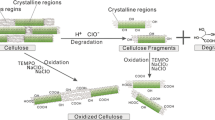
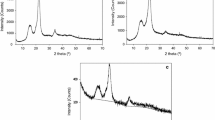
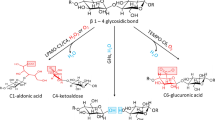
Abstract
The kinetics of periodate oxidation of cellulose was followed by exploiting the β-alkoxy degradation of oxidised units in alkaline medium, that brings about a decrease of viscometric degree of polymerisation. The parallel strong weight loss after NaOH treatment indicates a non-random mechanism of oxidation. The obtained results are interpreted on the basis of an appositely derived kinetic model that can be applied to other mechanisms of bulk and surface degradation.




Similar content being viewed by others
Abbreviations
- CED:
-
Cupriethylenediamine
- DP:
-
Degree of polymerisation, with suffixes w weigth-average, v viscometric-average, n number-average
- GPC:
-
Gel permeation chromatography
- hmw:
-
High molecular weight
- kr:
-
First-order rate constant of the random scissions
- ks:
-
First-order rate constant of the surface erosion
- λ:
-
Fractional weight loss
- LODP:
-
Leveling-off degree of polymerisation
- lmw:
-
Low molecular weight
- n°:
-
Number of scissile units per chain (DP°/LODP−1)
- Rw:
-
Random scissions per chain of the high-molecular weight fraction
- Sw:
-
Experimental number of scissions per chain (DPw°/DPw−1)
References
Calvini P (2010) What went wrong with the kinetics of cellulose degradation? In: Lejeune A, Deprez T (eds) Cellulose: structure and properties, derivatives and industrial uses. Nova Science Publishers, NY, pp 417–426
Calvini P (2012) The role of the Ekenstam equation on the kinetics of cellulose hydrolytic degradation. Cellulose 19:313–318
Calvini P, Gorassini A, Luciano G, Franceschi E (2006a) FTIR and WAXS analysis of periodate oxycellulose: evidence for a cluster mechanism of oxidation. Vib Spectrosc 40:177–183
Calvini P, Conio G, Princi E, Vicini S, Pedemonte E (2006b) Viscometric determination of dialdeyde content in periodate oxycellulose part II. Topochemistry of oxidation. Cellulose 13:571–579
Calvini P, Gorassini A, Merlani AL (2008) On the kinetics of cellulose degradation: looking beyond the pseudo zero order rate equation. Cellulose 15:193–203
Herdan G (1953) Fitting of polymer distributions of molecular weights by the method of moments. J Polym Sci 10(1):1–18
Kim U-J, Kuga S, Wada M, Okano T, Kondo T (2000) Periodate oxidation of crystalline cellulose. Biomacromolecules 1:488–492
Potthast A, Kostic M, Schiehser S, Kosma P, Rosenau T (2007) Studies on oxidative modifications of cellulose in the periodate system: molecular weight distribution and carbonyl group profiles. Holzforschung 61:662–667
Potthast A, Schiehser S, Rosenau T, Kostic M (2009) Oxidative modifications in the periodate system—reduction and beta-elimination reactions. Holzforschung 63:12–17
Schnabel W (1981) Polymer degradation. Principles and practical applications. Hanser International, Wien, pp 20–23
Whitmore PM, Bogaard J (1994) Determination of the cellulose scission route in the hydrolytic and oxidative degradation of paper. Restaurator 15:26–45. http://www.cmu.edu/acrc/Publications/h%20and%20o%20degradations.pdf. Accessed 21 Dec 2011
Yoon J-S, Chin I-J, Kim M-N, Kim C (1996) Degradation of microbial polyesters: a theoretical prediction of molecular weight and polydispersity. Macromolecules 29:3303–3307
Acknowledgments
The authors wish to acknowledge the anonymous referee who underlined the differences and the similarities between the viscometric and the GPC analyses of periodate oxidised samples, allowing a better picture of the suggested mechanism for further specific analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Calvini, P., Gorassini, A. Surface and bulk reactions of cellulose oxidation by periodate. A simple kinetic model. Cellulose 19, 1107–1114 (2012). https://doi.org/10.1007/s10570-012-9717-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-012-9717-1