Abstract
Correctness and reliability of molar mass data by viscometry in organometallic solvents (cuen, cuoxam, cadoxen) are compromised by the alkalinity of these solvents which causes immediate depolymerization especially in the case of pulps with higher carbonyl content (oxidative damage). The viscosity values thus correspond to the molar mass after the beta-elimination reactions that underly these degradative processes, which is sometimes significantly smaller than the molar mass determined by gel permeation chromatography (GPC) in the non-degrading solvent system DMAc/LiCl. Despite this well-known drawback, viscosity measurements have become a standard approach for molar mass measurements due to their ease and fastness, especially in the pulp and paper industries. A potential way to reduce the inherent error of these molar mass determinations via viscosity measurements is a reductive treatment prior to dissolution of the pulp in the organometallic solvents, which converts the labile, alkali-sensitive carbonyl structures back to the respective alcohols. Using sodium borohydride (NaBH4) on different types of cellulosic pulps, we demonstrate the beneficial effects of such a reduction step on the determined degree of polymerization (DP) for all three common solvents: cuen, cuoxam and cadoxen. Molar mass distributions and profiles of carbonyl groups were determined by GPC and by carbonyl selective fluorescence labeling (“CCOA method”). Such a reductive treatment was especially valuable for hemicellulose-containing pulps. While the decreased measurement error according to the new protocol is beyond doubt, an immediate acceptance in the pulp and paper industries is at least questionable, because the new, more correct data would not agree with the old – wrong, but consistent – numbers accumulated over years and decades. In the long run, however, the new, improved protocol will prevail here as well due to its lower error rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the pulp and paper industry, viscometry is a key approach to characterization of the pulps´ molar mass. Alongside mechanical tests and ISO brightness determination, viscometry is almost ubiquitously used among pulp producers for quality control during manufacturing and to optimize production processes. The method is a fast, not overly demanding, robust technique, it gives consistent results if carried out in the same lab under constant conditions, and the output is easy to evaluate and interpret (Zellcheming 1957, 1960; ISO 1981 and 2010). The measured intrinsic viscosity value is easily transformed mathematically into the viscosity-derived degree of from the Ancient Greek roots of the word, hyphenation should be polymerization (but not polym - erization) (DPV) which serves as a sum parameter to characterize the molar mass of the pulps.
When considering cellulose pulps for viscometry-determination of their degree of polymerization (DPV), some aspects must be kept in mind. During pulping and bleaching, or even upon natural aging or storage, pulps inevitably undergo some oxidative modification due to the harsh processing conditions, oxidative chemicals involved or autoxidation/aging processes (Potthast et al. 2006, 2007). Therefore, cellulose carries oxidized functionalities along its backbone, aldehydes or carboxyl groups at C-6, ketone functions at C-2 and C-3, which in the case of commercial pulps range around 20–30 µmol/g or even up to 100 µmol/g in oxidatively stressed material (Potthast et al. 2005, 2007). To determine the intrinsic viscosity, from which DPv is derived, cellulose is required to be dissolved; for the use of DPv in polymer and cellulose science see Evans and Wallis (1989). Dissolution is usually achieved in one of three organometallic solvents, either the copper(II) complex with ethylenediamine (“cuen”) or with ammonia (“cuoxam”, Schweizer's reagent), or the cadmium(II) complex with ethylenediamine (“cadoxen”) (Olsson and Westman 2013; Saalwächter et al. 2000). All these solvents are strongly alkaline (pH > 10) and immediately trigger β-alkoxy elimination reactions that cleave the glycosidic bonds adjacent to any carbonyl group along the cellulose polymer (Ahn et al. 2019a, 2019b; Zaccaron et al. 2020). The eliminations become detectable by a molar mass loss starting at pH values around 8.5 (Haskins and Hogsed 1950; Hosoya et al. 2018); those starting from oxidized C-2 and C-3 are about 20 times faster at room temperature than those from C-6 (Hosoya et al. 2018), and are completed in less than a minute at pH 10 and room temperature. In principle, the rate of elimination is proportional to the concentration of hydroxyl ions (the catalyst) and strongly increasing with temperature.
Since the standard procedures for viscosity determination take 30 min (cuen) or 60 min (cuoxam, cadoxen), it is obvious that at the time of measurement, the β-elimination reactions have completed, and the measurement result reflects the state of the pulp after those chain scissions and not the state before. The effects are comparable for all three viscometry media. The viscosity of the shortened chain is determined, and the outcome is an underestimation of the DP, which gets more severe with increasing oxidative damage of the pulp, i.e., increasing content of carbonyl groups (Ahn et al. 2019a; Zaccaron et al. 2020).
