Abstract
Background
Titanium dioxide nanoparticles (TiO2NPs) are widely used in medical application. However, the relevant health risk has not been completely assessed, the potential of inducing arterial thrombosis (AT) in particular.
Methods
Alterations in platelet function and susceptibility to arterial thrombosis induced by TiO2NPs were examined using peripheral blood samples from healthy adult males and an in vivo mouse model, respectively.
Results
Here, using human platelets (hPLTs) freshly isolated from health volunteers, we demonstrated TiO2NP treatment triggered the procoagulant activity of hPLTs through phosphatidylserine exposure and microvesicles generation. In addition, TiO2NP treatment increased the levels of glycoprotein IIb/IIIa and P-selectin leading to aggregation and activation of hPLTs, which were exacerbated by providing physiology-mimicking conditions, including introduction of thrombin, collagen, and high shear stress. Interestingly, intracellular calcium levels in hPLTs were increased upon TiO2NP treatment, which were crucial in TiO2NP-induced hPLT procoagulant activity, activation and aggregation. Moreover, using mice in vivo models, we further confirmed that TiO2NP treatment a reduction in mouse platelet (mPLT) counts, disrupted blood flow, and exacerbated carotid arterial thrombosis with enhanced deposition of mPLT.
Conclusions
Together, our study provides evidence for an ignored health risk caused by TiO2NPs, specifically TiO2NP treatment augments procoagulant activity, activation and aggregation of PLTs via calcium-dependent mechanism and thus increases the risk of AT.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The titanium dioxide nanoparticles (TiO2NPs) that are smaller than 100 nm are widely utilized in biomedical fields such as photoimaging, drug delivery and biological analysis due to their antimicrobial properties, photocatalytic activity, excellent biocompatibility, and corrosion resistance(Najahi-Missaoui et al. 2020; Zhao and Castranova 2011). Often, intravenous injection is the norm, allowing the distribution of these nanoparticles to each organ via the bloodstream for therapeutic purposes. However, this process frequently leads to unexpected organ toxicity, including liver, kidney, and brain injuries(Shi et al. 2013). Given the significance of blood cells as the primary and essential contact component in the blood circulation process, it is noteworthy that toxicological research on these cells remains relatively scarce. Hence, the potential health risk of TiO2NP treatment, particularly from a perspective focusing on blood cells, is becoming a large concern(Lee et al. 2019; Naserzadeh et al. 2018; Qi et al. 2022; Shakeel et al. 2016).
The thrombosis, which is responsible for one in four deaths(2014), can be primarily categorized into venous, arterial, and microvascular thrombosis(Brandtner et al. 2021; Petri 2020). Our previous study have demonstrated that TiO2NPs have the ability to initiate procoagulant activity in red blood cells, ultimately culminating in the development of venous thrombosis(Bian et al. 2021). Although some reports have proposed that TiO2NPs can target platelets and cause microclots in microcirculation, their effects appear to be primarily restricted to platelet aggregation in vitro(Haberl et al. 2015). Nonetheless, in-depth thorough investigation of the underlying mechanism and the presence of other forms of platelet dysfunction still remain unexplored.
Recently, the activation and aggregation of platelets, as indicated by an elevated expression of the integrin αIIbβ3 (GPIIb/IIIa complex, CD41/CD61) and the transmembrane protein P-selectin within the alpha granules, have been strongly linked to thrombotic disorders(Huang et al. 2019; Qiao et al. 2018; Yeini and Satchi-Fainaro 2022). In addition, recent studies have indicated that the pro-coagulant activity of platelets (PLTs) plays a crucial role in the development of thrombosis, which is primarily attributed to the level of phosphatidylserine (PS) exposed on the outer membrane surface and the release of microvesicles (MVs)(Pang et al. 2018; Zlamal et al. 2023). The two processes are caused by an elevated calcium level, leading to hemostasis and thrombosis(Obydennyy et al. 2016; Varga-Szabo et al. 2009), ultimately pointing to the same outcome: the arterial thrombosis.
In this study, we initially established that intravenous administration of TiO2NPs swiftly instigates procoagulant activity within platelets by enhancing phosphatidylserine exposure and microvesicle production. Additionally, we observed prompt platelet activation and aggregation following TiO2NP treatment through the activation of GPIIb/IIIa and the expression of P-selectin. These findings indicate that TiO2NPs possess the potential to alter platelet function and promote the carotid artery thrombosis, a process that necessitates an elevated intracellular calcium level. Indeed, we also observed dysregulation of blood flow signals and arterial thrombosis along with increased PLT deposition using a carotid artery thrombosis mice model. These results provide clear evidence for the risk of PLT-related arterial thrombosis caused by TiO2NP treatment, and help to improve the understanding of the arterial thrombotic risk caused by TiO2NPs.
Methods
Materials
TiO2NPs (anatase, nanopowder, < 100 nm particle size), trisodium citrate, HEPES, prostaglandin E1 (PGE1), glutaraldehyde, EDTA, EGTA, ferric chloride, urethane, clopidogrel, and bovine serum albumin were obtained from Sigma-Aldrich (St. Louis, MO, USA). Thrombin and collagen were obtained from Calbiochem (San Diego, CA, USA) and Chrono-log (Havertown, PA, USA), respectively. Fluorescein isothiocyanate (FITC)-labeled anti-CD62P antibody (anti-CD62P-FITC Ab), FITC-labeled PAC-1 (PAC-1-FITC), and FITC-labeled annexin V (annexin V-FITC) were from BD Biosciences (San Jose, CA, USA). Fluo-4 acetoxymethyl ester (Fluo-4 AM) was obtained from Invitrogen (Carlsbad, CA, USA).
Blood collection and preparation of human platelets (hPLTs)
This work was supported by the Ethics Committee of the Health Service Center at Seoul National University with the approval from the Institutional Review Board of Seoul National University (IRB No. 1702/003–004, 5, March, 2019). To simplify our study design, we selected only healthy male donors aged 20–30 years, excluding risk factors for thrombosis such as gender, age, and disease, and taking any medication in the past 2 weeks. For platelet-rich-plasma (PRP) preparation, whole blood with 3.2% trisodium citrate was centrifuged at 150 × g for 15 min and platelet cell count in PRP was adjusted 3 × 108 cells/mL by diluting with platelet-poor-plasma (PPP) on the day of experiments.
Characterization of TiO 2 NPs
TiO2NPs were not surface treated and insoluble in water, hydrochloric acid, or nitric acid. The particles were dispersed in distilled water and sonication was performed before each experiment, using an ultrasonicator with a maximum output of 150–200 W for 15 s to prevent agglomeration. TiO2NPs were dried and observed with scanning electron microscope (SEM) (SU8010, Hitachi Limited, Japan) to examine the size distribution. Detailed statistical analysis (Nano measure 1.2 and OraginPro2021) of TiO2NPs were performed by random measurement of 100 nanoparticles in the images taken by SEM. The dynamic particle size of the nanoparticles was evaluated using a Malvern laser particle size analyzer (DLS-7000, Otsuka Electronics, Co., Osaka, Japan). The sample was weighed and dispersed in deionized water (0.1% mass fraction), sonicated for 3 min, adjusted to pH 7.4 with NaOH or HCl. The zeta potential was measured with a nanoparticle size analyzer (ZS-90, Malvern Instruments, UK). All data were repeated three times and averaged. Technical support was provided by Beijing Standard Spectrum Testing Technology Co.
