Abstract
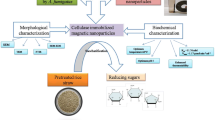
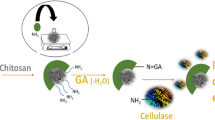
Production of bioethanol from various lignocellulosic biomass through enzymatic hydrolysis is considered as a promising approach to fulfill the global energy demand. In addition to overcoming the worldwide energy crisis, it also plays an important role in the management of lignocellulosic waste. We have synthesized iron oxide nanoparticles (magnetic nanoparticles—MNPs) using cell filtrate of fungus, Alternaria alternata. The synthesis of MNPs was initially confirmed by the visual observation followed by characterization using different analytical techniques. The NTA and TEM analysis showed the average size of 47 and 55 nm respectively. XRD analysis confirmed the FCC structure of nanoparticles and zeta potential for MNPs was − 7.06 mV, which indicated the stability of nanoparticles. Further, comparative evaluation of enzymatic hydrolysis of lignocellulosic biomass (sugarcane bagasse) using free and immobilized cellulase on MNPs at different temperatures was studied. The results obtained demonstrated that, in first cycle of hydrolysis, free enzyme was more efficient which showed about 78% conversion of cellulose to glucose at 40 °C after 24 h, whereas in case of immobilized enzyme it was found to be 72%. Moreover, immobilized cellulase was recovered by applying magnetic field and reused up to third cycle of hydrolysis. In second and third cycle, rate of conversion of cellulose to glucose was found to be 68 and 52% respectively. These findings suggest that immobilization of cellulase on MNPs facilitate the easy recovery and their reuse for more than one cycle of hydrolysis, thereby making the process economically viable. Further, optimization and modification of certain conditions will be helpful to increase the efficiency of immobilized enzyme.







Similar content being viewed by others
References
Abraham RE, Verma ML, Barrow CJ, Puri M (2014) Suitability of magnetic nanoparticles immobilized cellulases in enhancing enzymatic saccharification of pretreated hemp biomass. Biotechnol Biofuels 7:90. doi:10.1186/1754-6834-7-90
Ahmad R, Sardar M (2014) Immobilization of cellulase on TiO2 nanoparticles by physical and covalent methods: a comparative study. Indian J Biochem Biophys 51:314–320
Ahmad R, Sardar M (2015) Enzyme immobilization: an overview on nanoparticles as immobilization matrix. Biochem Anal Biochem 4:178. doi:10.4172/2161-1009.1000178
Ahmed M, Douek M (2013) The role of magnetic nanoparticles in the localization and treatment of breast cancer. Biomed Res Int. doi:10.1155/2013/281230 (2013: Article ID 281230)
Alftren J, Hobley TJ (2013) Covalent immobilization of β-glucosidase on magnetic particles for lignocellulose hydrolysis. Appl Biochem Biotechnol 169:2076–2087
Anwar Z, Gulfraz M, Irshad M (2014) Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: a brief review. J Rad Res Appl Sci 7(2):163–173
Asgher M, Ahmad Z, Iqbal HMN (2013) Alkali and enzymatic delignification of sugarcane bagasse to expose cellulose polymers for saccharification and bio-ethanol production. Ind Crops Prod 44:488–495
Asgher M, Bashir F, Iqbal HMN (2014a) A comprehensive ligninolytic pre-treatment approach from lignocellulose green biotechnology to produce bio-ethanol. Chem Eng Res Design 92(8):1571–1578
Asgher M, Shahid M, Kamal S, Iqbal HMN (2014b) Recent trends and valorization of immobilization strategies and ligninolytic enzymes by industrial biotechnology. J Mol Catal B: Enzymatic 101:56–66
Azhar SJM, Abdulla R, Jambo SA, Marbawi H, Gansau JA, Faik AAM, Rodrigues KF (2017) Yeasts in sustainable bioethanol production: a review. Biochem Biophys Reports 10:52–61