The consequences are as simple as severe: the higher the oxidative damage of a pulp, i.e., its content of carbonyl groups, the more incorrect the molar mass data determined with viscosity. Viscometry reports the molar mass of the material after β-elimination and not that of the starting material before dissolution. The values obtained are thus inevitably underestimated to a greater or lesser extent. Nevertheless, the method became the standard in the paper industry. This was certainly supported by the fact that no equally simple and generally applicable alternative to molar mass determination was or is available. Molar mass determination by means of gel permeation chromatography in the standard solvent DMAc/LiCl, which is known to leave the celluloses molar mass unchanged also in the case of oxidatively pulps, (Potthast et al. 2015; Henniges et al. 2011a) is far more demanding in terms of equipment, maintenance, measurement time and interpretation and thus no real alternative to a pulp-and-paper quality control method that first of all should be fast and easy.
A possible solution comes from reduction strategies which would convert “harmful carbonyls” back to “innocuous hydroxyls”. Among several reduction agents described in the literature as possible options, metal/acid combinations (generating hydrogen in statu nascendi), amine-borane complexes and sodium borohydride (NaBH4) are the ones having found applications. Metal/acid couples cause hydrolytic depolymerization of polysaccharides, which is advantageously used in analytical cases where depolymerization is required, such as approaches to determine the carbohydrate composition by acidic hydrolysis or methanolysis, but is otherwise unwanted. Amine-borane complexes in polysaccharide reductions are limited to special cases, such as paper conservation (Sanna et al. 2009; Henniges and Potthast 2009), due to their more complicated handling, the danger of glycosidic bond cleavage in the presence of Lewis acids (Gray et al. 1995; Hosoya et al. 2014) and sometimes toxicity issues. Sodium borohydride is the most prominent reducing agent in general polysaccharide analysis and chemistry because of its convenient handling in aqueous systems, its many applications in synthetic organic chemistry and – last but not least – its low price. NaBH4 was used to quantify carbonyl groups in polysaccharides (Abdel-Akher et al. 1952; Ströle 1956; Tihlarik and Pašteka 1991), in routes towards functionalized celluloses and polysaccharides (Errokh et al. 2018; Larsson et al. 2014; Larsson and Wågberg 2016; Leguy et al. 2018) and also in paper conservation contexts (Santucci and Zappal 2001), although in the latter field its application seems to be limited to a rather narrow window of reactions conditions (Henniges et al. 2011b). Sodium borohydride has also been used quite commonly in alkaline extractions of polysaccharides to prevent alkaline peeling (Hartler and Svensson 1965; Finne et al. 1979; Wigell et al. 2007; Wang et al. 2015). It has also been used to decrease the tendency towards yellowing (brightness reversion) and chromophore formation of highly bleached pulps (Rosenau et al. 2004; Korntner et al. 2015) by preventing the re-formation of chromophores in carbonyl-based, aldol-type condensation reactions (Rosenau et al. 2007). From the viewpoint of reaction mechanism, the reduction of an aldehyde or keto groups by sodium borohydride (and hydride reagents in general) involves nucleophilic hydride transfer to carbonyl-carbon, forming an alkoxide intermediate which is immediately protonated to give the corresponding alcohol (primary alcohol from aldehydes, secondary alcohol from ketones). The overall reaction corresponds to a hydrogenation, formally an addition of hydrogen to the C = O double bond (Johnson and Rickborn 1970; Organikum 2001).
In the present paper we would like to point out how a simple and fast reduction step, which can be most comfortably integrated into current protocols as a heterogeneous treatment prior to dissolution, can significantly improve the quality of molar mass data obtained by conventional viscosity measurements in cuen, cuoxam or cadoxen. We also point out how the benefits of less error-prone results must be balanced with problems of data comparability regarding earlier measurements, especially in industrial practice and quality control.
Materials and methods
General. Chemicals from Sigma-Aldrich (Schnelldorf, Germany) were of the highest grade available and used without further purification. Distilled water was used for all aqueous solutions.
Pulps. Two main types of pulp were used for the optimization the experiments, cotton linters (CL) with following parameters: kappa number 0.11, brightness 82.0% ISO, viscosity [cuen] 407 mL/g, and a bleached hardwood (beech) sulfite pulp (HBSP) with a kappa number of 0.36, brightness 91.2% ISO, and viscosity [cuen] 565 mL/g.
Pulp oxidation. Oxidatively-modified cellulose pulps were prepared using either sodium hypochlorite (NaOCl) or hydrogen peroxide (H2O2) according to the optimized procedure reported previously (Ahn et al. 2019a) which builds on previous reports by Lewin and Epstein (1962) and Lewin and Ettinger (1969), respectively..
Table 1 shows the molar mass (weight-averaged molar mass, Mw) and carbonyl data of the standard pulps used for the optimization of the reduction step. The values “after reduction” refer to the results of the standard reduction procedure given below.
