Abstract
Purinergic receptor P2Y11, a G protein-coupled receptor that is stimulated by extracellular ATP, has been demonstrated to be related to the chemotaxis of granulocytes, apoptosis of neutrophils, and secretion of cytokines in vitro. P2Y11 mutations were associated with narcolepsy. However, little is known about the roles of P2RY11 in the occurrence of narcolepsy and inflammatory response in vivo. In this study, we generated a zebrafish P2Y11 mutant using CRISPR/Cas9 genome editing and demonstrated that the P2Y11 mutant replicated the narcolepsy-like features including reduced HCRT expression and excessive daytime sleepiness, suggesting that P2Y11 is essential for HCRT expression. Furthermore, we accessed the cytokine expression in the mutant and revealed that the P2RY11 mutation disrupted the systemic inflammatory balance by reducing il4, il10 and tgfb, and increasing il6, tnfa, and il1b. In addition, the P2RY11-deficient larvae with caudal fin injuries exhibited significantly slower migration and less recruitment of neutrophils and macrophages at damaged site, and lower expression of anti-inflammatory cytokines during tissue damage. All these findings highlight the vital roles of P2RY11 in maintaining HCRT production and secreting anti-inflammatory cytokines in the native environment, and suggested that P2RY11-deficient zebrafish can serve as a reliable and unique model to further explore narcolepsy and inflammatory-related diseases with impaired neutrophil and macrophage responses.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extracellular ATP released from cellular stress or apoptosis can be recognized as a damage-associated molecular pattern (DAMP) and can lead to the coordinated activation of P2 purinergic receptors on immune cells (Klaver et al. 2022). Purinergic receptor P2Y11, belonging to the P2 purinergic receptors family of G protein-coupled receptors (GPCRs), is expressed in diverse immune cell types, including macrophages, dendritic cells, and neutrophils (Klaver and Thurnher 2021). It could couple to extracellular ATP and translate the ATP alarm into a cytoprotective response via immunomodulation (Di Virgilio et al. 2020). Activation of the P2Y11 receptor could trigger intracellular cAMP signaling and induce downstream protein kinase C (PKC) (Klaver et al. 2022).
Previous studies have reported that P2Y11-mediated purinergic signaling is involved in inflammatory response. The stimulation of P2Y11 could increase the chemotaxis of granulocytes, inhibit the apoptosis of neutrophils, and extend their survival (Kennedy 2017). Suppression of P2Y11 could inhibit the differentiation of the THP-1 cell line into M1 macrophages and the secretion of IL-6 following LPS stimulation (Sakaki et al. 2013). Interestingly, in human M2 macrophages, P2Y11 plays a role in the anti-inflammatory response via inhibiting TLR4-driven TNF-α secretion (Gruenbacher et al. 2021). The activation of P2Y11 reduced the increase in TNF-α induced by radiation and increased IL-10 release in the blood (Swennen et al. 2008). Recent evidence shows that P2Y11 functions as a key receptor for macrophages, playing a vital role in regulating cytokine expression and inhibiting virus replication upon nucleotide interactions (Andersen et al. 2023). However, almost all studies on the function of P2Y11 have only been conducted in various cell lines because the P2Y11 gene is absent in rodents. Thus, the function of P2Y11 in modulating the in vivo inflammatory response is still unknown.
SNP (rs2305795) of the P2RY11 gene is found to be related to narcolepsy with cataplexy (Narcolepsy type 1, NT1), which is an immune-related disease characterized by a significant loss of HCRT (hypocretin neuropeptide precursor; also known as orexin) neurons (Kornum et al. 2011). Besides rs2305795, other missense mutations of P2RY11 were also found in NT1 and led to a functional defect through both cAMP and Ca2+ signaling pathways (Degn et al. 2017). These studies have confirmed the correlation between the P2RY11 gene and narcolepsy. However, due to the lack of suitable animal models that can mimic the P2RY11 mutation in NT1, it has not been directly proven that the reduction of P2RY11 can lead to the occurrence of NT1.
