Abstract
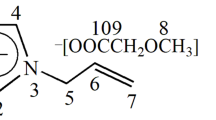
The amphiphilicity of solvent systems is realized for adjusting the dissolution of natural cellulose by making use of tetra-butylammonium hydroxide (TBAH) as an example. TBAH aqueous solution is found to have an obvious effect on adjusting its amphiphilicity, along with a flexible concentration ranging from 40 to 60 wt% for dissolving cellulose. With a suitable amphiphilic property, cellulose can be dissolved by a TBAH aqueous system . The experimental results demonstrate that with the introduction of urea (more than 0.2:1, w:v) into a TBAH aqueous system, the dissolution process of cellulose can be dramatically promoted, leading to a transparent solution of cellulose. Herein, a complex solvent of TBAH/urea has been proposed for mild and effective dissolution of cellulose under ambient conditions. In the TBAH/urea complex solvent, the structure of the hybrid hydrate of TBAH and urea formed. Urea served as a hydrophobic contributor adjusting the amphiphilicity of the solvent system, allowing interfacial resistance between the amphiphilic crystal surfaces of the natural cellulose and solvent to be reduced. After that, the crystal of natural cellulose could be fully infiltrated and subsequently dissolved by the TBAH/urea aqueous solvent. The performances of the aqueous solvent and ambient temperature dissolution make aqueous TBAH/urea a potential and green solvent of cellulose for broad applications, such as composites, films or wet spinning of cellulose, in laboratories or industries.







Similar content being viewed by others
References
Abe M, Fukaya Y, Ohno H (2012) Fast and facile dissolution of cellulose with tetrabutylphosphonium hydroxide containing 40 wt% water. Chem Commun 48:1808–1810. doi:10.1039/c2cc16203b
Abe M, Yamada T, Ohno H (2014) Dissolution of wet wood biomass without heating. RSC Adv 4:17136–17140. doi:10.1039/c4ra01038h
Abe M, Kuroda K, Ohno H (2015a) Maintenance-free cellulose solvents based on onium hydroxides. ACS Sust Chem Eng 3:1771–1776. doi:10.1021/acssuschemeng.5b00303
Abe M, Yamanaka S, Yamada H, Yamada T, Ohno H (2015b) Almost complete dissolution of woody biomass with tetra-n-butylphosphonium hydroxide aqueous solution at 60 C. Green Chem 17:4432–4438. doi:10.1039/c5gc00646e
Alves L, Medronho B, Antunes FE, Topgaard D, Lindman B (2015a) Dissolution state of cellulose in aqueous systems. 1. Alkaline solvents. Cellulose 23:247–258. doi:10.1007/s10570-015-0809-6
Alves L, Medronho BF, Antunes FE, Romano A, Miguel MG, Lindman B (2015b) On the role of hydrophobic interactions in cellulose dissolution and regeneration: colloidal aggregates and molecular solutions. Colloids Surf A 483:257–263. doi:10.1016/j.colsurfa.2015.03.011
Bao Y, Qian H-j Lu, Z-y Cui S (2015) Revealing the hydrophobicity of natural cellulose by single-molecule experiments. Macromolecules 48:3685–3690. doi:10.1021/acs.macromol.5b00260
Bergenstrahle M, Wohlert J, Himmel ME, Brady JW (2010) Simulation studies of the insolubility of cellulose. Carbohydr Res 345:2060–2066. doi:10.1016/j.carres.2010.06.017
Bergenstråhle-Wohlert M, Berglund LA, Brady JW, Larsson PT, Westlund P-O, Wohlert J (2012) Concentration enrichment of urea at cellulose surfaces: results from molecular dynamics simulations and NMR spectroscopy. Cellulose 19:1–12. doi:10.1007/s10570-011-9616-x
Biermann O, Hadicke E, Koltzenburg S, Muller-Plathe F (2001) Hydrophilicity and lipophilicity of cellulose crystal surfaces. Angew Chem Int Ed 40:3822–3825
Cai J, Zhang L (2005) Rapid dissolution of cellulose in LiOH/urea and NaOH/urea aqueous solutions. Macromol Biosci 5:539–548. doi:10.1002/mabi.200400222
Cai J et al (2008) Dynamic self-assembly induced rapid dissolution of cellulose at low temperatures. Macromolecules 41:9345–9351