Standard protocol for pulp reduction prior to viscosity measurement
Air-dried pulp (1 g) was suspended in water under vigorous stirring for 10 min and filtered off through a Buchner funnel. The fibers were suspended in an aqueous solution of sodium borohydride (0.1 M) in Sørensen buffer (pH = 8, 0.8 M, 100 mL) at room temperature and stirred for 30 min. Care should be taken that fresh, non-hydrolyzed reductant is used and that the solution is freshly prepared and used immediately. The pulp was thoroughly washed with bidistilled water on a Buchner funnel three times (100 mL each), filtered off under reduced pressure and immediately used for dissolution in the viscometry solvent. The pulps were stored in sealed Schott bottles at -18°C until GPC analysis.
The Sørensen buffer pH 8 is prepared from 2 stock solutions: A: 118.76 g of disodium hydrogenphosphate dihydrate (Na2HPO4*2H2O) filled up to 1000 mL with distilled water, B: 90.78 g of potassium dihydrogenphosphate (KH2PO4) filled up to 1000 mL with distilled water. 97.0 mL of solution A are mixed with 3 mL of solution B to give the buffer solution pH = 8 (0.667 M). Alternatively, ready-to-use buffer solution is commercially widely available.
Dissolution of the pulps in viscometry solvents and subsequent regeneration. Three different solvents commonly used for viscosity of cellulose were used according to standard protocols, namely copper ethylenediamine (cuen, ISO 5351–1, 1981 and 2010), cupric ammonia hydroxide (cuoxam, Zellcheming 1957), and cadmium ethylenediamine hydroxide (cadoxen, Zellcheming 1960). The detailed dissolution and regeneration procedures were reported previously (Ahn et al. 2019a; Zaccaron et al. 2020). In short, the concentration of pulps in the dissolution media was 5 mg/mL, 8 mg/mL and 5 mg/mL (rel. to air-dried pulp) for cuen, cuoxam and cadoxen, respectively, and the dissolution times were 30 min, 30 min and 60 min, respectively. After that time, cellulose was regenerated in excess water containing acetic acid (2 vol%), using 10 mL per mL of viscometry solvent, by slow stirring for 15 min. It was washed with bidistilled water until neutral, filtered off and immediately used for GPC measurements (solvent exchange water – ethanol – DMAc followed by dissolution in the eluant) or stored in sealed Schott bottles at –18 °C.
GPC analysis. Carbazole-9-Carbonyl-Oxy-Amine (CCOA) labeling of the carbonyl groups was performed as described earlier (Röhrling et al. 2002a and 2002b, Potthast et al. 2003). Gel permeation chromatography in the eluant N,N-dimethylacetamide/LiCl (Chrapava et al. 2003, Henniges et al. 2011a and 2013) with multi angle laser light scattering, refractive index and fluorescence detector analysis (GPC/MALLS-RI-Fluo) was used for the molecular characterization of the dissolved pulps. Details on sample preparation, analytical setup and GPC/MALLS-RI-Fluo system and conditions were as previously described (Potthast et al. 2015, Ahn et al. 2019b). The measurements provided the molar mass distribution, from which the molar mass parameters like Mw can be retrieved, in addition to the Mw-related profiles of oxidized groups and the total carbonyl content. Samples to be compared were prepared and measured in the same batch. All data evaluation was performed with Chromeleon, Astra 4.73, and GRAMS/32 software. The standard deviations for carbonyl group contents and Mw are below 5%, calculated from standard pulp measurements (N > 500).
Results and discussion
Reductive treatments and viscosity determination in cuen / cuoxam / cadoxen
In studies with aged papers as well as naturally and artificially aged (oxidized) cellulosic model pulps, reduction procedures with sodium borohydride have proven effective to preserve cellulose integrity and increase the stability of cellulose chains in alkaline media (Henniges and Potthast 2009; Tang 1986; Wang et al. 2015). Such treatments of cellulose and polysaccharides with NaBH4 are usually carried out in alkaline aqueous media in order to improve the stability of the reagent (Henniges et al. 2011b). Increasing acidity (= decreasing pH) causes consumption of the reagent by the disproportionation reaction with protons from the solvent (H+ + H– H2) and evolution of hydrogen. At room temperature, the kinetic rate constant for the reduction is less than one order of magnitude smaller than those of β-elimination reactions so that the reduction can well compete with alkali-triggered β-elimination (Potthast et al. 2009; Hosoya et al. 2018) if the conditions (temperature, alkalinity, concentration) are properly chosen. In general, pronounced molar mass losses due to β-elimination upon NaBH4 treatment occurs only in the case of highly oxidized celluloses (> 80 µmol/g), while it is tolerably low for conventional pulps with low degrees of oxidation (> 30 µmol/g).
In order to address the inherent error in the standard pulp viscosity protocols, we explored the efficacy of such reductive treatments before dissolving the pulp in the organometallic solvents. If the oxidized groups, namely aldehydes and ketones, were returned to their “hydroxyl state” before β-elimination sets in, the main error would be eliminated and the state of the pulp without chain shortening would be correctly reported. For optimization we used two cellulosic substrates, cotton linters and a bleached beached sulfite pulp, which had already been the test specimens in previous viscosity measurement studies. Both the original pulps and their counterparts with different degree of oxidative modification were used (see Materials and Methods section). As also the oxidant used in these modifications can influence the alkali-lability of the pulps, e.g., by introducing different ratios of carbonyl vs. carboxyl groups (Ahn et al. 2019b), we used either H2O2 or HOCl as in previous studies.