Zebrafish (Danio rerio) has emerged as a unique model for studying sleep/wake disorders and inflammation due to its advantages of rapid ex utero development, transparent embryos, daytime activity, and an inflammatory response similar to mammals’ (Novoa and Figueras 2012). More importantly, P2RY11 is present in the zebrafish genome and shows the same syntenic location as those of other mammalian species, implying that zebrafish P2RY11 is indeed an ortholog of human P2RY11 (Dreisig and Kornum 2016). Furthermore, the role of HCRT in promoting wakefulness was conserved between zebrafish and mammals.
To better evaluate the connection between the reduction of P2RY11 and NT1, as well as the effect of P2RY11 deficiency on the inflammatory response in vivo, we generated and characterized a zebrafish P2RY11 loss-of-function mutant. Mutant larvae exhibited excessive daytime sleepiness and a lower level of HCRT expression, implying that zebrafish P2RY11 mutants convincingly model human narcolepsy. Moreover, P2RY11 deficiency caused elevated levels of pro-inflammatory cytokines and reduced levels of anti-inflammatory cytokines. It also decreased the expression of genes that mediate the chemotaxis of neutrophils and macrophages, leading to diminished accumulation of macrophages and neutrophils at the injury site during non-infectious inflammation. These findings suggest important roles of P2RY11 in the inflammatory response in vivo.
Materials and methods
Zebrafish husbandry and morpholino injection
Adult zebrafish were raised at 28°C in a professional recirculating water system under a light-dark cycle (14-h light and 10-h dark) (Kimmel et al. 1995). Larvae were maintained in E3 medium with methylene blue. The following strains were used in this study: Tg(hcrt:GFP), Tg(lyz:DsRed), and Tg(mpeg1:GFP). Zebrafish P2RY11 (NM_001204454.1) MO (5′-TGCATAAACTGTCGTTCTTCATCTCT-3’) and mismatch MO (5′-TGGAAAAAGTGTCCTTGTTCATCTC-3’) were obtained from Gene-Tools, LLC. Approximately 0.6 ng of each was injected into one-cell stage embryos of Tg(hcrt:GFP), Tg(lyz:DsRed), or Tg(mpeg1:GFP) to generate the morphants.
Generation of P2RY11 mutant zebrafish
Mutagenesis was carried out using CRISPR/Cas9 technology, as previously described (Vejnar et al. 2016). Cas9 messenger RNA was synthesized using the mMESSAGE mMACHINE SP6 kit (Invitrogen) after the Cas9 plasmid was linearized. A mixture of sgRNA and Cas9 mRNA was injected into zebrafish embryos at the one-cell stage. The embryos were raised to adulthood as the F0 generation and crossed with wild-type (WT) zebrafish to obtain F1 generation embryos lacking P2RY11. Finally, an F1 founder line with an 8-base pair deletion in the P2RY11 gene was obtained and used in subsequent experiments.
Neutral red staining
A 2.5 ug/ml neutral red solution (Solarbio) was prepared using E3 solution containing 0.003% PTU. Zebrafish larvae at 3 dpf were immersed in the solution for 8 h at 28.5 °C in dark. Images were taken with a microscope. Quantification was performed manually.
Sudan Black staining
Fixed larvae of P2RY11−8 bp and siblings at 3 dpf were immersed in SB solution (G1691; Solabio) for 30 min followed by washing with 70% ethanol. Images were taken with a microscope. Quantification was performed manually.
Tail fin amputation
The tail fin was amputated using P2RY11−8 bp mutants and siblings, or MO-injected Tg(lyz:DsRed) and Tg(mpeg1:GFP). The tail fin was amputated with a sterile scalpel on Petri dishes coated with 1.5% agarose under a stereomicroscope. Amputated larvae were maintained in the E3 medium. MO-injected Tg(lyz:DsRed) and Tg(mpeg1:GFP) larvae were anesthetized and observed under a fluorescence microscope for neutrophils at 2 h post-injury (hpi) and macrophages at 6 hpi. P2RY11−8 bp and its siblings were used for Sudan black staining and neutral red staining. Regenerated tail fins of P2RY11−8 bp and its siblings were amputated at 6 hpi, and then harvested for RNA extraction.