Casarano R, El Seoud OA (2013) Successful application of an ionic liquid carrying the fluoride counter-ion in biomacromolecular chemistry: microwave-assisted acylation of cellulose in the presence of 1-allyl-3-methylimidazolium fluoride/DMSO mixtures. Macromol Biosci 13:191–202. doi:10.1002/mabi.201200296
Chanzy H, Dube M (1979) Crystallization of cellulose with N-methylmorpholine N-oxide: a new method of texturing cellulose. J Polym Sci Polym Lett Ed 17:219–226
Chrapava S, Touraud D, Rosenau T, Potthast A, Kunz W (2003) The investigation of the influence of water and temperature on the LiCl/DMAc/cellulose system. Phys Chem Chem Phys 5:1842–1847. doi:10.1039/b212665f
Chu ASGJ-W (2010) On the molecular origins of biomass recalcitrance: the interaction network and solvation structures of cellulose microfibrils. J Phys Chem B 114:13333–13341
Cuculo JA, Smith CB, Sangwatanaroj U, Stejskal EO, Sankar SS (1994a) A Study on the mechanism of dissolution of the cellulose/NH3/NH, SCN system. I. J Polym Sci Part A: Polym Chem 32:229–239
Cuculo JA, Smith CB, Sangwatanaroj U, Stejskal EO, Sankar SS (1994b) A study on the mechanism of dissolution of the cellulose/NH3/NH, SCN system. II. J Polym Sci Part A: Polym Chem 32:241–247
Dupont AL (2003) Cellulose in lithium chloride/N, N-dimethylacetamide, optimisation of a dissolution method using paper substrates and stability of the solutions. Polymer 44:4117–4126. doi:10.1016/s0032-3861(03)00398-7
Egal M, Budtova T, Navard P (2008) The dissolution of microcrystalline cellulose in sodium hydroxide-urea aqueous solutions. Cellulose 15:361–370. doi:10.1007/s10570-007-9185-1
Ema T, Komiyama T, Sunami S, Sakai T (2014) Synergistic effect of quaternary ammonium hydroxide and crown ether on the rapid and clear dissolution of cellulose at room temperature. RSC Adv 4:2523–2525. doi:10.1039/c3ra45888a
Fang Y, Duan B, Lu A, Liu M, Liu H, Xu X, Zhang L (2015) Intermolecular interaction and the extended wormlike chain conformation of chitin in NaOH/Urea aqueous solution. Biomacromolecules 16:1410–1417. doi:10.1021/acs.biomac.5b00195
Fink H-P, Weigel P, Purz HJ, Ganster J (2001) Structure formation of regenerated cellulose materials from NMMO-solutions. Prog Polym Sci 26:1473–1524
Glasser WG et al (2012) About the structure of cellulose: debating the Lindman hypothesis. Cellulose 19:589–598. doi:10.1007/s10570-012-9691-7
Hyvakko U, King AWT, Kilpelainen I (2014) Extraction of wheat straw with aqueous Tetra-n-butylphosphonium hydroxide. Bioresources 9:1565–1577
Isobe N, Noguchi K, Nishiyama Y, Kimura S, Wada M, Kuga S (2012) Role of urea in alkaline dissolution of cellulose. Cellulose 20:97–103. doi:10.1007/s10570-012-9800-7
Keijsers ER, Yilmaz G, van Dam JE (2013) The cellulose resource matrix. Carbohydr Polym 93:9–21. doi:10.1016/j.carbpol.2012.08.110
Klemm D, Heublein B, Fink H-P, Bohn A (2005) Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Int Ed 44:3358–3393. doi:10.1002/anie.200460587
Lau BBY et al (2015) Facile, room-temperature pre-treatment of rice husks with tetrabutylphosphonium hydroxide: enhanced enzymatic and acid hydrolysis yields. Bioresour Technol 197:252–259. doi:10.1016/j.biortech.2015.08.056
Li R, Wang S, Lu A, Zhang L (2015) Dissolution of cellulose from different sources in an NaOH/urea aqueous system at low temperature. Cellulose 22:339–349. doi:10.1007/s10570-014-0542-6
Lindman B, Medronho B (2015) The subtleties of dissolution and regeneration of cellulose: breaking and making hydrogen bonds. Bioresources 10:3811–3814
Lindman B, Karlström G, Stigsson L (2010) On the mechanism of dissolution of cellulose. J Mol Liq 156:76–81. doi:10.1016/j.molliq.2010.04.016
Lue A, Zhang L, Ruan D (2007) Inclusion complex formation of cellulose in NaOH–thiourea aqueous system at low temperature. Macromol Chem Phys 208:2359–2366. doi:10.1002/macp.200700177