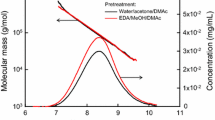
To determine the molar mass distribution of the pulps, gel permeation chromatography in the solvent/eluant DMAc/LiCl was employed which is known to be completely non-degrading if used according to the optimized protocol (Potthast et al. 2002; Chrapava et al. 2003; Potthast et al. 2015). In addition, molar mass-related profiles of carbonyl groups according to the CCOA method (Röhrling et al. 2002a, 2002b; Potthast et al. 2003) were used to directly monitor the dynamic changes in the carbonyl group contents. By dissolving the pulp directly in the GPC solvent, we can monitor the unchanged molar mass distributions of the pulps (black graphs in Figs. 1 and 2). Alternatively, the pulps were treated exactly as during viscometry measurements, i.e., dissolved and kept in solution for 30 min (cuen) or 60 min (cuoxam, cadoxen), then precipitated in water and dissolved in DMAc/LiCl for GPC measurement. Now, GPC shows the state of the pulp after all processes that might have occurred in the viscometry solvents (colored graphs in Figs. 1 and 2).
Effect of NaBH4 reduction on the molar mass distributions (left y-axes) and carbonyl profiles (DSCO, right y-axes) exemplified by a bleached (HOCl) hardwood sulfite pulp. Dissolution media: cuen (orange, CuEN), cadoxen (green, CdEN), cuoxam (purple, CuAM). Top left: sample directly dissolved in DMAc/LiCl (9%, w/v) and measured by GPC, without contact to the organometallic viscosity solvents
Effect of NaBH4 reduction on the molar mass distributions (left y-axes) and carbonyl profiles (DSCO, right y-axes) exemplified by a bleached (H2O2) hardwood sulfite pulp. Dissolution media: cuen (orange, CuEN), cadoxen (green, CdEN), cuoxam (purple, CuAM). Top left: sample directly dissolved in DMAc/LiCl (9%, w/v) and measured by GPC, without contact to the organometallic viscosity solvents
Figures 1 and 2 present clear proof that a reduction step prior to dissolution in the viscosity solvents is beneficial with regard to molar mass preservation. The experiments underlying these figures employed a bleached hardwood sulfite pulp (HBSP), into which oxidized functionalities were introduced beforehand, with oxidation by NaOCl in Fig. 1 (starting carbonyl content 52 µmol/g) and with H2O2 in Fig. 2 (starting carbonyl content 50 µmol/g). The reduction procedure itself is innocuous with regard to the molar mass distribution. The curves before and after reduction are nearly congruent (Fig. 1, black graphs). Under special circumstances, the reduction even has a beneficial effect on the direct dissolution in DMAC/LiCl which normally does not affect the molar mass distribution at all: in the special case of an H2O2-oxidized pulp, which contains many C-2/C-3 keto groups that are notoriously labile, the molar mass distribution is slightly shifted to higher values indicating the protective effect of the reduction (Fig. 2, black graph). Such minor degradative effects upon dissolution in DMAc/LiCl – like the one seen here, which is eliminated by the reduction treatment – usually occur in pulps with significantly increased contents of oxidized groups and/or with eluants that have not been especially purified and contain some traces of water and alkaline impurities (dissolved N,N-dimethylamine). This result suggests that for pulps with carbonyl contents greater than about 30 µmol/g, a reduction step can be considered even for direct GPC measurements in DMAc/LiCl. Besides the effect on the molar mass distribution, it was evident that the carbonyl groups were largely removed through the reduction, and the carbonyl contents were significantly reduced (from 52 to 14 µmol/g in Fig. 1 and from 50 to 12 µmol/g in Fig. 2, see also Table1). It should be noted that these carbonyl contents can be further lowered by excessively long reduction times, but these long times are not practical for standard protocols, and the very low contents that can be achieved are bought at the cost of molar mass losses caused by extended contact with the alkaline reduction medium.
The colored subgraph in Figs. 1 and 2, each containing three molar mass curves, refer to the viscosity solvents. The solid, grey distributions show the molar mass distribution without dissolution in the viscosity solvent, after direct dissolution in the GPC solvent. The solid lines give the molar mass distributions after dissolution in the viscosity solvent, and the dashed lines after reduction and subsequent dissolution in the viscosity solvent. As expected, the gray, colored distributions (no viscosity solvent) correspond to the highest molar mass and the solid lines (viscosity solvent, no reduction) to the lowest. The dashed lines lie between these two extremes, clearly showing that the molar mass loss caused by the viscosity solvents is diminished by the reduction treatment. The dashed curves are not congruent with the gray colored distributions (which would imply complete prevention of any degradation), but are reasonably close to them and lie at significantly higher molar mass values than the solid curves (without reduction treatment).