Observation of cellular uptake of TiO2NPs under transmission electron microscope (TEM)
Cellular uptake of TiO2NPs by PLTs was observed using TEM following these procedures. After incubating isolated PLTs for 24 h with distilled water (as a control) and 25 µg/mL of TiO2NPs dispersed in distilled water as a colloidal suspension, cell fixation was carried out using a 2% glutaraldehyde solution in the refrigerator for 1 h, followed by post-fixation using 1% osmium tetroxide for 2 h. Subsequently, en-bloc staining was done with 0.5% uranyl acetate for 30 min, followed by serial dehydration steps with 30, 50, 70, 80, 90% ethanol (1 time each) and 100% ethanol (3 times). Transition and infiltration were then gradually performed using propylene oxide (10 min, twice), once with propylene oxide and Spurr's resin (1:1) for 2 h, and finally with Spurr's resin in a desiccator overnight. The next day, infiltration was completed with fresh Spurr's resin for 2 h in the desiccator, and samples were then kept in a 70 °C oven overnight for polymerization. Finally, samples were examined under TEM (JEOL, JEM 1010).
Measurement of PLT aggregation
After incubation with TiO2NPs (0.5, 1, 5, 10, 25, and 100 μg/ml; 5 min, 30 min, and 60 min) at 37 °C, the number of individual PLTs per microliter was calculated using optical microscopy and the degree of PLT aggregation was assessed based on the count of single cells. Data were presented as percentages of PLT aggregation. Besides, PLT aggregation induced by TiO2NP treatment under physiology-mimicking conditions was observed by the above method under stimulation with thrombin (0.6–0.8 U/ml), collagen (2–4 μg/ml). PLT suspension was exposed to a shear rate of 1500 s−1 for 10 min at 37 °C using a programmable cone-plate viscometer. The shear-stressed platelets were fixed with 0.5% glutaraldehyde in Tyrode's buffer (134 mM NaCl, 2.9 mM KCl, 1.0 mM MgCl2, 10.0 mM HEPES, 5.0 mM glucose, 12.0 mM NaHCO3, 0.34 mM Na2HPO4, and 0.3% bovine serum albumin, pH 7.4) to evaluate platelet aggregation. The number of single platelets per microliter was counted under a phase-contrast light microscope. Prior to counting, platelet suspensions were diluted to approximately 300 to 500 particles in 5/25 squares. The variations between two different fixed samples from the same platelet suspension were typically less than 3% from the mean. Optical observation and counting methods can effectively exclude the potential influence of nanomaterial agglutination on platelet aggregation, ensuring accurate results.
Evaluation of LDH leakage
Lactate dehydrogenase (LDH) leakage from PLT was measured by spectrophotometry. After incubation with TiO2NPs for 5 min, the supernatant obtained from the centrifuge reaction mixture was used for LDH determination (digitonin 50 μM treatment for 1 h was used as positive control). The degree of cell lysis was expressed as a percentage of total enzyme activity compared to control incubation with cleavage with digitonin.
Flow cytometry analysis
The PLTs were obtained by centrifugation as described above. After PLTs were exposed with TiO2NPs at 37 °C for 10 min, the level of P-selectin and the activation of glycoprotein GPIIb/IIIa were determined by staining with CD62P-FITC and PAC-1-FITC for 20 min, respectively. PS exposure and MV generation in PLTs were examined by staining with both annexin V-FITC and anti-CD42b-PE Ab (as PLT identifier). MVs are described as cells with a diameter of less than 1 µm. Therefore, the cell population of MVs was differentiated by flow cytometry SSC versus FSC, while MV production in PLTs was confirmed by the PLT surface marker CD42b. Intracellular calcium level was determined by pre-loading fluo-4/AM (5 µM) for 45 min. All the determinations were conducted using FACS Calibur (Becton Dickinson, USA), and Cell Quest Pro software was used to collect and analyze data from 10,000 events. When analyzing, draw the part of annexin V-FITC positive staining and CD42b positive staining in the FACS program, and then export the percentage. Upon analyzing, for example, the PS exposure on platelets, we identified the portion of annexin V-FITC positive staining and CD42b positive staining in the FACS program. Subsequently, we exported the corresponding percentage.
A prothrombinase assay
The prothrombinase assay was applied to assess procoagulant activity. Specifically, TiO2NP-treated PLTs were further induced into thrombin generation by adding 5 nM Xa factor, 10 nM Va factor and 2 μM prothrombin. Then, chromogenic substrate S2238 (Chromogenic, Milan, Italy) was used to measure the generated thrombin after adding a stop buffer (50 mM Tris–HCl, 120 mM NaCl, 2 mM EDTA, pH 7.9). Calculation of thrombin production rate was based on the absorbance change at 405 nm from the calibration curve.
Animals
The experimental C57BL/6 J mice (male, 12 weeks old) were kept in a clean environment without specific pathogens and fed with SPF chow diet and distilled water. The indoor temperature was controlled at 23 ± 1 ℃, the humidity was 55–70%, and the circadian rhythm was alternated (12 h/12 h). Mice were randomly divided into two groups: control group and exposed group (TiO2NPs, 25 mg/kg, intravenous injection). Mice were anesthetized using 3–5% isoflurane by inhalation, and blood samples and other relevant indicators were collected from the mice under anesthesia with a mercy endpoint. All animal experiments were reviewed and approved by the Animal Ethics Committee of China Medical University (CMU20231000). For this specific experiment, we strictly adhered to the triple-blind methodology, ensuring that neither the experimental observer, the research subject, nor the data analyst was aware of the experimental specifics until the official release of the analysis results. Furthermore, taking into account the blinding techniques employed in animal studies, mice were subsequently randomized and assigned to either the control or exposure groups (TiO2NPs, 25 mg/kg, intravenous administration).
Ex vivo assessment using blood cell analyzer
Blood (30 μl) was collected from mice tail with EDTA-K2 -containing tube 1 h post TiO2NP injection. Then fresh blood samples were tested by an automated five-classification animal blood cell analyzer (IDEXX ProCyte Dx, Japan) for the following indicators: PLT count (performed by both impedance (PLT-I) and optical (PLT-O) method), percentage of PLT-larger cell ratio (L-PCR%), PLT crit (PLT%), mean PLT volume (MPV), mean PLT volume/PLT count (MPV/P), PLT distribution width (PDW), red blood cell count, percentage of hematocrit, hemoglobin, and white blood cell-related indexes.
Ex vivo measurement of mPLT aggregation was conducted by an animal blood cell analyzer with impedance method, which only allows one single cell go through. The PLT aggregation rate was calculated by the formula: (PLTCont-PLTTiO2NPs)/PLTCont × 100)%.
Arterial thrombosis in mice
Arterial thrombosis mouse model: The left common carotid artery was isolated from the left side of the trachea using 3 × 5 mm tin foil to isolate the surrounding tissues. Afterwards, 1 × 2 mm filter paper was fully submerged with 5% FeCl3 and applied to the left common carotid artery for 15 min.
Ultrasound observation: The blood flow signal by the Doppler ultrasound in the mouse model was slightly adjusted based on previous study(Jing et al. 2023; Wang and Xu 2005). Mice were anesthetized with isoflurane inhalation at 3% and maintained at 2% throughout the procedure and then cleaned of hair on the neck and chest to facilitate ultrasound. AVINNO6 LAB small animal Doppler ultrasound system (VINNO Co., China) was used. The left common carotid artery was imaged and the flow signals (including velocity, flow volume, internal diameter, perfusion index, heart rate) were quantified.
