II: Polysaccharides from Eukaryotes. De Baets S, Vandamme E, Steinbüchel A. (eds) Wiley VCH
McCormick CL, Lichatowich DK (1979) Homogeneous solution reactions of cellulose, chitin, and other polysaccharides to produce controlled-activity pesticide systems. J Polym Sci Polym Lett Ed 17(8):479–484
McCormick CL, Shen TS (1982) In: Seymour RB, Stahl GS (eds) Macromolecular solutions. Pergamon: New York, p. 101
McCormick CL, Callais PA, Hutchinson BH (1985) Solution studies of cellulose in lithium chloride and N,N-dimethylacetamide. Macromolecules 18(12):2394–2401
Morgenstern B (2001) Habilitation thesis, Dresden University of Technology, Germany
Morgenstern B, Berger W (1993) Investigations about dissolution of cellulose in the LiCl/N,N-dimethylformamide system. Acta Polym 44(2):100–102
Morgenstern B, Kammer HW (1996) Solvation in cellulose-LiCl-DMAc solutions. TRIP 4:87–92
Morgenstern B, Kammer HW (1998) On the particulate structure of cellulose solutions. Polymer 40(5):1299–1304
Mori S (1998) Problems associated with SEC measurements of polymers among laboratories: summary on cooperative determination of molecular weight averages of polystyrenes by SEC in Japan. Int J Polym Anal Charact 4(6):531–546
Mori S, Barth HG (1999) Size exclusion chromatography. Springer Laboratory Series, Berlin
Mourey T (2004) SEC molecular-weight-sensitive detection. Intl J Polymer Anal Char 9(1–3):97–135
Podzimek S (2011) Light scattering, size exclusion chromatography and assymetric field flow fractionation. Wiley, New York
Potthast A, Rosenau T, Sartori J, Sixta H, Kosma P (2002) Hydrolytic processes and condensation reactions in the cellulose solvent system N, N-dimethylacetamide/lithium chloride. Part 2: degradation of cellulose. Polymer 44(1):7–17
Qiu H, Mallik AK, Takafuji M, Jiang S, Ihara H (2012) Ionic liquids in liquid chromatography. In: Handbook of ionic liquids: properties, applications and hazards, pp 325–350
Röder T, Morgenstern B (1999) The influence of activation on the solution state of cellulose dissolved in N-methylmorpholine-N-oxide-monohydrate. Polymer 40(14):4143–4147
Röhrling J, Potthast A, Rosenau T, Lange T, Ebner G, Sixta H, Kosma P (2002) A novel method for the determination of carbonyl groups in cellulosics by fluorescence labeling. 1. Method development. Biomacromolecules 3(5):959–968
Rosenau T, Potthast A, Hofinger A, Sixta H, Kosma P (2005) Hydrolytic processes and condensation reactions in the cellulose solvent system N, N-dimethylacetamide/lithium chloride. Part 1. Holzforschung 55(6):661–666
Rosenau T, Potthast A, Krainz K, Hettegger H, Henniges U, Yoneda Y, Rohrer C, French AD (2014) Chromophores in cellulosics, XI: isolation and identification of residual chromophores from bacterial cellulose. Cellulose 21(4):2271–2283
Schelosky N, Röder T, Baldinger T (1999) Molecular mass distribution of cellulosic products by size exclusion chromatography in DMAc/LiCl. Das Papier 53(12):728–738
Siller M, Ahn K, Pircher N, Rosenau T, Potthast A (2014) Dissolution of rayon fibers for size exclusion chromatography: a challenge. Cellulose 21(5):3291–3301
Sinner M, Puls J (1978) Non-corrosive dye reagent for detection of reducing sugars in borate complex ion-exchange chromatography. J Chromatogr 156:197–204
Sjöholm E, Gustafsson K, Eriksson B, Brown W, Colmsjö A (2000) Aggregation of cellulose in lithium chloride/N,N-dimethylacetamide. Carbohydr Polym 41(2):153–161
Stol R, Pedersoli JL Jr, Poppe H, Kok WT (2002) Application of size exclusion electrochromatography to the microanalytical determination of the molecular mass distribution of celluloses from objects of cultural and historical value. Anal Chem 74(10):2314–2320
Strlic M, Kolar J (2003) Size exclusion chromatography of cellulose in LiCl/N,N-dimethylacetamide. J Biochem Biophys Methods 56:265–279
Strlic M, Kolenc J, Kolar J, Pihlar B (2002) Enthalpic interactions in size exclusion chromatography of pullulan and cellulose in LiCl–N,N-dimethylacetamide. J Chromatogr A 964:47–54
Taguchi R, Kikuchi Y, Sakano Y, Kobayashi T (1973) Polysaccharide production by pullularia pullulans. I. Structural uniformity of pullulan produced by several strains of pullularia pullulans. Agric Biol Chem 37(7):1583–1588