Balamurughan MG, Mohanraj S, Kodhaiyolii S, Pugalenthi V (2014) Ocimum sanctum leaf extract mediated green synthesis of iron oxide nanoparticles: spectroscopic and microscopic studies. J Chem Pharma Sci 4:201–204
Bhargava A, Jain N, Barathi LM, Akhtar MS, Yeoung-Sang Y, Panwar J (2013) Synthesis, characterization and mechanistic insights of mycogenic iron oxide nanoparticles. J Nanopart Res 15:2031. doi:10.1007/s11051-013-2031-5
Bilal M, Asgher M, Iqbal HMN, Hu H, Zhang X (2017) Biotransformation of lignocellulosic materials into value-added products: a review. Int J Biol Macromol 98:447–458
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Cherian E, Dharmendirakumar M, Baskar G (2015) Immobilization of cellulase onto MnO2 nanoparticles for bioethanol production by enhanced hydrolysis of agricultural waste. Chin J Catal 36:1223–1229
Cutzu R, Bardi L (2017) Production of bioethanol from agricultural wastes using residual thermal energy of a cogeneration plant in the distillation phase. Fermentation 3:24. doi:10.3390/fermentation3020024
de Barros EM, Carvalho VM, Rodrigues THS, Rocha MVP, Gonçalves LRB (2017) Comparison of strategies for the simultaneous saccharification and fermentation of cashew apple bagasse using a thermotolerant Kluyveromyces marxianus to enhance cellulosic ethanol production. Chem Eng J 307:939–947
Dussan KJ, Silva DDV, Moraes EJC, Arruda PV, Felipe MGA (2014) Dilute-acid hydrolysis of cellulose to glucose from sugarcane bagasse. Chem Eng Trans 38:433–438
Gade A, Gaikwad S, Duran N, Rai M (2014) Green synthesis of silver nanoparticles by Phoma glomerata. Micron 59:52–59
Gaikwad S, Ingle A, Gade A, Rai M, Falanga A, Incornato N, Russo L, Galdiero S, Galdiero M (2013) Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int J Nanomed 8:4303–4314
Goh WJ, Makam VS, Hu J, Kang L, Zheng M, Yoong SL, Udalagama CN, Pastorin G (2012) Iron oxide filled magnetic carbon nanotube-enzyme conjugates for recycling of amyloglucosidase: toward useful applications in biofuel production process. Langmuir 28(49):16864–16873
Gokhale AA, Lu J, Lee I (2013) Immobilization of cellulase on magneto responsive graphene nano-supports. J Mol Catal B: Enzymatic 90:76–86
Guo R, Ding M, Zhang SL, Xu GJ, Zhao FK (2008) Molecular cloning and characterization of two novel cellulase genes from the mollusc Ampullaria crossean. J Comp Physiol B 178:209–215
Hartmann M, Kostrov X (2013) Immobilization of enzymes on porous silicas: benefits and challenges. Chem Soc Rev 42:6277–6289
Husain Q (2017) Nanomaterials as novel supports for the immobilization of amylolytic enzymes and their applications: a review. Biocatalysis 3:37–53
Iqbal HMN, Kyazze G, Keshavarz T (2013) Advances in the valorization of lignocellulosic materials by biotechnology: an overview. Bioresources 8(2):3157–3176
Jonsson LJ, Martin C (2016) Pretreatment of lignocellulose: formation of inhibitory by-products and strategies for minimizing their effects. Bioresour Technol 199:103–112
Kumar AK, Sharma S (2017) Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour Bioprocess 4(1):7. doi:10.1186/s40643-017-0137-9
Lavarack BP, Griffin GJ, Rodman D (2002) The acid hydrolysis of sugarcane bagasse hemicellulose to produce xylose, arabinose, glucose and other products. Biomass Bioenerg 23:367–380
Li C, Jiang S, Zhao X, Liang H (2017) Co-immobilization of enzymes and magnetic nanoparticles by metal-nucleotide hydrogel nanofibers for improving stability and recycling. Molecules 22:179. doi:10.3390/molecules22010179