Thrombuspathological experiment
The tissue of the common carotid artery was collected for the pathological experiment. The thrombus tissue was embedded using the frozen section embedding agent Sakura OCT (a water-soluble mixture of polyethylene glycol and polyvinyl alcohol), sectioned at 6 μm thickness with frozen sectioning machine (CM 1950, LEICA, Germany), and collected using adhesive slides to observe thrombus morphology under a general light microscope. By measuring the percentage of each blank area in the thrombus (the area that blood flow can pass through), it is artificially divided into the following groups: 0–1, 1–5, and 5–10% of the total area. Less blank area means more compact thrombus.
Immunofluorescence observations for frozen sections were examined by adding α-fibronogen with Alexa Fluor® 488 and CD42b with Alexa Fluor® 568, respectively. Then, the samples were sealed with a blocking buffer containing a liquid with an anti-fluorescent cracking agent.
Statistical analysis
All data are presented as the mean and standard deviation. Data were subjected to Student's t-test or two-way ANOVA followed by Duncan's multiple range test. In all cases, a P value < 0.05 was considered statistically significant. The asterisk represents significant differences from the control group (***P < 0.001; **P < 0.01; *P < 0.05). The pound represents significant differences from the TiO2NP treatment group (###P < 0.001; ##P < 0.01; #P < 0.05).
Results
Characterization of TiO 2 NPs and the uptake by human platelets (hPLTs)
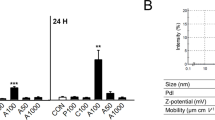
The physicochemical characterization of TiO2NPs was examined by SEM and DLS. The size distribution of TiO2NPs was in the range of 20 to 70 nm with an average diameter of 35.7 nm (Fig. 1a), which was randomly calculated from 100 particles shown in SEM images. DLS data further showed that the average size by intensity was 148.3 nm in saline (with 10% FBS) and 197.2 nm in PBS, respectively (Fig. 1b). The zeta potential of TiO2NPs was + 11.7 mV at pH 7.4 (Fig. 1c). Interestingly, TEM observation showed that TiO2NPs were within hPLTs (dashed circles) or adhered to hPLT membrane (dashed box) (Fig. 1d), indicating altered consequences linked to PLT functions in response to TiO2NP treatment.
Characterization of titanium dioxide nanoparticles (TiO2NPs) and the uptake by human platelets (hPLTs). (a) SEM observation showed the size of TiO2NPs between 20 and 70 nm with an average diameter of 35.7 nm. Scale bar: 100 nm. (b) The dynamic size distribution of TiO2NPs by the intensity in saline and in PBS with a peak of distribution at 148.3 nm and 197.2 nm, respectively. (c) The zeta potential of TiO2NPs in distilled water was + 11.7 mV as tested by a nanoparticle size analyzer. (d) TEM observation of uptake of control (distilled water) and TiO2NPs by hPLTs. Yellow arrowhead: hPLT; black scale bar: 500 nm; dashed circles: TiO2NPs within hPLTs; dashed box: TiO2NPs adhered to hPLT membrane surface
TiO 2 NPs enhance hPLT procoagulant activity through PS exposure and MVs generation
In resting PLTs, PS are commonly in the inner leaflet of the membrane. Upon stimuli, PS are externalized to the outer leaflets and microvesicles (MVs, < 1 μm) are released, both of which participate in coagulation pathway and then promote the production of thrombin from prothrombin under prothrombinase complex (Va, Xa)(Lentz 2003). To estimate procoagulant activity of PLTs induced by TiO2NPs, we treated hPLTs with TiO2NPs for 10 min, determined PS exposure and MVs generation using FACs, and estimated thrombin generation under prothrombinase complex (Va, Xa) (Fig. 2a). PS exposure and MVs were significantly increased in a concentration-dependent manner after TiO2NP treatment (Fig. 2b and 2c). In parallel, with a prothrombinase assay, TiO2NP treatment accelerated thrombin generation in hPLTs, reflecting increased procoagulant activity (Fig. 2d).
TiO2NPs enhance hPLT procoagulant activity through phosphatidylserine (PS) exposure. (a) A diagram of prothrombinase assay showing PS exposure of PLTs in the process of thrombin generation indicating increased procoagulant activity after TiO2NP treatment. (b-c) Isolated hPLTs were treated with various concentrations (1, 10, 25, and 100 μg/mL) of TiO2NPs at 37 °C for 10 min. Both (b) PS exposure and (c) MV generation were measured by flow cytometry. (d) The procoagulant activity was determined by thrombin generation using prothrombinase assays as mentioned in Methods. Values are mean ± SD of the independent experiments from different blood donors (n = 5–7). The asterisk represents significant differences from the control group (***P < 0.001; **P < 0.01; *P < 0.05)
TiO 2 NPs induce hPLT aggregation and activation via GPIIb/IIIa activation and increased P-selectin level
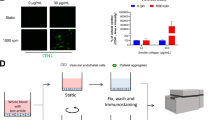
To exam whether TiO2NPs induce PLT aggregation, we used PRP freshly isolated from healthy volunteers and incubated hPLTs with TiO2NPs for 5 min, 30 min or 60 min as shown in Fig. 3a. In consequence, TiO2NPs caused PLT aggregation at 5 min in vitro (Fig. 3b). Upon 30 and 60 min exposure, TiO2NPs also caused PLT aggregation but did not show obvious time-dependent effects even when compared with 5 min exposure (Fig. 3c), indicating that TiO2NPs rapidly exaggerate PLT aggregation which occurs within 5 min. Next, we examined the leakage of LDH from hPLTs and found that no cytotoxicity was induced by TiO2NP treatment (digitonin was used as a positive control) (Fig. 3d). Glycoprotein IIb/IIIa (GPIIb/IIIa) plays a key role in the maintenance of PLT aggregation(Aliotta et al. 2021). In addition, P-selectin is an adhesion molecule belonging to the selectin family expressed on PLTs, and activated PLTs express high levels of P-selectin(Yeini and Satchi-Fainaro 2022). As shown in Fig. 3e and 3f, GPIIb/IIIa activation and P-selectin expression were both significantly induced by TiO2NP treatment. Together with Fig. 2 b and d, all the markers suggesting procoagulant activity are matched well with Scientific and Standardization Committee of the ISTH defined procoagulant platelets (Josefsson et al. 2023).