Terbojevich M, Cosani A, Camilot M, Focher B (1995) Solution studies of cellulose tricarbanilates obtained in homogeneous phase. J Appl Polym Sci 55(12):1663–1671
Timpa JD (1991) Application of universal calibration in gel permeation chromatography for molecular weight determinations of plant cell wall polymers: cotton fiber. J Agric Food Chem 39(2):270–275
Turbak AF, Hammer RB, Davies RE, Hergert HL (1980) Cellulose solvents. Chemtech 10(1):51–57
Turbak AF, El-Kafrawy A, Snyder FW, Auerbach AB (1981) US patent 4,302,252
Wang H, Gurau G, Rogers RD (2012) Ionic liquid processing of cellulose. Chem Soc Rev 41(4):1519–1537
Westermark U, Gustafsson K (1994) Molecular size distribution of wood polymers in birch kraft pulps. Holzforschung 48(Suppl.):146–150
Willför S, Pranovich A, Tamminen T, Puls J, Laine C, Suurnäkki A, Saake B, Uotila K, Simolin H, Hemming J, Holmbom B (2009) Carbohydrate analysis of plant materials with uronic acid-containing polysaccharides: a comparison between different hydrolysis and subsequent chromatographic analytical techniques. Ind Crops Prod 29:571–580
Yanagisawa M, Isogai A (2008) SEC-MALS-QELS study on the molecular conformation of cellulose in LiCl/Amide solutions. Biomacromolecules 6(3):1258–1265
Yanagisawa M, Shibata I, Isogai A (2004) SEC–MALLS analysis of cellulose using LiCl/1,3-dimethyl-2-imidazolidinone as an eluent. Cellulose 11(2):169–176
Yanagisawa M, Shibata I, Isogai A (2005) SEC-MALLS analysis of softwood kraft pulp using LiCl/1,3-dimethyl-2-imidazolidinone as an eluent. Cellulose 12(2):151–158
Zhang C, Liu R, Xiang J, Kang H, Liu Z, Huang Y (2014) Dissolution mechanism of cellulose in N, N-dimethylacetamide/lithium chloride: revisiting through molecular interactions. J Phys Chem B 118(31):9507–9514
Acknowledgments
The authors are grateful to the EPNOE association which provided frame and travel support for the present study. In addition we like to thank the association Zellcheming (subcommittee pulp analysis). The authors appreciate the help of Alexander Tischer with statistics.
Author information
Authors and Affiliations
Corresponding author
Appendix: Round robin test on gel permeation chromatography of cellulose
Appendix: Round robin test on gel permeation chromatography of cellulose
Dissolution of cellulosic pulps for measurement
The following describes two representative procedures for the process of dissolving celluloses, the first by derivatization as tricarbanilate and the second by direct dissolution in DMAc/LiCl.
Optimized carbanilation and measurement in THF
Pulp sheets were disintegrated in deionized water by high-speed homogenisation (20,000 rpm). The sample was recovered on a glass frit (G3), applying only slight vacuum. The pulp was soaked in acetone two times followed by filtration, and the sample was dried in vacuo at 30 °C. The sample was fluffed in a non-cutting laboratory mill and further dried in a desiccator over phosphorus pentoxide. 50 mg of pulp were transferred into a 100 ml Duran bottle, anhydrous pyridine (50 ml) was added, and the bottle was placed in a vacuum oven for 10 min, in order to desorb air from the fibres. After addition of phenyl isocyanate (3.5 ml) the bottle was closed with a thermo-stable PTFE cap and the mixture was allowed to stand in order to react in a heating oven at 80 °C for 48 h. During that time the bottles had to be shaken manually approximately eight times. The reaction was stopped by addition of methanol (10 ml). The mixture was cooled to room temperature and added dropwise into a mixture of methanol (250 ml), water (150 ml), and glacial acetic acid (5 ml). To improve the precipitation, the mixtures were kept in a refrigerator overnight. The precipitate was recovered by centrifugation in 500 ml beakers at 20,000 g (centrifuge with 11,000 U min−1). The supernatant was decanted and the precipitate was dissolved in acetone (50 ml) in a laboratory shaker overnight. The resulting solution was added dropwise to a solution of methanol (250 ml) and water (150 ml). The mixture was again stored overnight in the refrigerator, the precipitate was recovered by centrifugation as described before, and the products were dried in vacuo at 30 °C.