The changes in molar mass and carbonyl content of the HBSP pulp caused by the reduction process are summarized in Fig. 3. In all cases, the total amount of carbonyl groups, i.e., the “predetermined breaking points” of the cellulose chain under alkaline conditions, decreased significantly, and the effect of the subsequent alkaline dissolution types of—loss of molar mass—was thus considerably attenuated. For cotton linters, a similar positive effect of the sodium borohydride reduction step was observed (Fig. 4). There was no clear difference between the viscosity solvents, the NaBH4 reduction step was similarly effective for all three.
Reduction treatment (NaBH4) prior to pulp dissolution in viscosity solvents. Decrease of alkali-triggered molar weight loss (as differential weight-average molar mass, ∆Mw, left axis, columns) and carbonyl group content (C = O, right axis, dots) compared for two differently oxidized hardwood sulfite pulps and three viscosity solvents: cuen, cadoxen and cuoxam. Error bars: standard deviation 5% for C = O and 10% for ∆Mw
Reduction treatment (NaBH4) prior to pulp dissolution in viscosity solvents. Decrease of alkali-triggered molar weight loss (as differential weight-average molar mass, ∆Mw, left axis, columns) and carbonyl group content (C = O, right axis, dots) compared for two differently oxidized cotton linters samples and three viscosity solvents: cuen, cadoxen and cuoxam. Error bars: standard deviation 5% for C = O and 10% for ∆Mw
At the same time, it became evident that both type of the pulp and nature of oxidative damage had an influence on the reduction, which is, after all, carried out as a heterogeneous process. The influence of the pulp type is simply explained by the different accessibility and crystallinity of the pulps, as well as the influence of the pre-oxidation, since different oxidants have different selectivity (C-2, C-3, C-6) and cause different ratios between oxidized groups (keto/aldehyde vs. carboxylic acids). This is fully consistent with similar observations from preceding work on the effects of viscometry solvents on cellulose integrity (Ahn et al. 2019a, Zaccaron et al. 2020). The results also made clear that the reduction is not a panacea that can completely eliminate the negative effect of viscosity solvent alkalinity on cellulose integrity. The protective effect was obvious, but even with the additional reduction step, the molar masses measured in cuen/cuoxam/cadoxen were still lower than those determined directly (GPC in DMAc/LiCl). Thus, it is important to keep in mind that the reduction step is a significant improvement with respect error prevention, although viscosity determination still cannot compete with GPC in terms of maintaining cellulose integrity.
Optimization of the standard reduction protocol
The reduction protocol to be used as a step in the DP-determination by viscometry must meet certain requirements. Of course, it should reduce the molar mass loss due to the solvent-induced (alkali-induced) β-elimination reactions as much as possible. However, this must be reconciled with operational requirements in daily lab practice. Viscometric DP determination is a standard procedure in the pulp and paper industries. Any change or addition to the established protocol must be as short, easy and economic as possible to find acceptance. No complicated procedures, lengthy work-ups or harmful chemicals should be involved.
We have optimized the reduction procedure with respect to temperature, reagent concentration and ratio, pH of the aqueous medium and reaction time (Fig. 5). In order to make the measurements of different pulps comparable and allow consistent evaluation, the molar mass preservation without dissolution in the viscometry solvent (the minimum possible loss) was designated as 100% and the molar mass preservation in the viscometry solvent without reduction step was designated as 0% (the maximum “achievable” loss). The beneficial effect of the reduction then lies between 0 and 100% – the greater the number, the greater the molar mass-preserving effect of the reductive treatment. By taking these relative values, we could average the data over different pulps, similar to the later application of the procedure within viscosity measurements, which would also encompass all types of pulps samples.
The influence of the temperature (5 °C, 22 °C (r.t.), 40 °C) was very small. The results for 5 °C and 22 °C were the same, so that the additional effort to cool the mixture below room temperature did not seem justified. The reduction effect (decrease of carbonyl content) was somewhat higher at 40 °C, while the molar-mass-preserving effect – the decisive factor – was slightly lower than at the other two temperatures. At the same time, degradation of the reductant through reaction with protons from the medium became considerable at 40 °C so that this temperature was ruled out. The influence of the pH appeared to be large enough to justify the additional complication of using a pH buffer instead of water, in order to keep the alkali-induced degradation at bay. At pH 6, considerable degradation of the reductant occurred, evident from hydrogen evolution, but at the same time a very good conservation of Mw was achieved. At pH 7 and 8, both effects were slightly weaker. Thus, pH 8 was the best compromise. At pH 9 and above, very little degradation of NaBH4 occurred, but Mw loss increased greatly. The pH effects were tested with reductant concentrations up to 5 M (see below). In daily lab practice, pH can be adjusted very easily by a buffer system. Sørensen phosphate buffers are common, readily available (also as premixed solutions with different ionic strength / buffer capacity and easy to prepare even in labs not specially equipped for chemical work.