TiO2NPs induce hPLT aggregation and activation through increased GPIIb/IIIa activation and P-selectin level. (a) In vitro experimental method using PLTs freshly isolated from health volunteers. (b-c) Percentage of hPLT aggregation after hPLTs were treated to various concentrations (0.5, 1, 5, 10, 25, and 100 μg/ml) of TiO2NPs for (b) 5 min, (c) 30 min and 60 min. (d) LDH leakage of hPLTs induced by TiO2NPs. DIG, digitonin 50 μM treatment for 1 h, used as positive control. (e) GPIIb/IIIa activation and (f) P-selectin level were measured by flow cytometry. Values are mean ± SD of the independent experiments from different blood donors (n = 3–5). The asterisk represents significant differences from the control group (***P < 0.001; **P < 0.01; *P < 0.05)
TiO 2 NP-induced hPLT aggregation is exacerbated under physiology-mimicking conditions
In the physiological system, the presence of physiological aggregators (e.g. thrombin and collagen) contributes to the heterogeneity in PLT responses (Aslam et al. 2013; Petzold et al. 2016; Sang et al. 2021). Additionally, vascular shear force plays an existential role in the physiological function of PLTs(Casa et al. 2015; Yagi et al. 2017). To assess the effect of TiO2NPs on PLT aggregation more accurately, we determined PLT aggregation using an in vitro experimental method with adjustable physiological simulating conditions (Fig. 4a, i) and compared the results with those from a basic in vitro experimental method (Fig. 4a, ii). After adding thrombin or collagen in vitro, accelerated TiO2NP-induced PLT aggregation was found (Fig. 4b). Similarly, after adding shear stress in vitro that simulates physiological high shear flow, enhanced PLT aggregation was observed (Fig. 4c). More significantly, the existence of physiological aggregators (thrombin and collagen) and high shear stress reduced the minimum toxic level of TiO2NPs for PLT aggregation from 1 μg/mL to 0.1 μg/mL (Fig. 4b, insert) and 0.5 μg/mL (Fig. 4c, insert), respectively, as compared to the basic condition. These data indicate that TiO2NPs boost more severe PLT aggregation under physiology-mimicking conditions.
TiO2NP-induced hPLT aggregation is exacerbated under physiology-mimicking conditions. (a) A diagram showing a basic in vitro experimental method versus an in vitro experimental method with adjustable physiological simulating conditions. (b-c) Aggregation of hPLTs after adding thrombin (0.6–0.8 U/mL), collagen (2–4 μg/mL) or shear stress (1500 s.−1 for 3 min) under various concentrations (0.1, 0.5, 1, and 10 μg/ml) of TiO2NPs. Values are mean ± SD of the independent experiments from different blood donors (n = 3–5). The asterisk represents significant differences from the no stimuli or no shear stress group (***P < 0.001; *P < 0.05)
Involvement of Ca 2+ in hPLT aggregation and procoagulant activity induced by TiO 2 NPs
Intracellular Ca2+ plays a key role in PLT activation, aggregation and procogulant activity(Back et al. 2022; Xiang et al. 2021; Zhu et al. 2016). Here, we observed a concentration-dependent increase in intracellular Ca2+ level after TiO2NP treatment (Fig. 5a). To further assess the role of Ca2+ in PLT dysfunction, we treated hPLTs with EGTA (a chelator of Ca2+) prior to TiO2NP treatment. In consequence, increased PS exposure (Fig. 5b) and procoagulant activity (Fig. 5c) induced by TiO2NPs were both effectively reduced by EGTA. Concurrently, TiO2NP-induced hPLT aggregation was markedly blocked by EGTA (Fig. 5d). Meanwhile, elevated GPIIb/IIIa activation (Fig. 5e) and P-selectin level (Fig. 5f) by TiO2NP treatment were both significantly inhibited by EGTA.
Involvement of Ca2+ in procoagulant activity, activation and aggregation of hPLT induced by TiO2NPs. (a) Intracellular Ca2+ level in hPLTs was measured by adding Fluo-4 AM after hPLTs were treated with 100 μg/mL TiO2NPs for 10 min. (b) PS exposure, (c) procoagulant activity, (d) hPLT aggregation, (e) GPIIb/IIIa activation, and (f) P-selectin level were performed by preloading a calcium chelating agent (EGTA) prior to TiO2NP treatment. Values are mean ± SD of the independent experiments from different blood donors (n = 3–6). The asterisk represents significant differences from the control group (***P < 0.001; **P < 0.01; *P < 0.05). The pound represents significant differences from the TiO2NP treatment group (###P < 0.001; ##P < 0.01; #P < 0.05)
TiO 2 NP treatment decreases PLT counts and increases large PLT ratio in mice
To elucidate the in vivo effect of TiO2NPs, mice were intravenously injected with TiO2NPs and after 1 h, blood was collected and analyzed using a blood cell analyzer. It is clear from the data that PLT counts (Fig. 6a) detected by the impedance method (Fig. 6a, left) and the optical method (Fig. 6A, right), respectively, as well as PCT% (Fig. 6b), were significantly declined in TiO2NP-exposed mice. Meanwhile, P-LCR% was increased apparently in TiO2NP-exposed mice (Fig. 6c). In addition to P-LCR, MPV, PDW and MPV/P were all considered as potential indicators of PLT shape and function(Azab et al. 2011; Gasparyan et al. 2011; Han et al. 2013). Accordingly, increases in MPV (Fig. 6d) and MPV/P (Fig. 6f) after TiO2NP treatment were observed, but there was no change in PDW (Fig. 6e).
TiO2NP treatment results in decreased PLT counts and increased large PLT ratio in mice. TiO2NPs were intravenously injected into mice, and 1 h later, blood was collected from mice for hemocyte analysis. (a) PLT counts by impedance method (PLT-I), PLT counts by optical method (PLT-O), (b) percentage of plateletcrit (PCT%), (c) percentage of large PLTs (P-LCR%), (d) mean PLT volume by impedance (MPV-I) and by optical (MPV-O), (e) PLT volume distribution width (PDW), and (f) mean PLT volume/PLT count (MPV/P). At the same time, red blood cell-related indexes, such as (g) red blood cell count, percentage of hematocrit, (h) hemoglobin, and (i) white blood cell-related indexes, including white blood cell count and classification percentage were tested separately. Values are mean ± SD of the independent experiments from mice (n = 4–6). The asterisk represents significant differences from the control group (***P < 0.001; *P < 0.05)
Moreover, TiO2NP-exposed mice showed no significant change in red blood cells (RBC) index including RBC counts (Fig. 6g, left), hematocrit (HCT%) (Fig. 6g, right), hemoglobin (HGB) (Fig. 6h), or white blood cells (WBC) index including neutrophils, lymphocytes, monocytes, eosinophils and basophils (Fig. 6i).
TiO 2 NPs trigger PLT dysfunction and locally alter arterial blood flow signals in mice
To further determine whether TiO2NPs cause PLT aggregation and activation, we firstly did ex vivo study using PLTs isolated from mice (mPLTs) treated with TiO2NPs (Fig. 7a). TiO2NPs resulted in increased mPLT aggregation (Fig. 7b) and P-selectin (Fig. 7c) level 1 h post treatment. Numerous studies have shown that PLT activation and aggregation are closely related to thrombosis(Nayak et al. 2021; Yeung et al. 2018). Therefore, we established an AT-initiating mice model 1 h after TiO2NP treatment, and phenotypes were determined by color doppler ultrasonography (Fig. 7d, i). As shown in Fig. 7e, TiO2NP-exposed group showed different flow signals compared with control group. Specifically, TiO2NP-exposed mice represented obvious accelerated arterial blood flow velocity (Fig. 7f), increased blood flow volume (Fig. 7g), and a slightly declined trend in the vascular resistance index (Fig. 7i) with unconspicuous alterations in artery internal diameter (Fig. 7h). Such doppler data indicate thrombi ahead, which is consistent with early reports on the phenomena of increased blood flow signals and thrombus formation(He et al. 2023). Briefly, these all point to a critical clue that TiO2NP treatment increases the risk of AT.