Cautionary notes:
-
Before injection, the sample solution should be filtered to remove particles that might block the columns;
-
Isocyanate is toxic and therefore care should be taken when handling.
Optimized direct dissolution and measurement in DMAc/LiCl
To achieve a molecularly disperse dissolution in DMAc/LiCl (9 %, m/V) overnight at room temperature, pulp samples must be activated using the following steps. The pulp samples (20 mg air dried) are disintegrated in a kitchen mixer with 250 mL of deionized water by mixing three times for 10 s. Extended mixing, milling, or the use of ultra-turrax or ultrasound all must be avoided as they cause cellulose degradation. The water is filtered off and exchanged for ethanol or acetone and finally DMAc. The DMAc-wet sample is placed into excess DMAc (4-5 ml) and agitated overnight in a shaker at room temperature. The excess DMAc is filtered off using black ribbon cellulose filter paper and the sample dissolved in DMAc/LiCl 9 % applying gentle shaking and few times vortexing (the DMAc and the LiCl are used as received; no additional drying is necessary). DMAc is highly hygroscopic, and even a few seconds’ exposure causes rewetting of the dried solvent and renders previous drying useless (Chrapava et al. 2003). Traces of water will not interfere with the dissolution.
The DMAc-wet sample is dissolved in 2 ml of DMAc/LiCl (9 %). Dissolution should proceed at room temperature; for special samples, such as highly oriented fibers like viscose, moderate heating (up to 40 °C) may be applied. The samples cannot be heated above that levels, since the cellulose integrity would be severely affected (Potthast et al. 2002). Dissolution times vary depending on the material; for a study on different pulp samples and their dissolution times, see Henniges et al. (2011, 2013). In general, sulfite pulps dissolve very quickly, whereas annual plant fibers need longer dissolution times. Normally, dissolution times beyond 24 h offer no additional improvement to the MMD profile. For measurement, the dissolved cellulose is diluted with pure DMAc (3:1), filtered through a 0.45 µm disk filter and injected. For dissolution of viscose or lyocell fibers different protocols have to be applied (Siller et al. 2014). This is also true for nano-fibrillated cellulose samples (Hasani et al. 2013), but this is beyond the scope of this protocol.
Cautionary notes:
-
DMAc is toxic and should be handled with due caution, refer to the MSDS (Material Safety Data Sheet) for safety precautions;
-
Insufficient activation of the cellulose will lead to difficulties in dissolution;
-
DMAc/LiCl is a powerful solvent. Careless handling or leaks will lead to damage of the analytical hardware;
-
Not all analytical devices (e.g. stainless steel pistons in pumps) can withstand DMAc/LiCl and might be rapidly damaged due to corrosion. Check with the beforehand manufacturer if the system is compatible.
-
Stainless steel capillaries should not be used to connect chromatography equipment (pump, autosampler, or detectors), use PEEK instead.
-
Operating a chromatography system in DMAc/LiCl at higher temperature (T > 30 °C) will increase the risk of corrosion. Operation at room temperature is recommended and does not cause back pressure problems.
Calibration with standards
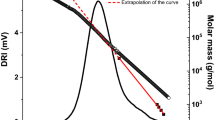
The classical calibration approach in GPC of (synthetic) polymers—the use of narrow distributed polymer standards and the direct correlation of their retention time to the molar mass of the analytes—is not readily applicable in the case of cellulose, simply because of a lack of appropriate standards. For cellulose tricarbanilates, the chemical similarity likely justifies the use of polystyrene standards. However, retention times naturally differ for different polymers and thus the validity of using polystyrene standards is also limited. For cellulose being directly dissolved without preceding derivatization, pullulan standards are available up to a molar mass of 2.5 × 106 g mol−1, which can be used with the solvent system DMAc/LiCl. Pullulan is a polysaccharide composed of α-1,6-glycosidic linked α-1,4-maltotriose units with a small number of randomly distributed α-1,4-maltotetraose subunits (Carolan et al. 1983; Leathers 2004; Taguchi et al. 1973; Catley et al. 1986) and cellulose is a β-1,4-linked glucan. Therefore these polysaccharides have different structures in solution and likely different hydrodynamic radii at specific molar masses. Hence, the direct correlation of molar mass and retention volume based on pullulan standards gives only relative molar masses (pullulan-related).