RNA extraction and quantitative real-time PCR
P2RY11−8 bp embryos and their siblings were harvested at 3 dpf. Amputated regenerated tail fins were harvested at 6 hpi. Total RNA was extracted using TRIzol reagent and then transcribed into cDNA with a HiFiScript cDNA Synthesis Kit (CWBIO, China). qRT-PCR was performed using a SYBR labeling system (CWBIO, China) on an Applied Biosystems system (Thermo Fisher Scientific, US). The relative expression was calculated using the 2-ΔΔCt method, normalized to the beta-actin level. The primer pairs were summarized in Table S1.
RNA sequencing analysis
Total RNA was extracted from 50 embryos of P2RY11−8 bp and siblings, quantified, and its integrity was checked (n = 3). The high-throughput RNA-sequencing (RNA-seq) analysis was performed on an Illumina HiSeq 4000 by Shanghai Aksomics Biotech in Shanghai, China. Briefly, RNA libraries were constructed and quantified with an Agilent 2100 Bioanalyzer and an ABI StepOnePlus RT-PCR system (Thermo Fisher, MA, USA). The raw trimmed reads were aligned to the zebrafish reference genome (GRCz11) using HISAT2 software (v2.1.0). The transcript abundance for each sample was estimated using StringTie (v1.3.3), and the FPKM values for gene and transcript levels were calculated using the R package Ballgown (v2.10.0). Differential expressed genes were identified based on the following criteria: fold change > 1.5 and P-value < 0.05.
Sleep/wake behaviors
A video‐tracking system was modified from a previous study and was applied to record 48-h continuous sleep/wake behaviors (Rihel et al. 2010). 12 larvae of P2RY11−8 bp and their siblings at 96 hpf were placed in a 96‐ well plate, with one larva in each well. 400 μL of E3 solution in each well can help maintain a nearly flat surface at the top of the wells, ensuring clear larval images with high resolution, brightness, and contrast. The 96‐well plate was illuminated with diffuse white light (7:30 AM to 9:30 PM) and constant infrared LED light. Images were recorded utilizing a video camera, which was equipped with a stationary megapixel lens (MP5018) and an infrared-penetrable filter, enabling the capture of infrared light. The entire system was kept at a steady temperature of 28°C and monitored for 48 h from 96 to 144 hpf.
Western blot and antibodies
Zebrafish larvae at 5 dpf or adult tail fins were lysed in RIPA (CWBIO, China) with PMSF (Sigma-Aldrich). The proteins were quantified, and subjected to SDS-PAGE. They were then transferred onto a PVDF membrane. The primary antibodies are listed as follows: mouse anti- GAPDH (1:5000; Abmart, China), mouse anti- actin (1:1000; Millipore), rabbit anti- zHCRT (1:1000; produced by HuaBio, Hangzhou, China), and rabbit anti- zP2RY11(1:1000 produced by HuaBio, Hangzhou, China).
Image acquisition and processing
The injured MO-injected Tg(lyz:DsRed) and Tg(mpeg1:GFP) larvae were mounted in 1% agarose after being anesthetized. Subsequently, the injured fin was imaged using a confocal microscope at 10 × . Images were taken every 3 min until 6 hpi for macrophages in Tg(mpeg1: GFP) or 2 hpi for neutrophils in Tg(lyz:DsRed). The images were used for analyzing the motility of macrophage and neutrophil with the software ImageJ (NIH, USA).
Data analysis
All experiments were performed in triplicate. One-way ANOVA and unpaired two-tailed t-tests were used to evaluate differences among the various groups. Graph Prism 6 and MATLAB were utilized for statistical analysis and chart creation. The data is presented as the mean ± standard deviation (SD). Significance was set as P < 0.05 (*), P < 0.01 (**), and P < 0.001 (***). A phylogenetic tree was constructed using the neighbor-joining method with 1000 bootstrap replicates in MEGA 7.0. Heatmaps were generated with Heatmapper. Gene Ontology (GO) term enrichment analysis was conducted using the sources in the Metascape website.