TiO2NPs trigger PLT dysfunction and locally accelerate arterial blood flow signals in mice. (a-c) A diagram of ex vivo study: 1 h after TiO2NP intravenous injection, (b) percentage of PLT aggregation was calculated by the impedance method and (c) P-selectin was measured using flow cytometry. (d) A diagram of the FeCl3-initiated AT mouse model. (e-i) Blood flow signals in the common carotid artery of mice were measured using small animal doppler color ultrasound. (f) Blood flow velocity, (g) blood flow volume, (h) internal diameter of the common carotid artery, and (i) vascular resistance index, were detected and quantified. Values are mean ± SD of the independent experiments from mice (n = 3–5). The asterisk represents significant differences from the control group (**P < 0.01; *P < 0.05)
TiO 2 NP treatment leads to carotid artery thrombosis with PLT deposition in mice
In addition to the doppler ultrasonic detection, we conducted pathological sections of the arterial thrombus in mouse AT model (Fig. 7d, ii), and found increased thrombus formation in TiO2NP-exposed mice (Fig. 8a). Besides, we noticed that thrombi formed in TiO2NP-exposed mice were fuller and more compact, reflecting that the blood flow became more sluggish. Hence, we further analyzed the blank area of aorta (area that blood flow can go through) as mentioned in methods, to reflect the potential unobstructed capacity of blood flow. The total counts were separated into three distinguished levels including the tiny (less than 1% of the total aorta), the moderate (1–5% of the total aorta) and the loose blank area (5–10% of the total aorta). Consistent with the increase in thrombosis, mice exposed to TiO2NPs showed significantly less total blank areas or blank areas at each level compared to control mice, reflecting a decrease in blood flow patency in mice with AT induced by TiO2NPs (Fig. 8b). Finally, we stained the thrombi by immunofluorescence with CD42b (red color) to reflect the active role of PLTs in TiO2NP-induced AT. The red fluorescence indicating PLT deposition within the thrombi was increased in TiO2NP-exposed mice (Fig. 8c).
TiO2NP treatment leads to PLT-involved AT in mice. (a) Immunofluorescence staining of arterial thrombi was obtained using AT mouse model and (b) the blank area of aorta (area that blood flow can go through) was quantified as described in Methods. (c) The pathological composition of the thrombus was observed using immunofluorescence staining for PLTs (red, CD42b), α-fibrinogen (green) and DAPI (blue), respectively. Scale bar: 100 μm. Thrombus were obtained from independent mouse samples (n = 3)
Discussion
The growing medical application of TiO2NPs has sparked critical consideration of their bio-safety. This study, for the first time, reveals that TiO2NPs can enhance procoagulant activity in isolated human platelets by increasing exposure of PS and the generation of MVs. Additionally, exposure to TiO2NPs rapidly triggers platelet activation and aggregation through increased expression of P-selectin and activation of GPIIb/IIIa. Here, intracellular calcium plays a role in both processes. Furthermore, TiO2NPs alter blood flow and exacerbate arterial thrombus formation in mice, accompanied by increased platelet deposition, underscoring the relevance of our findings to real in vivo conditions (Scheme 1).
Suggested mechanism for the dysfunction of PLTs by TiO2NP. TiO2NPs can induce hPLT aggregation by elevating P-selectin, GPIIb/IIIa levels, and procoagulant activity through PS externalization and MV generation, involving intracellular calcium. PLT aggregation sensitization takes place within a physiology-mimicking system, thereby intensifying arterial thrombosis in mice via PLT deposition
Nanoparticles are often used intravenously in medical use, which inevitably have unintended side effects on blood cells. Numerous studies have shown that these particles can impact macrophages(Dey et al. 2021), neutrophils(Yamano et al. 2022), and lymphocytes. In addition, our previous research revealed that TiO2NPs can contribute to venous thrombosis by enhancing the procoagulant activity of red blood cells(Bian et al. 2021). In peripheral blood, platelets also play key roles in hemostasis and thrombosis(Koupenova et al. 2018). In this study, using isolated human samples, we discovered that platelets are more sensitive to TiO2NPs than red blood cells were in our previous finding. This heightened sensitivity was evident at concentrations as low as 1 μg/mL of TiO2NPs (after less than 10 min in vitro), which preceded the dysfunction of red blood cells, which occurred relatively high at 10 μg/mL after approximately 24 h in vitro. The findings of this study indicate that intravenous administration of TiO2NPs may pose a significant risk to blood cell function and homeostasis. It is crucial for future research to further investigate the potential long-term effects of these nanoparticles on blood cell function and cardiovascular health.
The reports on toxicity of NPs are always controversial due to the ability to release ionic form from metal NPs. Some studies claim that the toxicity of metal NPs is due to the metal ions released by NPs, while others argue that the toxicity of metal NPs is due to the small particle size of NPs themselves and other physicochemical properties as additional influencing factors(Bian et al. 2019; Ye et al. 2018; Zhang et al. 2020). For instance, zinc oxide and copper oxide nanoparticles (ZnONPs and CuONPs) are easy to release their ionic forms and their main toxicity is considered to be caused by the metal ions(Liu et al. 2016; Wang et al. 2016). However, with respect to silver nanoparticles (AgNPs), toxic effects are currently attributed into two aspects, the metal ion (Ag+) effect and the NP effect, respectively(Poynton et al. 2012). Most earlier studies suggest that it is Ag+ released from the AgNPs exerting a significant influence on protein regulation and the induction of cellular stress(Zhang et al. 2020). Different from this perspective, our previous study found that the level of Ag+ released from AgNPs as detected by ICP-MS method was negligible (< 0.01%) and hardly initiated a significant response of the cell(Bian et al. 2019), indicating that the effect of NPs themselves rather than the ion effect is the main contributor of AgNP toxicity. A recent study has revealed that though the ions of AgNPs cause a certain degree of toxicity, but not as severe as the toxicity due to their physical characteristics as NPs(Cvjetko et al. 2017). Unlike those typical metal NPs possessing the capability of releasing ionic forms, the level of metal titanium ions (Ti4+) dissociated from TiO2NPs is extremely low due to the special nature of titanium(Prokopiuk et al. 2023; Qin et al. 2017). Generally speaking, the toxic effects of TiO2NPs are proved to be mainly based on their unique NP properties.
TiO2NPs possess a range of physicochemical attributes, including diverse particle sizes, various crystalline forms (such as anatase and rutile), and distinct surface modifications (including surface charge and coatings). These characteristics play a pivotal role in determining the NPs' biological properties. Surface functionalization of TiO2NPs with negatively charged moieties has been demonstrated to mitigate the erythrocyte aggregation effects elicited by these NPs, potentially attributed to the formation of a complex system on the NP surface resulting from surface modification (Han et al. 2012). Notably, anatase TiO2NPs is renowned for its superior bioactivity compared to the rutile form, and in combination with smaller particle sizes, it may exacerbate toxicity (De Matteis et al. 2016; Shi et al. 2013), as evidenced by our prior investigation using human erythrocytes (Bian et al. 2021). Furthermore, previous studies have consistently reported that rutile-type TiO2NPs do not significantly affect PLT activation(Bihari et al. 2010). However, our novel research reveals that anatase-type TiO2NPs induce notable PLT activation, even at lower concentrations. Given these intriguing findings, we believe that further exploration of the thrombotic risks mediated by PLTs in future studies involving various TiO2NPs is imperative. Such investigations could provide valuable insights into the safety profile and potential therapeutic applications of these nanoparticles.