Mai NL, Koo Y-M (2015) Computer-aided design of ionic liquids for high cellulose dissolution. ACS Sust Chem Eng 4:541–547. doi:10.1021/acssuschemeng.5b00958
Mazza M, Catana D-A, Vaca-Garcia C, Cecutti C (2009) Influence of water on the dissolution of cellulose in selected ionic liquids. Cellulose 16:207–215. doi:10.1007/s10570-008-9257-x
McCormick CL, Callais PA, Hutchinson BH Jr (1985) Solution studies of cellulose in lithium chloride and N, N-dimethylacetamide. Macromolecules 18:2394–2401
Medronho B, Lindman B (2014) Competing forces during cellulose dissolution: from solvents to mechanisms. Curr Opin Colloid Interface Sci 19:32–40. doi:10.1016/j.cocis.2013.12.001
Medronho B, Romano A, Miguel MG, Stigsson L, Lindman B (2012) Rationalizing cellulose (in)solubility: reviewing basic physicochemical aspects and role of hydrophobic interactions. Cellulose 19:581–587. doi:10.1007/s10570-011-9644-6
O’Sullivan AC (1997) Cellulose: the structure slowly unravels. Cellulose 4:173–207
Pinkert A, Marsh KN, Pang S, Staiger MP (2009) Ionic liquids and their interaction with cellulose. Chem Rev 109:6712–6728
Qi H, Chang C, Zhang L (2008) Effects of temperature and molecular weight on dissolution of cellulose in NaOH/urea aqueous solution. Cellulose 15:779–787. doi:10.1007/s10570-008-9230-8
Rosenau T, Potthast A, Sixta H, Kosma P (2001) The chemistry of side reactions and byproduct formation in the system NMMO/cellulose (Lyocell process). Prog Polym Sci 26:1763–1837
Sen S, Martin JD, Argyropoulos DS (2013) Review of cellulose non-derivatizing solvent interactions with emphasis on activity in inorganic molten salt hydrates. ACS Sustainable Chem Eng 1:858–870. doi:10.1021/sc400085a
Swatloski RP, Spear SK, Holbrey JD, Rogers RD (2002) Dissolution of cellulose with ionic liquids. J Am Chem Soc 124:4974–4975
Wang H, Gurau G, Rogers RD (2012) Ionic liquid processing of cellulose. Chem Soc Rev 41:1519–1537. doi:10.1039/c2cs15311d
Wei W et al (2015) Improved dissolution of cellulose in quaternary ammonium hydroxide by adjusting temperature. RSC Adv 5:39080–39083. doi:10.1039/C5RA04247J
Weng L, Zhang L, Ruan D, Shi L, Xu J (2004) Thermal gelation of cellulose in a NaOH/thiourea aqueous solution. Langmuir ACS J Surfaces Colloids 20:2086–2093
Wernersson E, Stenqvist B, Lund M (2015) The mechanism of cellulose solubilization by urea studied by molecular simulation. Cellulose 22:991–1001. doi:10.1007/s10570-015-0548-8
Xiong B, Zhao P, Cai P, Zhang L, Hu K, Cheng G (2013) NMR spectroscopic studies on the mechanism of cellulose dissolution in alkali solutions. Cellulose 20:613–621. doi:10.1007/s10570-013-9869-7
Xiong B, Zhao P, Hu K, Zhang L, Cheng G (2014) Dissolution of cellulose in aqueous NaOH/urea solution: role of urea. Cellulose 21:1183–1192. doi:10.1007/s10570-014-0221-7
Zhong C, Wang C, Huang F, Jia H, Wei P (2013) Wheat straw cellulose dissolution and isolation by tetra-n-butylammonium hydroxide. Carbohydr Polym 94:38–45. doi:10.1016/j.carbpol.2013.01.043
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (No. 51303151), the National Key Technology R&D Program of the Ministry of Science and Technology of China (No. 2011BAE11B01), the Science and Technology Planning Project of Sichuan Province (Nos. 2013RZ0036, 2015RZ0003 and 2016GZ0222) and Entrepreneurial Talents of Science and Technology Project of Sichuan Province (No. 2014GZ0099).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wei, W., Meng, F., Cui, Y. et al. Room temperature dissolution of cellulose in tetra-butylammonium hydroxide aqueous solvent through adjustment of solvent amphiphilicity. Cellulose 24, 49–59 (2017). https://doi.org/10.1007/s10570-016-1113-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-016-1113-9