Results
Establishment of P2RY11 mutant
To elucidate the relationship between zebrafish P2Y11 and its corresponding orthologues in different taxa, a phylogenetic analysis were performed using the protein sequences of 8 families of P2Y receptors across 7 species (shrew, zebrafish, human, macaques, rat, mouse, and frog) using the neighbor-joining method (Fig. 1A). The P2Y receptors can be categorized into two clades. The P2Y11 cluster forms one clade with clusters of P2Y1, P2Y2, P2Y4, and P2Y6. Clusters of P2Y8, P2Y12, P2Y13, and P2Y14 forms another clade. As expected, zebrafish P2Y11 is located in the cluster of P2Y11.
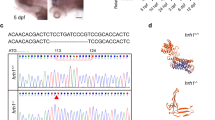
Phylogenetic trees of the P2YR family and the generation of a P2RY11−/− mutation in zebrafish using CRISPR-Cas9 editing. A. Phylogenetic analysis was conducted on the predicted protein sequences of 8 families of P2Y receptors across 7 species using the neighbor-joining method. B. Structure of the zebrafish P2RY11 gene and protein. A guide RNA was designed to specifically target exon 2. The P2RY11−/− allele was created by deleting 8 bases in exon 2. The "-" indicates the deleted nucleotides. The mutation results in a frameshift and premature truncation of the protein. The blue boxes represent the transmembrane domains of P2RY11 protein. C. Western blot and statistical analysis (n = 3) of the P2RY11 protein in the tail of the P2RY11−/− and P2RY11+/+ adult (3 mpf) zebrafish. D. The expression level of P2RY11 mRNA in the 3-dpf P2RY11−/− and P2RY11+/+ larvae was analyzed by qRT- PCR (n = 20). T-test, ***p < 0.001, **** p < 0.0001
To investigate the role of P2RY11 in sleep and inflammation, a P2RY11mutated zebrafish line was generated by targeting the exon 2 of P2RY11 (Fig. 1B). A P2RY11 mutation with an 8- bp deletion was identified, causing a frameshift mutation in the protein-coding region, which consequently triggered premature termination of translation. To verify the mutation, the expression of P2RY11 mRNA and protein were measured in WT and P2RY11 mutants. The results showed that the expression of P2RY11 was decreased in mRNA level and undetected at the protein level in P2RY11 mutants (Fig. 1C, D). Therefore, the generated P2RY11 (-8 bp) mutant is a loss-of-function mutation.
Morphological analysis of P2RY11-deficient zebrafish larvae
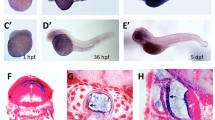
To examine whether P2RY11 deficiency would affect early development, we observed morphologic characteristics from 4-cell stage. No difference was found between the P2RY11−8 bp mutant and its siblings from 4-cell stage to 24 hpf. With development progressing, the eyes and head of P2RY11−8 bp larvae became significantly smaller than those of their siblings at 48, 72 and 96 hpf (Fig. 2A-C). Pericardial edema was also evident in P2RY11−8 bp larvae (Fig. 2A). The size of the pericardium was significantly larger when compared with the control siblings at 48, 72, and 96 hpf (Fig. 2D). In summary, the deficiency of P2RY11 could not affect the early embryonic development but impact later growth of the eye, head, and heart regions.
Morphological analysis of P2RY11−/− zebrafish larvae. A, Morphology of P2RY11−/− and P2RY11+/+ larvae at 48, 72, and 96 hpf. B-D, Statistical analysis for the head (B), eye (C), and pericardium (D) of P2RY11−/− and P2RY11+/+ larvae at 48 hpf (n = 28), 72 hpf (n = 23), and 96 hpf (n = 28). Scale bar: 500 μm. T-test for analysis, *p < 0.05, ** p < 0.01 ***p < 0.001, ****p < 0.0001
Deficiency of P2RY11 decreases the expression of HCRT
Given the association of P2RY11 with NT1, the expression of HCRT was examined in P2RY11-deficient larvae at 3 dpf. Both hcrt mRNA and HCRT protein were reduced in P2RY11−8 bp larvae compared to their WT controls (Fig. 3A, B). In order to investigate the impact of P2RY11 deficiency on HCRT neurons, we knocked down the expression of P2RY11 in Tg(hcrt:GFP) and observed a decrease in number of HCRT neurons and mRNA levels of hcrt in the P2RY11 deficient larvae (Fig. 3C-E).
