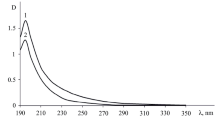
Abstract
The kinetics of periodate oxidation of cellulose was followed through the alkaline degradation of the dialdehyde groups by measuring the viscometric degree of polymerisation and the alkali consumption. The obtained results show that a fast but limited attack of periodate occurs in the amorphous region of cellulose, causing the decrease of degree of polymerisation to its levelling-off value. The alkali consumption indicates at least two further slower reactions, that lead to the asymptotic complete oxidation of cellulose units. With the pseudo first-order approximation, the oxidation half-time of these three reactions can be calculated, corresponding to 1.2, 20 and 854 h respectively. In spite of the high oxidation of the analysed samples (up to about 46%), the residue after alkaline degradation shows a relatively high value of degree of polymerisation rather than the narrow molecular weight distribution of oligomers expected from a random oxidation, thus indicating that periodate oxidises cellulose in isolated domains. The sequence of analyses over the same sample utilised in this work (titrimetry, weight loss and viscometry), performed at room temperature in mild conditions, makes it possible to investigate the topochemistry of oxidation of paper and textiles of historic and artistic value with microdistructive techniques on a single, very small fragment of material.
Similar content being viewed by others
Abbreviations
- α:
-
fractional weight loss
- DAU:
-
dialdehyde units
- DP:
-
viscosity average degree of polymerisation (with suffixes ac – acidity degraded samples; hmw – high molecular weight fraction; lmw – low molecular weight fraction; ox – oxidised samples; oxred – oxidised and reduced samples)
- S:
-
number of β-alkoxy scissions
- t 0.5 :
-
oxidation half-time
References
Aimin T., Hongwey Z., Gang C., Guohui X. and Wenzhi L. (2005). Influence of ultrasound treatment on accessibility and regioselective oxidation reactivity of cellulose. Ultrason. Sonochem. 12(6):467–472
Bicchieri M., Bella M. and Sementilli F.M. (1999). A quantitative measure of borane tert-butylamine complex effectiveness in carbonyl reduction of aged papers. Restaurator 20:22–29
Calvini P., Conio G., Lorenzoni M., and Pedemonte E. (2004). Viscometric determination of dialdehyde content in periodate oxycellulose. Part I. Methodology. Cellulose 11: 99–107
Calvini P. 2005. The influence of levelling-off degree of polymerisation on the kinetics of cellulose degradation. Cellulose 12: 445–447
Calvini P., Gorassini A., Luciano G., and Franceschi E. 2006. FTIR and WAXS analysis of periodate oxycellulose: evidence for a cluster mechanism of oxidation. Vibr. Spectrosc. 40(2): 177–183
Casu B., Naggi A., Torri G., Allegra G., Meille S.V., Cosani A. and Terbojevich M. (1985). Stereoregular acyclic polyalcohols and polyacetates from cellulose and amylose. Macromolecules 18:2762–2767
Chavan V.B., Sarwade B.D. and Varma A.J. (2002). Morphology of cellulose and oxidised cellulose in powder form. Carbohydr. Polym. 50:41–45
Guthrie J.P. (1975). Carbonyl addition reactions: factors affecting the hydrate-hemiacetal and hemiacetal-acetal equilibrium constants. Can. J. Chem. 53:898–906
Herdan G. (1953). Fitting of polymer distributions of molecular weight by the method of moments. J. Polym. Sci. 10(1):1–18
Hofreiter B.T., Alexander B.H. and Wolff I.A. (1955). Rapid estimation of dialdehyde content of periodate oxystarch through quantitative alkali consumption. Anal. Chem. 27(12):1930–1931
Ishak M.F. and Painter T. (1971). Formation of inter-residue hemiacetals during the oxidation of polysaccharides by periodate ion. Acta Chem. Scand. 25(10):3875–3877
Kim U.-J., Kuga S., Wada M., Okano T. and Kondo T. (2000). Periodate oxidation of crystalline cellulose. Biomacromolecules 1:488–492