Physiological factors play important regulatory roles in the functional performance of PLTs(Sang et al. 2021). Flowing blood generates a frictional force called shear stress(Souilhol et al. 2020), and a common pathological symptom for myocardial infarction and ischemic stroke is thrombotic blood flow obstruction that forms at high shear rates in the arteries(Casa and Ku 2017). Thrombin, collagen, and ADP are currently considered to be the most potent physiological agonists of PLTs. In the present study, under physiology-mimicking conditions, we found that TiO2NP-induced hPLT aggregation was dramatically exacerbated. Although we tried our best to simulate in vivo system by adding physiological aggregators and imposing high shear stress, and as expected, observed increased PLT aggregation, the real human body is a large complex system quite difficult to completely and perfectly simulate.
Maintaining the intracellular concentration and distribution of free calcium ions in dynamic equilibrium is vital for cellular life activities. This balance is achieved through complex calcium ion permeation and transport mechanisms within the cell membrane and organelles. However, EGTA primarily chelates extracellular calcium, thus limiting its accuracy in assessing [Ca2+]i levels. In past studies, MAPTAM (Korchak et al. 1998) has been used to block the increase in [Ca2+]i, but its recent application is uncommon. Briefly, both agents ultimately affect intracellular calcium levels. Given the widespread use of EGTA, we utilized it in the current study. Nevertheless, considering the importance of [Ca2+]i regulation in therapeutic drug development, it is crucial to explore alternative approaches. A multifaceted approach is necessary to effectively target and modulate [Ca2+]i levels, including exploring novel agents or techniques that can directly influence [Ca2+]i dynamics. By doing so, we can gain deeper insights into calcium-related cellular processes and potentially develop more effective therapeutic strategies for treating calcium dysregulation-related diseases.
Calcium signaling and its network of interactions are involved in mediating many cellular physiological functions. Studies have shown that the agonist-induced Ca2+ level is critical for PLT activation in hemostasis and thrombosis(Varga-Szabo et al. 2009). Increased procoagulant activity of PLTs can be initiated by integrin αIIbβ3 (GPIIb/IIIa)/Gα13-mediated co-stimulation of outward-inward signaling and GPVI signaling, leading to intracellular Ca2+ release above a threshold(Kaiser et al. 2022). In a study on procoagulant PLTs, it was mentioned that only the procoagulant PLTs showed high cytoplasmic Ca2+ level as detected by fluorescent probes(Abbasian et al. 2020). Although the crucial significance of calcium ions in platelets and thrombosis is well-established, the potential perils associated with their interaction with TiO2NPs, particularly pertaining to calcium involvement, have remained relatively unexplored. This article endeavors to fill this gap in knowledge and enhance our understanding of this intricate relationship by demonstrating that exposure to TiO2NPs elevates intracellular Ca2+ levels, which are vital for triggering platelet activation, aggregation, and procoagulant activity, ultimately culminating in arterial thrombosis in mice. Thus, it is noteworthy that our study may provide new clues on the preventive and therapeutic strategies of AT caused by TiO2NP treatment.
Conclusions
Our study reveals a pro-thrombotic effect of TiO2NPs by pro-coagulant activity and activation/aggregation of PLTs. Mechanistically, TiO2NPs initiate PS exposure and MVs generation, ultimately leading to a heightened pro-coagulant activity. Simultaneously, these particles boost the expression of P-selectin and activate GPIIb/IIIa, thereby facilitating the activation/aggregation of platelets. This entire process involves an increase in intracellular Ca2+ levels, making it calcium-dependent. Most importantly, we demonstrated the relevance of our findings in vivo by showing that intravenous administration of TiO2NPs can increase arterial thrombosis in mouse carotid arteries, emphasizing the need for caution during medical applications of TiO2NPs.
Data availability
No datasets were generated or analysed during the current study
References
Abbasian N, Millington-Burgess SL, Chabra S, Malcor JD, Harper MT. Supramaximal calcium signaling triggers procoagulant platelet formation. Blood Adv. 2020;4:154–64. https://doi.org/10.1182/bloodadvances.2019000182.
Aliotta A, Bertaggia Calderara D, Zermatten MG, Alberio L. High-Dose Epinephrine Enhances Platelet Aggregation at the Expense of Procoagulant Activity. Thromb Haemost. 2021;121:1337–44. https://doi.org/10.1055/a-1420-7630.
Aslam M, Sedding D, Koshty A, Santoso S, Schulz R, Hamm C, Gündüz D. Nucleoside triphosphates inhibit ADP, collagen, and epinephrine-induced platelet aggregation: role of P2Y1 and P2Y12 receptors. Thromb Res. 2013;132:548–57. https://doi.org/10.1016/j.thromres.2013.08.021.
Azab B, Torbey E, Singh J, Akerman M, Khoueiry G, McGinn JT, Widmann WD, Lafferty J. Mean platelet volume/platelet count ratio as a predictor of long-term mortality after non-ST-elevation myocardial infarction. Platelets. 2011;22:557–66. https://doi.org/10.3109/09537104.2011.584086.
Back V, Asgari A, Franczak A, Saito M, Castaneda Zaragoza D, Sandow SL, Plane F, Jurasz P. Inhibition of platelet aggregation by activation of platelet intermediate conductance Ca(2+) -activated potassium channels. J Thromb Haemost. 2022;20:2587–600. https://doi.org/10.1111/jth.15827.
Bian Y, Kim K, Ngo T, Kim I, Bae ON, Lim KM, Chung JH. Silver nanoparticles promote procoagulant activity of red blood cells: a potential risk of thrombosis in susceptible population. Part Fibre Toxicol. 2019;16:9. https://doi.org/10.1186/s12989-019-0292-6.
Bian Y, Chung HY, Bae ON, Lim KM, Chung JH, Pi J. Titanium dioxide nanoparticles enhance thrombosis through triggering the phosphatidylserine exposure and procoagulant activation of red blood cells. Part Fibre Toxicol. 2021;18:28. https://doi.org/10.1186/s12989-021-00422-1.
Bihari P, Holzer M, Praetner M, Fent J, Lerchenberger M, Reichel CA, Rehberg M, Lakatos S, Krombach F. Single-walled carbon nanotubes activate platelets and accelerate thrombus formation in the microcirculation. Toxicology. 2010;269:148–54. https://doi.org/10.1016/j.tox.2009.08.011.
Brandtner AK, Lehner GF, Pircher A, Feistritzer C, Joannidis M. Differential procoagulatory response of microvascular, arterial and venous endothelial cells upon inflammation in vitro. Thromb Res. 2021;205:70–80. https://doi.org/10.1016/j.thromres.2021.07.002.
Casa LDC, Ku DN. Thrombus Formation at High Shear Rates. Annu Rev Biomed Eng. 2017;19:415–33. https://doi.org/10.1146/annurev-bioeng-071516-044539.
Casa LD, Deaton DH, Ku DN. Role of high shear rate in thrombosis. J Vasc Surg. 2015;61:1068–80. https://doi.org/10.1016/j.jvs.2014.12.050.
Cvjetko P, Milošić A, Domijan AM, Vinković Vrček I, Tolić S, Peharec Štefanić P, Letofsky-Papst I, Tkalec M, Balen B. Toxicity of silver ions and differently coated silver nanoparticles in Allium cepa roots. Ecotoxicol Environ Saf. 2017;137:18–28. https://doi.org/10.1016/j.ecoenv.2016.11.009.
De Matteis V, Cascione M, Brunetti V, Toma CC, Rinaldi R. Toxicity assessment of anatase and rutile titanium dioxide nanoparticles: The role of degradation in different pH conditions and light exposure. Toxicol Vitro. 2016;37:201–10. https://doi.org/10.1016/j.tiv.2016.09.010.