Deficiency of P2RY11 reduces the HCRT expression. A, Reduced expression of hcrt mRNA in P2RY11−/− mutants at 3 dpf detected by RT-qPCR (n = 20). B, Western blot (n = 10) and statistical analysis (n = 3) of the HCRT protein in P2RY11−/− mutants at 3 dpf detected by western blot. C, Decreased HCRT neurons in P2RY11 MO injected larvae at 3 dpf (n = 20). D, Reduced expression of hcrt mRNA in P2RY11 MO morphants at 3dpf (n = 20). E, Western blot (n = 20) and statistical analysis (n = 3) of the P2RY11 protein in P2RY11 MO morphants at 3dpf. T-test (A, B, E) and one way ANOVA (D) were conducted. *p < 0.05, **p < 0.01 ***p < 0.001
Deficiency in P2RY11 increases sleepiness and reduced waking activity during the daytime
To examine whether the deficiency of P2RY11 could affect the sleep patterns of zebrafish larvae, we conducted a sleep/wake analysis from 96 to 144 hpf. The results showed that P2RY11−8 bp larvae exhibited reduced activity and increased rest during both night and daytime compared to their WT controls (Fig. 4A, B). The parameters regarding sleep/wake behavior were analyzed. P2RY11 deficiency could significantly increase duration of rest, the number of rest bouts, and length of rest bouts only during the daytime period (Fig. 4C-E). Accordingly, both total activity and waking activity of P2RY11−8 bp larvae were considerably lower than those of their WT controls in either daytime recorded (Fig. 4F; Fig. S1). Thus, P2RY11−8 bp larvae exhibited a changed sleep/wake pattern similar to the excessive daytime sleepiness observed in narcolepsy patients.
Abnormal sleep/wake pattern in P2RY11−/− mutants. A, B, showing time sequence photos of rest total (A) and waking activity (B). The red line represents the mutant group, while the blue line indicates the WT group. C-F, Parameter analysis showed that rest time (C) and rest bout length (D) were prolonged in P2RY11−/− mutants, with increased sleep frequency (E) during the daytime. Meanwhile, the waking activity (F) were decreased in the daytime recordings. The bars in black and white blow the x-axis indicate the tested nighttime and daytime periods, respectively. (t-test, n = 12, **p < 0.01, ***p < 0.001, ****p < 0.0001)
The lack of P2RY11 affects the expression of genes involved in the immune system process
Since P2RY11 is expressed in different immune cell types, and its activation exerts both pro- and anti-inflammatory effect (Gruenbacher et al. 2021), here we conducted a qRT-PCR analysis to measure the mRNA levels cytokines to detect the impact of P2RY11 deficiency on inflammatory status. Comparing with WT controls, the mRNA levels of il6, tnfa, and il1b were up-regulated, while the mRNA levels of il4, il10 and tgfb were down-regulated in the P2RY11−8 bp larvae (Fig. 5A).