As for the borohydride concentration, the Mw was better preserved at lower concentrations than at higher concentrations, an effect that seemed paradoxical at first glance. However, it must be kept in mind that the reduction is a heterogeneous process. It is well-known from general cellulose chemistry that high concentrations of ionic reagents can be counterproductive in heterogeneous cellulose reactions. Ions are adsorbtively enriched at the surface and further access of other ions into the matrix and the pore system is hindered by electrostatic repulsion. Borohydride concentrations between 0.1 M and 1 M worked best, with the lower concentration of 0.1 M chosen to not overload the capacity of the buffer and to save chemicals. Stoichiometrically, the amount of carbonyl groups to be reduced is minute compared to the amount of reagent introduced, but the heterogeneous reaction impedes the process. Very low concentrations would mean extended reaction times, increasing the importance of the slower β-elimination reactions. A ratio of 1 g pulp per 100 mL of solvent was maintained throughout, which is also the usual proportion used in viscometry measurements. The Mw-preserving effect was nearly constant between reaction times of 30 min and 2 h. At longer times, the Mw loss increased. Shorter reaction times at higher reagent concentrations did not improve the outcome because the heterogeneous reaction is determined by accessibility (reagent diffusion). Therefore, a reaction time of 30 min was set.
As a result of optimization, the general reduction procedure was carried out using 0.1 M sodium borohydride in phosphate buffer pH = 8 for 30 min at r.t., which combines optimum Mw retention with maximum robustness and ease of use.
Conclusions
This study demonstrated that a reduction step with sodium borohydride is able to improve the results of viscometry measurements in cuen/cuoxam/cadoxen, which are a standard analytical procedure in the pulp and paper industries and cellulose-related research labs. The alkalinity of the viscometry solvents induces β-elimination reactions at carbonyl groups along the cellulose chain so that the determined molar lass values are underestimated. The higher the content of carbonyl groups, i.e., the oxidative damage of the pulp, the more severe the error. This negative effect of the solvents is mitigated by a preceding reduction step, which cannot completely eliminate the triggered degradation, but at least significantly suppress it. The reduction step lowers the amount of the pulps´ carbonyl groups, and as a consequence the stability of cellulose in these organometallic media was significantly enhanced, for all three viscosity solvents (cuen, cuoxam, and cadoxen) and all types of pulps tested. The resulting molar mass (DPV) values became much closer to those determined by direct GPC measurements, i.e., without the alkaline degradation effect. The parameters of the reduction were optimized, balancing the requirement of a fast and easy application with maximum preservation of the molecular integrity of the cellulose. The optimized reduction step can easily be implemented into the current viscometry protocols, as it has very little demands with regard to time requirements, costs, chemicals, complexity of the work flow and involvement of non-standard techniques, hazardous chemicals or safety-related issues.
Despite the undeniable advantages, it seems questionable, realistically speaking, whether the reduction step will quickly find widespread application in the pulp and paper industry, where viscometry has been used for years, following largely unchanged protocols. This means that the data collected based on it, while all subject to the inherent “β-elimination error”, are readily comparable with each other. The improved protocol with the reduction step now provides values that are closer to the true molar mass of the pulps, but they are no longer comparable with the earlier values. The question for manufacturers is therefore what is more important: the improved absolute values or maintaining the comparability with earlier measurement series. It is thus to be expected that the modified protocol will be adopted rather slowly in industrial practice, while a rapid introduction is to be anticipated in research labs.
Both in research laboratories as well as in the industrial practice a reduction treatment prior viscosity-DP determination of pulps in any organometallic solvent is generally advisable to improve the reliability of the molecular mass information gained.
Data availability
Data is available from the authors upon reasonable request.
Code availability
Not applicable.