Dey AK, Gonon A, Pécheur EI, Pezet M, Villiers C, Marche PN. Impact of Gold Nanoparticles on the Functions of Macrophages and Dendritic Cells. Cells. 2021;10:96. https://doi.org/10.3390/cells10010096.
Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des. 2011;17:47–58. https://doi.org/10.2174/138161211795049804.
Haberl N, Hirn S, Holzer M, Zuchtriegel G, Rehberg M, Krombach F. Effects of acute systemic administration of TiO2, ZnO, SiO2, and Ag nanoparticles on hemodynamics, hemostasis and leukocyte recruitment. Nanotoxicology. 2015;9:963–71. https://doi.org/10.3109/17435390.2014.992815.
Han Y, Wang X, Dai H, Li S. Nanosize and surface charge effects of hydroxyapatite nanoparticles on red blood cell suspensions. ACS Appl Mater Interfaces. 2012;4:4616–22. https://doi.org/10.1021/am300992x.
Han JS, Park TS, Cho SY, Joh JH, Ahn HJ. Increased mean platelet volume and mean platelet volume/platelet count ratio in Korean patients with deep vein thrombosis. Platelets. 2013;24:590–3. https://doi.org/10.3109/09537104.2012.748187.
He B, Lei J, Lang X, Li Z, Cui W, Zhang Y. Ultra-fast ultrasound blood flow velocimetry for carotid artery with deep learning. Artif Intell Med. 2023;144:102664. https://doi.org/10.1016/j.artmed.2023.102664.
Huang J, Li X, Shi X, Zhu M, Wang J, Huang S, Huang X, Wang H, Li L, Deng H, Zhou Y, Mao J, Long Z, Ma Z, Ye W, Pan J, Xi X, Jin J. Platelet integrin αIIbβ3: signal transduction, regulation, and its therapeutic targeting. J Hematol Oncol. 2019;12:26. https://doi.org/10.1186/s13045-019-0709-6.
Jing B, Carrasco DI, AuYong N, Lindsey BD. A Transverse Velocity Spectral Estimation Method for Ultrafast Ultrasound Doppler Imaging. IEEE Trans Ultrason Ferroelectr Freq Control. 2023;70:1749–60. https://doi.org/10.1109/tuffc.2023.3316748.
Josefsson EC, Ramström S, Thaler J, Lordkipanidzé M. Consensus report on markers to distinguish procoagulant platelets from apoptotic platelets: communication from the Scientific and Standardization Committee of the ISTH. J Thromb Haemost. 2023;21:2291–9. https://doi.org/10.1016/j.jtha.2023.05.001.
Kaiser R, Escaig R, Kranich J, Hoffknecht ML, Anjum A, Polewka V, Mader M, Hu W, Belz L, Gold C, Titova A, Lorenz M, Pekayvaz K, Kääb S, Gaertner F, Stark K, Brocker T, Massberg S, Nicolai L. Procoagulant platelet sentinels prevent inflammatory bleeding through GPIIBIIIA and GPVI. Blood. 2022;140:121–39. https://doi.org/10.1182/blood.2021014914.
Korchak HM, Vosshall LB, Haines KA, Wilkenfeld C, Lundquist KF, Weissmann G. Activation of the human neutrophil by calcium-mobilizing ligands. II. Correlation of calcium, diacyl glycerol, and phosphatidic acid generation with superoxide anion generation. J Biol Chem. 1998;263:11098–105.
Koupenova M, Clancy L, Corkrey HA, Freedman JE. Circulating Platelets as Mediators of Immunity, Inflammation, and Thrombosis. Circ Res. 2018;122:337–51. https://doi.org/10.1161/circresaha.117.310795.
Lee J, Jeong JS, Kim SY, Park MK, Choi SD, Kim UJ, Park K, Jeong EJ, Nam SY, Yu WJ. Titanium dioxide nanoparticles oral exposure to pregnant rats and its distribution. Part Fibre Toxicol. 2019;16:31. https://doi.org/10.1186/s12989-019-0313-5.
Lentz BR. Exposure of platelet membrane phosphatidylserine regulates blood coagulation. Prog Lipid Res. 2003;42:423–38. https://doi.org/10.1016/s0163-7827(03)00025-0.
Liu J, Feng X, Wei L, Chen L, Song B, Shao L. The toxicology of ion-shedding zinc oxide nanoparticles. Crit Rev Toxicol. 2016;46:348–84. https://doi.org/10.3109/10408444.2015.1137864.
Najahi-Missaoui W, Arnold RD, Cummings BS. Safe Nanoparticles: Are We There Yet? Int J Mol Sci. 2020;22:385. https://doi.org/10.3390/ijms22010385.
Naserzadeh P, Ghanbary F, Ashtari P, Seydi E, Ashtari K, Akbari M. Biocompatibility assessment of titanium dioxide nanoparticles in mice fetoplacental unit. J Biomed Mater Res A. 2018;106:580–9. https://doi.org/10.1002/jbm.a.36221.
Nayak MK, Ghatge M, Flora GD, Dhanesha N, Jain M, Markan KR, Potthoff MJ, Lentz SR, Chauhan AK. The metabolic enzyme pyruvate kinase M2 regulates platelet function and arterial thrombosis. Blood. 2021;137:1658–68. https://doi.org/10.1182/blood.2020007140.
Obydennyy SI, Sveshnikova AN, Ataullakhanov FI, Panteleev MA. Dynamics of calcium spiking, mitochondrial collapse and phosphatidylserine exposure in platelet subpopulations during activation. J Thromb Haemost. 2016;14:1867–81. https://doi.org/10.1111/jth.13395.
Pang A, Cui Y, Chen Y, Cheng N, Delaney MK, Gu M, Stojanovic-Terpo A, Zhu C, Du X. Shear-induced integrin signaling in platelet phosphatidylserine exposure, microvesicle release, and coagulation. Blood. 2018;132:533–43. https://doi.org/10.1182/blood-2017-05-785253.
Petri M. Antiphospholipid syndrome. Transl Res. 2020;225:70–81. https://doi.org/10.1016/j.trsl.2020.04.006.
Petzold T, Thienel M, Konrad I, Schubert I, Regenauer R, Hoppe B, Lorenz M, Eckart A, Chandraratne S, Lennerz C, Kolb C, Braun D, Jamasbi J, Brandl R, Braun S, Siess W, Schulz C, Massberg S. Oral thrombin inhibitor aggravates platelet adhesion and aggregation during arterial thrombosis. Sci Transl Med. 2016;8:367ra168. https://doi.org/10.1126/scitranslmed.aad6712.
Poynton HC, Lazorchak JM, Impellitteri CA, Blalock BJ, Rogers K, Allen HJ, Loguinov A, Heckman JL, Govindasmawy S. Toxicogenomic responses of nanotoxicity in Daphnia magna exposed to silver nitrate and coated silver nanoparticles. Environ Sci Technol. 2012;46:6288–96. https://doi.org/10.1021/es3001618.
Prokopiuk V, Yefimova S, Onishchenko A, Kapustnik V, Myasoedov V, Maksimchuk P, Butov D, Bespalova I, Tkachenko A. Assessing the Cytotoxicity of TiO(2–x) Nanoparticles with a Different Ti(3+)(Ti(2+))/Ti(4+) Ratio. Biol Trace Elem Res. 2023;201:3117–30. https://doi.org/10.1007/s12011-022-03403-3.