Effect of P2RY11 mutation on the expression of genes related to the immune system process. A, Quantitative analysis of the expression of il6, tnfa, il1b, il4, il10 and tgfb. (n = 20). B, Gene Ontology analysis of the DE genes related to the immune system process. C, The relationship of the enriched GO terms. D, Heatmap demonstrating downregulation of genes in P2RY11−/− mutants involved in the main enriched terms (n = 3). E, qRT-PCR validation of the RNA-seq data. Downregulation of cxcl20, cora1a, ccl39.3, ccl34b.1, cyba, mpeg1, mmp13a, lyz (n = 20). T-test was performed, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
To analyze the function of P2RY11, RNA sequencing analysis was performed and 1693 differential expressed (DE) genes were identified. GO term analysis were further conducted on DE genes using the Metascape database. The results showed that the plasma membrane signaling receptor complex (GO:0098802), calcium ion binding (GO:0005509), and protein refolding (GO:0042026) were the most enriched terms in the up-regulated DE genes (Fig. S2A, B). While metabolic processes (GO:008152), cellular locations (GO:0051179) and cellular processes (GO:009987) were the most enriched terms in the down-regulated DE genes (Fig. S2C). In addition, the immune system process (GO:0002376) was also enriched in down-regulated DE genes of P2RY11−8 bp compared to WT controls (Fig. S2C). Based on the genes related to the immune system process, we further conducted GO term enrichment analysis and found that the defense response, leukocyte chemotaxis, and inflammatory response were the main enriched terms (Fig. 5B, C). Clustering analysis of the enriched GO term of the immune system process was conducted and showed that genes involved in neutrophil chemotaxis (cxcl8a, cxcl20, ccl25b, ccl39.3, wasb, lta4h, ccl44), inflammatory response (pycard, caspb, lta4h, anxa1c), defense response (tfa, mpeg1, hamp, lygl1, npsn, tnfrsf14l, wfdc2), and macrophage chemotaxis (mmp13a, cyba, cxcr3.2) were downregulated in P2RY11−8 bp compared to their WT controls (Fig. 5D). The expression of ccl34.b, coro1a, ccl39.3, cxcl20, cyba, mmp13a, lyz, and mpeg1 were detected to valid the RNA- seq data. It was found that mRNA levels of the selected genes were significantly reduced in P2RY11−8 bp larvae, consistent with the RNA-seq results (Fig. 5E). Therefore, the above results show that the absence of P2RY11 reduces the mRNA expression of several genes related to the chemotaxis of macrophages and neutrophils, inflammatory response, and defense response.
The lack of P2RY11 decreases the number of macrophages and neutrophils, as well as their accumulation at the injury site following fin amputation
Since P2RY11−8 bp larvae showed decreased expression of mpeg1 and lyz, which are specifically expressed in macrophages and neutrophils, respectively, we used Sudan Black staining and Neural red staining to label macrophages and neutrophils in P2RY11−8 bp larvae. We found that mutation of P2RY11 obviously reduced the numbers of neutrophils and macrophages in the caudal hematopoietic tissue (CHT) located at the ventral side of the tail (Fig. 6A, B).
Deficiency of P2RY11 reduced macrophages and neutrophils and their accumulation at the injury site following the fin amputation. A, The Sudan black B (SB) signal in siblings (upper) and P2RY11 mutants (lower) at 3 dpf (t-test, n = 10). B, The neutral red signal in siblings (upper) and P2RY11 mutants (lower) at 3 dpf in CHT (t-test, n = 10). C, recruited neutrophils to wounds following tail fin amputation. SB staining (upper) showed the recruitment of neutrophils at 2 hpi in 3dpf-siblings and P2RY11 mutants (t-test, n = 10). SB+ cells were significantly reduced in P2RY11−/− mutants in the quantified region (the black rectangle). The recruitment of neutrophils (RPF+ cells in the white rectangle) was also abolished in P2RY11 MO morphants at 3 dpf in the transgenic lyz:DsRed compared with the control morpholino-injected group (cm) and wild type group (WT) (one-way ANOVA, n = 11). D, recruited macrophages to wounds following tail fin amputation. Neutral red staining revealed the recruitment of macrophages at 6 hpi in 3dpf-siblings and P2RY11 mutants (t-test, n = 12). Neutral red+ cells were significantly reduced in P2RY11 mutants within the quantified region (the black rectangle). The recruitment of macrophages (GFP+ cells in the white rectangle) was also abolished in P2RY11 MO morphants at 3 dpf in the transgenic mpeg1: GFP compared with the control morpholino-injected group (CM) and wild- type group (WT) (one-way ANOVA, n = 12). E, Migration speed of neutrophils and macrophages in P2RY11 knock-down larvae during the process toward the wound edge (one-way ANOVA, n = 5). F, Quantitative analysis of il6, tnfa, il1b, il4, il10, and tgfb from tails of WT and mutants at 6 hpi (t-test, n = 20). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, Scale bar: 200 μm
Given the reduction of genes related to the chemotaxis of macrophage and neutrophil in P2RY11−8 bp larvae, a caudal fin injury was induced to stimulate non-infectious inflammatory responses and access the chemotaxis of macrophages and neutrophils. The numbers of neutrophils and macrophages at the damaged site reached their peaks at 2 hpi and 6 hpi, respectively. Thus, larvae were collected at 2 hpi to detect the number of neutrophils in the cut region. SB staining revealed that depletion of P2RY11 could significantly reduce the accumulation of neutrophils in the wound at 2 hpi, consistent with the findings in the P2RY11MO-injected transgenic line Tg (lyz: Dsred) (Fig. 6C). The macrophages were also examined in the cut region at 6 hpi in P2RY11−8 bp larvae and P2RY11MO-injected transgenic line Tg (mpeg1: GFP). Neutral red staining revealed that the number of macrophages at the wounded site of P2RY11−8 bp larvae was conspicuously lower than the control group (Fig. 6D). Similarly, larvae injected with P2RY11MO displayed a reduced accumulation of macrophages in the cut region at 6 hpi compared with the WT groups (Fig. 6D). Additionally, the migration of macrophages and neutrophils was detected and found to be slower in P2RY11 knocked-down larvae compared to WT larvae.