Kim U.-J. and Kuga S. (2000). Reactive interaction of aromatic amines with dialdehyde cellulose gel. Cellulose 7:287–297
Kim U.-J. and Kuga S. (2001). Thermal decomposition of dialdehyde cellulose and its nitrogen-containing derivatives. Termochimica Acta 369:79–85
Kim U.-J., Wada M. and Kuga S. (2004). Solubilization of dialdehyde cellulose by hot water. Carboydr. Polym. 56:7–10
Maekawa E. and Koshijima T. (1991). Preparation and structural consideration of nitrogen-containing derivatives obtained from dialdehyde celluloses. J. Appl. Polym. Sci. 42:169–178
Margutti S., Conio G., Calvini P. and Pedemonte E. (2001). Hydrolytic and oxidative degradation of paper. Restaurator 22(2):67–83
Mašura V. (1974). Preparation of alkali cellulose with suitable DP without ageing. Cellulose Chem. Technol. 8:21–25
O’Meara D. and Richards G.N. 1958. Alkaline degradation of polysaccharides. Part V. Periodate oxycellulose. J. Chem. Soc. (London) Paper No 909: 4504–4508
Painter T. and Larsen B. (1970). Transient hemiacetal structures formed during the periodate oxidation of xylan. Acta Chem. Scand. 24:2366–2378
Pommerening K., Rein H., Bertram D. and Müller R. (1992). Estimation of dialdehyde groups in 2,3 dialdehyde bead-cellulose. Carbohydr. Res. 233:219–223
Potthast A., Röhrling J., Rosenau T., Bogards A., Sixta H. and Kosma P. (2003). A novel method for the determination of carbonyl groups in cellulosics by fluorescence labeling. 3. Monitoring oxidative processes. Biomacromolecules 4:743–749
Princi E., Vicini S., Pedemonte E., Proietti N., Capitani D., Segre A.L., D’Orazio L., Gentile G., Polcaro C., and Martuscelli E. (2004). Physical and chemical characterization of cellulose based textiles modified by periodate oxidation. Macromol. Symp. 218:343–352
Rahn K. and Heinze TH. (1998). New cellulosic polymers by subsequent modification of 2,3-dialdehyde cellulose. Cellulose Chem. Technol. 32:173–183
Rowland S.P. and Cousins E.R. (1966). Periodate oxidative decrystallization of cotton cellulose. J. Polym. Sci. part A-1 4:793–799
Sharples A. (1954). The hydrolysis of cellulose. Part II. Acid sensitive linkages in Egyptian cotton. J. Polym. Sci. 14:95–104
Sihtola H. (1960). Chemical properties of modified celluloses. Makromol. Chem. 35(1):250–265
Sihtola H., Neimo L. and Sumiala R. (1963). Classification of carbonyl groups in cellulose on the basis of their reaction rates at oximation. J. Polym. Sci. part C 2:289–310
Tiziani S., Sussich F. and Cesàro A. (2003). The kinetics of periodate oxidation of carbohydrates 2. Polymeric substrates. Carbohydr. Res. 338:1083–1085
Varma A.J. and Chavan V.B. (1995). A study of crystallinity changes in oxidised celluloses. Polym. Degrad. Stabil. 49:245–250
Varma A.J., Chavan V.B., Rajmohanan P.R. and Ganapathy S. (1997). Some observations on the high-resolution solid-state CP-MAS 13C-NMR spectra of periodate-oxidised cellulose. Polym. Degrad. Stab. 58:257–260
Varma A.J. and Kulkarni M.P. (2002). Oxidation of cellulose under controlled conditions. Polym. Degrad. Stab. 77:25–27
Veelaert S., de Wit D. and Tournois H. (1994). An improved kinetic model for the periodate oxidation of starch. Polymer 35 (23):5091–5097
Whitmore P.M. and Bogaard J. (1994). Determination of the cellulose scission route in the hydrolytic and oxidative degradation of paper. Restaurator 15:26–45
Whitmore P.M. and Bogaard J. (1995). The effect of oxidation on the subsequent oven aging of filter paper. Restaurator 16(1995):10–30
Zhbankov R.G. (1966). Infrared Spectra of Cellulose and its Derivatives. Consultants Bureau/Plenum Publishing Co., New York
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Calvini, P., Conio, G., Princi, E. et al. Viscometric determination of dialdehyde content in periodate oxycellulose Part II. Topochemistry of oxidation. Cellulose 13, 571–579 (2006). https://doi.org/10.1007/s10570-005-9035-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-005-9035-y