Qi Y, Wei S, Xin T, Huang C, Pu Y, Ma J, Zhang C, Liu Y, Lynch I, Liu S. Passage of exogeneous fine particles from the lung into the brain in humans and animals. Proc Natl Acad Sci USA. 2022;119:e2117083119. https://doi.org/10.1073/pnas.2117083119.
Qiao J, Wu X, Luo Q, Wei G, Xu M, Wu Y, Liu Y, Li X, Zi J, Ju W, Fu L, Chen C, Wu Q, Zhu S, Qi K, Li D, Li Z, Andrews RK, Zeng L, Gardiner EE, Xu K. NLRP3 regulates platelet integrin αIIbβ3 outside-in signaling, hemostasis and arterial thrombosis. Haematologica. 2018;103:1568–76. https://doi.org/10.3324/haematol.2018.191700.
Qin Q, Li J, Wang J. Antibacterial Activity Comparison of Three Metal Oxide Nanoparticles and their Dissolved Metal Ions. Water Environ Res. 2017;89:378–83. https://doi.org/10.2175/106143017x14839994523262.
Sang Y, Roest M, de Laat B, de Groot PG, Huskens D. Interplay between platelets and coagulation. Blood Rev. 2021;46:100733. https://doi.org/10.1016/j.blre.2020.100733.
Shakeel M, Jabeen F, Shabbir S, Asghar MS, Khan MS, Chaudhry AS. Toxicity of Nano-Titanium Dioxide (TiO2-NP) Through Various Routes of Exposure: a Review. Biol Trace Elem Res. 2016;172:1–36. https://doi.org/10.1007/s12011-015-0550-x.
Shi H, Magaye R, Castranova V, Zhao J. Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol. 2013;10:15. https://doi.org/10.1186/1743-8977-10-15.
Souilhol C, Serbanovic-Canic J, Fragiadaki M, Chico TJ, Ridger V, Roddie H, Evans PC. Endothelial responses to shear stress in atherosclerosis: a novel role for developmental genes. Nat Rev Cardiol. 2020;17:52–63. https://doi.org/10.1038/s41569-019-0239-5.
Varga-Szabo D, Braun A, Nieswandt B. Calcium signaling in platelets. J Thromb Haemost. 2009;7:1057–66. https://doi.org/10.1111/j.1538-7836.2009.03455.x.
Wang X, Xu L. An optimized murine model of ferric chloride-induced arterial thrombosis for thrombosis research. Thromb Res. 2005;115:95–100. https://doi.org/10.1016/j.thromres.2004.07.009.
Wang D, Lin Z, Wang T, Yao Z, Qin M, Zheng S, Lu W. Where does the toxicity of metal oxide nanoparticles come from: The nanoparticles, the ions, or a combination of both? J Hazard Mater. 2016;308:328–34. https://doi.org/10.1016/j.jhazmat.2016.01.066.
Xiang B, Zhang G, Zhang Y, Wu C, Joshi S, Morris AJ, Ware J, Smyth SS, Whiteheart SW, Li Z. Calcium Ion Chelation Preserves Platelet Function During Cold Storage. Arterioscler Thromb Vasc Biol. 2021;41:234–49. https://doi.org/10.1161/atvbaha.120.314879.
Yagi H, Yamaguchi N, Shida Y, Sugimoto M, Tubaki K, Fujimura Y, Matsumoto M. Highly elevated plasma level of von Willebrand factor accelerates the formation of platelet thrombus under high shear stress in plasma with deficient ADAMTS13 activity. Thromb Res. 2017;159:91–5. https://doi.org/10.1016/j.thromres.2017.10.007.
Yamano S, Goto Y, Takeda T, Hirai S, Furukawa Y, Kikuchi Y, Kasai T, Misumi K, Suzuki M, Takanobu K, Senoh H, Saito M, Kondo H, Umeda Y. Pulmonary dust foci as rat pneumoconiosis lesion induced by titanium dioxide nanoparticles in 13-week inhalation study. Part Fibre Toxicol. 2022;19:58. https://doi.org/10.1186/s12989-022-00498-3.
Ye N, Wang Z, Wang S, Peijnenburg W. Toxicity of mixtures of zinc oxide and graphene oxide nanoparticles to aquatic organisms of different trophic level: particles outperform dissolved ions. Nanotoxicology. 2018;12:423–38. https://doi.org/10.1080/17435390.2018.1458342.
Yeini E, Satchi-Fainaro R. The role of P-selectin in cancer-associated thrombosis and beyond. Thromb Res. 2022;213(Suppl 1):S22–8. https://doi.org/10.1016/j.thromres.2021.12.027.
Yeung J, Li W, Holinstat M. Platelet Signaling and Disease: Targeted Therapy for Thrombosis and Other Related Diseases. Pharmacol Rev. 2018;70:526–48. https://doi.org/10.1124/pr.117.014530.
Zhang J, Xiang Q, Shen L, Ling J, Zhou C, Hu J, Chen L. Surface charge-dependent bioaccumulation dynamics of silver nanoparticles in freshwater algae. Chemosphere. 2020;247:125936. https://doi.org/10.1016/j.chemosphere.2020.125936.
Zhao J, Castranova V. Toxicology of nanomaterials used in nanomedicine. J Toxicol Environ Health Part B. 2011;14:593–632. https://doi.org/10.1080/10937404.2011.615113.
Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WHW, DiDonato JA, Brown JM, Lusis AJ, Hazen SL. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell. 2016;165:111–24. https://doi.org/10.1016/j.cell.2016.02.011.
Zlamal J, Singh A, Weich K, Jaffal H, Uzun G, Pelzl L, Althaus K, Bakchoul T. Platelet phosphatidylserine is the critical mediator of thrombosis in heparin-induced thrombocytopenia. Haematologica. 2023;108:2690–702. https://doi.org/10.3324/haematol.2022.282275.
Funding
This research was funded by the National Natural Science Foundation of China 82241090 (Y.X.), 82022063 (Y.X.), 82003500 (Y.B), 82211540403 (Y.B.) and Department of Science and Technology of Liaoning Province 2022JH2/20200035(Y.X.), 2022JH2/20200017 (Y.B.), 2023JH1/10400031 (L.X.).
Author information
Authors and Affiliations
Contributions
Yiying Bian: designed and performed most part of in vitro experiments, writing-review & editing, Funding acquisition, Supervision. Qiushuo Jin: writing original manuscript, and performed most part of animal experiments. Jinrui He and Thien Ngo: did partial animal experiments and data analysis. Ok-Nam Bae, Jingbo Pi and Han Young Chung: validation, data analysis and review. Liguo Xing: funding acquisition and revision. Yuanyuan Xu: writing - review & editing, funding acquisition, data interpretation, and supervision. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
With the approval from the Ethics Committee of the Health Service Center at Seoul National University, human blood was obtained from healthy male donors. All the animal protocols used in vivo experiments were approved by the Ethics Committee of the Animal Service Center at China Medical University (CMU20231000).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bian, Y., Jin, Q., He, J. et al. Biomedical application of TiO2NPs can cause arterial thrombotic risks through triggering procoagulant activity, activation and aggregation of platelets. Cell Biol Toxicol 40, 67 (2024). https://doi.org/10.1007/s10565-024-09908-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10565-024-09908-y