References
Abdel-Akher M, Hamilton JK, Montgomery R, Smith F (1952) A new procedure for the determination of the fine structure of polysaccharides. J Am Chem Soc 74(19):4970–4971
Ahn K, Zaccaron S, Rosenau T, Potthast A (2019a) How Alkaline solvents in viscosity measurements affect data for oxidatively damaged celluloses: cupri-ethylenediamine. Biomacromol 20(11):4117–4125
Ahn K, Zaccaron S, Zwirchmayr NS, Hettegger H, Hofinger H, Bacher M, Henniges U, Hosoya T, Potthast A, Rosenau T (2019b) Yellowing and brightness reversion of celluloses: CO or COOH, who is the culprit? Cellulose 26:429–444
Chrapava S, Touraud D, Rosenau T, Potthast A, Kunz W (2003) The investigation of the influence of water and temperature on the LiCl/DMAc/cellulose system. Phys Chem Chem Phys 5:1842–1847
Errokh A, Magnin A, Putaux JL, Boufi S (2018) Morphology of the nanocellulose produced by periodate oxidation and reductive treatment of cellulose fibers. Cellulose 25(7):3899–3911
Evans R, Wallis AFA (1989) Cellulose molecular-weights determined by viscometry. J Appl Polym Sci 37:2331–2340
Finne J, Krusius T, Margolis RK, Margolis RU (1979) Novel mannitol-containing oligosaccharides obtained by mild alkaline borohydride treatment of a chondroitin sulfate proteoglycan from brain. J Biol Chem 254(20):10295–10300
Gray GR, In HO (1995) Reductive cleavage of glycosides with borane complexes in the presence of boron trifluoride etherate. Carbohydr Res 278(2):329–338
Hartler N, Svensson IL (1965) Alkali stability of some uronic acids and its implications in borohydride and polysulfide cooking. I&EC Chem Prod Res Develop 4(2):80–82
Haskins J, Hogsed M (1950) The alkaline oxidation of cellulose. I. Mechanism of the degradative oxidation of cellulose by hydrogen peroxide in presence of alkali. J Org Chem 15(6):1264–1274
Henniges U, Potthast A (2009) Bleaching revisited: Impact of oxidative and reductive bleaching treatments on cellulose and paper. Restaurator 30(4):294–320
Henniges U, Kostic M, Borgards A, Rosenau T, Potthast A (2011a) Dissolution behaviour of different celluloses. Biomacromol 12(4):871–879
Henniges U, Bjerregaard L, Ludwig B, Potthast A (2011b) Controversial influence of aqueous treatments on historic textiles. Polym Degrad Stab 96(4):588–594
Henniges U, Hasani M, Potthast A, Westman G, Rosenau T (2013) Electron beam irradiation of cellulosic materials-opportunities and limitations. Materials 6(5):1584–1598
Hosoya T, Takano T, Kosma P, Rosenau T (2014) Theoretical evidence for the presence of oxacarbenium ions in chemical glycoside synthesis. J Org Chem 79(17):7889–7894
Hosoya T, Bacher M, Potthast A, Elder T, Rosenau T (2018) Insights into degradation pathways of oxidized anhydroglucose units in cellulose by beta-alkoxy-elimination – a combined theoretical and experimental approach. Cellulose 25(7):3797–3814
ISO5351–1 (1981 and 2010). Cellulose in dilute solutions. Determination of limiting viscosity number. Part 1: Method in cupri-ethylene-diamine (CED) solution.
Johnson MR, Rickborn B (1970) Sodium borohydride reduction of conjugated aldehydes and ketones. J Org Chem 35(4):1041–1045
Korntner P, Hosoya T, Dietz T, Eibinger K, Reiter H, Spitzbart M, Röder T, Borgards A, Kreiner W, Mahler AK, Winter H, French AD, Henniges U, Potthast A, Rosenau T (2015) Chromophores in lignin-free cellulosic materials belong to three compound classes, chromophores in cellulosics. XII. Cellulose 22(2):1053–1062
Larsson PA, Wågberg L (2016) Towards natural-fibre-based thermoplastic films produced by conventional papermaking. Green Chem 18(11):3324–3333
Larsson PA, Berglund LA, Wågberg L (2014) Ductile all-cellulose nanocomposite films fabricated from core–shell structured cellulose nanofibrils. Biomacromol 15(6):2218–2223
Leguy J, Diallo A, Putaux JL, Nishiyama Y, Heux L, Jean B (2018) Periodate oxidation followed by NaBH4 reduction converts microfibrillated cellulose into sterically stabilized neutral cellulose nanocrystal suspensions. Langmuir 34(37):11066–11075
Lewin M, Epstein JA (1962) Functional groups and degradation of cotton oxidized by hypochlorite. J Polym Sci 58(166):1023–1037
Lewin M, Ettinger A (1969) Oxidation of cellulose by hydrogen peroxide. Cellulose Chem Technol 3(1):9–20
Organikum, Author collective (2001) 21st ed. Wiley-VCH, Weinheim, New York. ISBN 3-527-29985-8, pp.568.