A possible approach to improve the calibration with standards was introducing correction factors for the pullulan calibration to better reflect the molar mass of celluloses. Such factors have to be determined beforehand by an absolute method, such as light scattering (Sjöholm et al. 2000). A set of pullulan-to-cellulose conversion factors is available from Berggren et al. (2003).
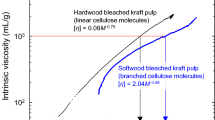
Viscosity detection and universal calibration
Another way to obtain information on the molar mass of celluloses by GPC is the use of a viscosity detector. The value of the intrinsic viscosity [η] for a polymer in a specific solvent is directly related to the molar mass of the polymer through the Kuhn–Mark–Houwink–Sakurada equation (KMHS, Eq. 6):
with K and α being constants which have to be determined from a double logarithmic plot of [η] versus Mw. These constants vary with solvent type, temperature, and type of polymer. They reflect the hydrodynamic interaction between polymer and solvent. In plots of log[η] × Mw versus retention time, data points from different polymers in the same solvent merge into a single curve (Gallot-Grubisic et al. 1967). This plot is called the universal calibration curve and can be used for both cellulose solutions and solutions of cellulose derivatives (such as cellulose tricarbanilates in THF), provided that the corresponding KMHS constants are available. Such sets of constants for polymers in solution can be obtained from literature (Ganster and Fink 1999); for solutions of cellulose in DMAc/LiCl the KMHS constants were reviewed by Bikova and Treimanis (2002). Further α and K values are listed in Mori and Barth (1999). However, caution has to be exercised when values for α and K from the literature are used. These values may be faulty, and it is advisable to double-check that the solvents and conditions match properly. The use of a viscosity detector also allows obtaining additional information on the shape of the molecule in solution, because the viscosimetric radius Rη can be derived from the intrinsic viscosity. The viscosimetric radius is reported to resemble the hydrodynamic value (RH) as determined by dynamic light scattering (Burchard 1999; Mourey 2004).
Possible pitfalls are that KMHS parameters have to be known for identical conditions. Literature data to apply the KMHS-equation might not be suitable for the equipment available in the respective laboratory. Thoughtless application of available parameters from literature and/or inappropriate molecules for calibration might lead to under/overestimation of the samples’ molar mass.
Determination of the refractive index increment (dn/dc)µ in DMAc/LiCl
It is evident that knowledge of the exact specific refractive index increment (dn/dc)µ is an essential prerequisite to precise measurement of the molar mass by light scattering. As the (dn/dc)µ parameter is also wavelength-dependent, its determination should be performed at the same wavelength as the actual GPC light scattering experiment, i.e., the light source of the RI detector should be a laser of the same wavelength as that used for the light scattering. In practice, tungsten lamps do the job just as well. There is a dependence on the molar mass, which comes into play especially at low molar masses below 2 × 103 g mol−1, and a dependence on the solute composition, which is important for copolymer analysis. In the analysis of cellulosic substrates, the latter might be significant for the analysis of non-uniformly substituted cellulose derivatives.
n = refractive index; no = refractive index of pure solvent; k = detector constant; U = output voltage of RI detector; c = concentration of the polymer.
The (dn/dc)µ value is the only constant required by a GPC-MALLS system in order to measure the molar mass in an absolute way, without calibration by standards. The (dn/dc)µ value is independent of concentration and molar mass at very dilute solutions and sufficiently high Mw (Mori and Barth 1999). At low Mw values (monomer to oligomers up to ~2000 g mol−1), (dn/dc)µ depends highly on Mw. In addition, (dn/dc)µ is required to measure the concentration of the sample with an RI detector (Eqs. 7 and 8).
The (dn/dc)µ value can be a measure of the polarisability of the respective molecule which influences the interaction with the electromagnetic waves of the laser and hence the light scattering intensity. The (dn/dc)µ value has a large effect on the final data obtained for the absolute values of the molar mass, as (dn/dc)µ is part of the Zimm equation where (dn/dc)µ2 is part of the denominator, e.g. with a (dn/dc)µ being 0.077 ml g−1 in 0.5 % LiCl/DMAc (Dupont and Harrison 2004) compared to 0.140 ml g−1 (Röhrling et al. 2003) in 0.9 % LiCl/DMAc, the final value for Mw will deviate to a large extent (compare Eqs. 9 and 10).