Miao X, Pi L, Fang L, Wu R, Xiong C (2016) Application and characterization of magnetic chitosan microspheres for enhanced immobilization of cellulase. Biocatal Biotransform 34(6):272–282
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
Misson M, Zhang H, Jin B (2015) Nano-biocatalyst advancements and bioprocessing applications. J R Soc Interface. doi:10.1098/rsif.2014.0891
Rai M, dos Santos JC, Soler MF, Marcelino PRF, Brumano LP, Ingle AP, Gaikwad S, Gade A, da Silva SS (2016a) Strategic role of nanotechnology for production of bioethanol and biodiesel. Nanotechnol Rev 5(2):231–250
Rai M, Ingle AP, Gaikwad S, Dussán KJ, Silvério da Silva S (2016b) Role of nanoparticles in enzymatic hydrolysis of lignocellulose in ethanol. In: Rai M, Silvério da Silva S (eds) Nanotechnology for bioenergy and biofuel production. Springer International Publishing, Basel, pp 153–171
Raja M, Pearlin (2015) Green synthesis of iron nanoparticles and investigation of their effect on degradation of dyes. J. Biol Inform Sci 4(2):6–8
Rezende CA, de-Lima MA, Maziero P, de Azevedo ER, Garcia W, Polikarpov I (2011) Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility. Biotechnol Biofuels 4:54. doi:10.1186/1754-6834-4-54
Rorke DCS, Kana EBG (2017) Kinetics of bioethanol production from waste sorghum leaves using Saccharomyces cerevisiae BY4743. Fermentation 3:19. doi:10.3390/fermentation3020019
Sánchez-Ramírez J, Martínez-Hernández JL, Segura-Ceniceros P, López G, Saade H, Medina-Morales MA, Ramos-González R, Aguilar CN, Ilyina A (2017) Cellulases immobilization on chitosan-coated magnetic nanoparticles: application for Agave Atrovirens lignocellulosic biomass hydrolysis. Bioprocess Biosyst Eng 40(1):9–22
Smuleac V, Varma R, Sikdar S, Bhattacharyya D (2011) Green synthesis of Fe and Fe/Pd bimetallic nanoparticles in membranes for reductive degradation of chlorinated organics. J Membr Sci 379(1–2):131–137
Tharani K, Nehru LC (2015) Synthesis and characterization of iron oxide nanoparticle by precipitation method. Int J Adv Res Phys Sci 2:47–50
Tiwari N, Pandit R, Gaikwad S, Gade A, Rai M (2017) Biosynthesis of zinc oxide nanoparticles by petals extract of Rosa indica L., its formulation as nail paint and evaluation of antifungal activity against fungi causing onychomycosis. IET Nanobiotechnol 11(2):205–211
Verma ML, Barrow CJ, Puri M (2013) Nanobiotechnology as a novel paradigm for enzyme immobilization and stabilization with potential applications in biodiesel production. Appl Microbiol Biotechnol 97:23–39
Wang YX, Dong MJ, Zhuang WC (2012) Enzymatic saccharification of cellulose pretreated from lignocellulosic biomass: status and prospect. Adv Mat Res 446:2809–2814
Xu J, Ju C, Sheng J, Wang F, Zhang Q, Sun G, Sun M (2013) Synthesis and characterization of magnetic nanoparticles and its application in lipase immobilization. Korean Chem Soc 34(8):2408–2412
Yang B, Wyman C (2008) Pretreatment: the key to unlocking low-cost cellulosic ethanol. Biofuels Bioprod Biorefin 2(2):26–40
Acknowledgments
Mahendra Rai and Silvio Silvério da Silva thankfully acknowledge the financial help rendered by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-Brazil, Process No. 449609/2014-6). Raksha Pandit is grateful to the Department of Science and Technology, Government of India for award of INSPIRE fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ingle, A.P., Rathod, J., Pandit, R. et al. Comparative evaluation of free and immobilized cellulase for enzymatic hydrolysis of lignocellulosic biomass for sustainable bioethanol production. Cellulose 24, 5529–5540 (2017). https://doi.org/10.1007/s10570-017-1517-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-017-1517-1