Potthast A, Rosenau T, Sartori J, Sixta H, Kosma P (2002) Hydrolytic processes and condensation reactions in the cellulose solvent system N, N-dimethylacetamide/lithium chloride. Part 2: degradation of cellulose. Polymer 44(1):7–17
Potthast A, Röhrling J, Rosenau T, Borgards A, Sixta H, Kosma P (2003) A novel method for the determination of carbonyl groups in cellulosics by fluorescence labelling. 3. Monitoring oxidative processes. Biomacromolecules 4(3):743–749
Potthast A, Rosenau T, Kosma P, Saariaho AM, Vuorinen T, Sixta H (2005) On the nature of carbonyl groups in cellulosic pulps. Cellulose 12(1):43–50
Potthast A, Rosenau T, Kosma P (2006) Analysis of oxidized functionalities in cellulose. In: Klemm D (ed) Polysaccharides II. Springer, Heidelberg, pp 1–48
Potthast A, Rosenau T, Kosma P (2007) Carbonyl and carboxyl profiles as two novel parameters in advanced cellulose analytics. In: Argyropoulos D (ed) Materials, chemicals and energy from forest biomass materials, chemicals and energy from forest biomass. American Chemical Society, Washington, pp 513–530
Potthast A, Schiehser S, Rosenau T, Kostic M (2009) Oxidative modifications of cellulose in the periodate system–reduction and beta-elimination reactions. Holzforschung 63(1):12–17
Potthast A, Rosenau T, Henniges U, Schiehser S, Kosma P, Saake B, Lebioda S, Radosta S, Vorwerg W, Wetzel H, Koschella A, Heinze T, Strobin G, Sixta H, Strlic M, Isogai A (2015) Comparison testing of methods for gel permeation chromatography of cellulose: coming closer to a standard protocol. Cellulose 22(3):1591–1613
Röhrling J, Potthast A, Rosenau T, Lange T, Borgards A, Sixta H, Kosma P (2002) A novel method for the determination of carbonyl groups in cellulosics by fluorescence labelling. 2. Validation and applications. Biomacromolecules 3(5):969–975
Röhrling J, Potthast A, Rosenau T, Lange T, Ebner G, Sixta H, Kosma P (2002) A novel method for the determination of carbonyl groups in cellulosics by fluorescence labelling. 1. Method development. Biomacromolecules 3(5):959–968
Rosenau T, Potthast A, Milacher W, Hofinger A, Kosma P (2004) Isolation and identification of residual chromophores in cellulosic materials. Polymer 45(19):6437–6443
Rosenau T, Potthast A, Kosma P, Suess HU, Nimmerfroh N (2007) First isolation and identification of residual chromophores from aged bleached pulp samples. Holzforschung 61(6):656–661
Sanna C, Sodo A, Laguzzi G, Mancini G, Bicchieri M (2009) Tert-butyl amine borane complex: an unusual application of a reducing agent on model molecules of cellulose based materials. J Cult Heritage 10(3):356–361
Santucci L, Zappal M (2001) Cellulose viscometric oxidometry, restaurator. Int J Preserv Library Archiv Mat 22(1):51–65
Ströle VU (1956) Bestimmung der carbonylgruppen in oxydierter cellulose. Makromolekulare Chemie: Macromol Chem Phys 20(1):19–36
Tang LC (1986) Stabilization of paper through sodium borohydride treatment. In: Needles HL, Zeronian SH (eds) Historic textile and paper materials. Advances in Chemistry Series 212. American Chemical Society, Washington, pp 427–41
Tihlarik K, Pašteka M (1991) Determination of the carbonyl groups in oxidized starches by sodium borohydride. Starch 43(3):83–85
Wang Y, Azhar S, Lindström ME, Henriksson G (2015) Stabilization of polysaccharides during alkaline pre-treatment of wood combined with enzyme-supported extractions in a biorefinery. J Wood Chem Technol 35(2):91–101
Wigell A, Brelid H, Theliander H (2007) Degradation/dissolution of softwood hemicellulose during alkaline cooking at different temperatures and alkali concentrations. Nordic Pulp Paper Res J 22(4):488–494
Zaccaron S, Henniges U, Potthast A, Rosenau T (2020) How Alkaline solvents in viscosity measurements affect data for oxidatively damaged cellulose. Cuoxam and Cadoxen Carbohydr Polym 240:116251–116259
Zellcheming (1957) Merkblatt IV/30/62: Schnellbestimmung der Kupferviskosität von Zellstof-fen (Betriebsmethode)
Zellcheming (1960) Merkblatt IV/52/71: Prüfung von Zellstoff und Papier - Auflösung in Cadoxen
Acknowledgments
Lenzing AG, Sappi, Mondi AG and Zellstoff Pöls AG are gratefully acknowledged for donation of cellulosic pulps and interesting discussions. We would like to thank the Austrian research promotion agency (FFG) for financially supporting this work in the framework of the project series “Chromophores” (Projects #847169, 855644, and 861863). The financial support by the Austrian Biorefinery Center Tulln (ABCT) is gratefully acknowledged.
Funding
Open access funding provided by University of Natural Resources and Life Sciences Vienna (BOKU).
Author information
Authors and Affiliations
Contributions
SZ, AP and TR contributed to the study conception and design. Material preparation, data collection and analysis were performed by all authors. The first draft of the manuscript was written by SZ and TR and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zaccaron, S., Ahn, K., Henniges, U. et al. An improved, less erroneous protocol for the classical “cuen”, “cuoxam” or “cadoxen” viscosity measurements of pulps. Cellulose 29, 3733–3744 (2022). https://doi.org/10.1007/s10570-022-04505-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-022-04505-w