The refractive index increment depends on the solvent used and the laser wavelengths applied. However, there is a linear relationship between the laser wavelength and (dn/dc)µ in the same solvent. If the (dn/dc)µ values are known for two wavelengths in the same solvent, the third wavelength’s (dn/dc)µ can be estimated with sufficient accuracy. The (dn/dc)µ value also relies on a number of other parameters, such as temperature, solvent, pressure, and salt concentration in the case of salt-containing eluants. Some specific problems arise from the salt dependence in determining the (dn/dc)µ value for cellulose dissolved in DMAc/LiCl. In this specific system, the rather high salt concentration compared to the concentration of cellulose and the polyelectrolyte character of the net solvent system combine to render a straightforward offline measurement of (dn/dc)µ inapplicable for cellulose in DMAc/LiCl.
The change in the (dn/dc)µ value is much more pronounced for polymers dissolved in organic polymers as compared to polymers in water. For polysaccharides a (dn/dc)µ of 0.146 ml g−1 is more or less valid for all polysaccharides dissolved in aqueous solution including those containing different salt concentrations. For many synthetic polymers, the (dn/dc)µ constant can be found in the literature for a given solvent and given wavelength. When it comes to biopolymers, the situation is unfortunately quite different. Some data are available for cellulose derivatives in THF. However, incorrect (dn/dc)µ values have often been published, so values solely from the literature should never be relied on without corroboration. This is especially critical when it comes to the solvent system DMAc/LiCl. Here two different concentrations influence the (dn/dc)µ value, the concentration of the salt (LiCl) and the concentration of the cellulose which is varied in the determination of the (dn/dc)µ value. The concentration of the salt is usually in the range of 0.5–0.9 % and hence an order of magnitude larger compared to the concentration of the cellulose itself. The ordinary procedure to determine a (dn/dc)µ value would consist in the direct injection of different concentrations of the polymer (e.g. cellulose) in the respective solvent into the cell of the refractive index detector in order to obtain a plot of dV over dc in which the slope corresponds to (dn/dc)µ for a known RI-detector constant (this constant is measured with the same procedure for a compound with a known (dn/dc)µ, often saccharose, with 0.15009 ml g−1, see Fig. 13). For a thorough discussion on that topic refer to Kes and Christensen (2013) and references sited therein.
This offline procedure is only applicable, however, if the solvent does not contain any salt or if these differences are levelled out. If the solvent contains salt, a solvent gradient between the polymer and the surrounding solution is generated, even if the polymer is not a polyelectrolyte and hence not charged per se (Fig. 14). An indication of such a process occurring may be the fact that the slope of the dV over dc curve used to determine the (dn/dc)µ with the offline RI detection does not cross the dV axis close to zero (see right Graph in Fig. 13). To level the concentration gradient induced by the salt and the polymer, dialysis is normally applied. However, this is not possible or very tedious with DMAc/LiCl, as the cellulose solvent will dissolve the dialysis membranes as well. Beyond the high concentration of LiCl that has a large influence on the (dn/dc)µ value itself, the determination of (dn/dc)µ requires very precise concentrations of cellulose. Before cellulose can be dissolved in DMAc/LiCl, it has to go through an activation step comprising a solvent exchange, which significantly hampers accurate determination of the cellulose concentration. To overcome this problem, an easily-soluble cellulose, e.g. a sulfite dissolving pulp may be used after activation followed by freeze-drying. These pulps dissolve instantly and can be used directly as test substrates.
In order to overcome the dialysis problem, the column approach can be employed (Podzimek 2011); the levelling effect of the column material can be used in the same way that dialysis operates. The only prerequisite is the knowledge of the exact cellulose concentration in the solution injected. The approach noted above with freeze-dried cellulose may be applied to ensure a correct concentration in the solution. The major hurdle consists in knowing whether the injection system (e.g. autosampler or manual valve) actually meets the nominal setting. This should be tested in any case (e.g. by calibration with a UV- or fluorescence-active substance without columns). With a known RI detector constant and a reliable concentration of injected cellulose and assuming 100 % recovery after the columns, the (dn/dc)µ value can be determined with light scattering software. Experience with (dn/dc)µ determination by the column approach shows that the value obtained also depends on the age of the columns, obviously due to decreased recovery ratios of older columns.
Rights and permissions
About this article
Cite this article
Potthast, A., Radosta, S., Saake, B. et al. Comparison testing of methods for gel permeation chromatography of cellulose: coming closer to a standard protocol. Cellulose 22, 1591–1613 (2015). https://doi.org/10.1007/s10570-015-0586-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-015-0586-2