The cytokines in the tail at 6 hpi was also examined using qRT-PCR. It was found that tnfa and il1b was much higher in P2RY11−8 bp larvae than in the WT group, whereas il6 showed no difference between WT and P2RY11−8 bp larvae. Il4, il10, and tgfb showed decreased expression in P2RY11−8 bp larvae, especially conspicuously for tgfb expression. This suggests that the deficiency of P2RY11 exaggerated inflammatory responses and affect the balance of the inflammatory response during tissue damage (Fig. 6E).
Taken together, the above results show that the depletion of P2RY11 could affect the production of cytokines and the accumulation of the two types of leucocytes in the damaged tissue.
Discussion
In this study, we generated a P2RY11-deficient zebrafish line and revealed that a P2RY11 mutation exhibited similar features to NT1. Moreover, P2RY11 deficiency significantly promoted a systemic inflammatory response and affected gene expression related to the recruitment of both neutrophils and macrophages. Furthermore, the P2RY11 mutant exhibited deficiency in the accumulations of neutrophils and macrophages following tissue damage. All these findings highlight the significant roles of P2RY11 in maintaining HCRT expression and inflammatory response in vivo, which suggested that P2RY11 mutated zebrafish can function as an animal model to further explore the narcolepsy and inflammatory-related diseases with impaired neutrophil and macrophage responses.
Although the association between P2RY11 mutations or variants and NT1 has been confirmed in patients with narcolepsy, little is known about the function of P2RY11 in the development of the disease due to the absence of P2RY11 gene in rodents. In this study, P2RY11 mutant zebrafish exhibited the significant deformities such as microcephaly, microphthalmia, and pericardial edema initially at 48 hpf. Although the defects in P2RY11−/− larvae became less prominent in adulthood, the morphological abnormalities of larvae still suggest that the P2RY11 gene may be involved in other aspects beyond sleep regulation. We also demonstrated the reduction of HCRT expression and excessive daytime sleepiness in zebrafish P2RY11−/− mutants. Combined with the etiology and symptoms of NT1, it can be concluded that P2RY11 deletion can cause narcolepsy-like symptoms by reducing the production of HCRT. Given the significance of HCRT production during NT1 development, the zebrafish P2RY11−8 bp mutant serves as a dependable and distinctive model for NT1, which enables us to investigate HCRT abnormalities resulting from the mutant P2RY11 for the first time.
Previous studies have suggested that P2Y11-dependent inflammatory signaling exhibits either proinflammatory or anti-inflammatory effects depending on the specific in vitro conditions. In our study, we observed an increase in pro-inflammatory cytokines and a decrease of anti-inflammatory cytokines in the native environment of P2RY11−/− mutants. Both IL-4 and IL-10 are crucial anti-inflammatory cytokines that inhibit the release of TNF-α, IL-1β, and IL-6 in monocytes (te Velde et al. 1990, Zhang and An 2007). TGFb can counteract IL-1, IL-2, IL-6, and TNF, and shifting the state from active inflammation to resolution and repair (Zhang and An 2007). Therefore, the drastic decrease in anti-inflammatory cytokines in P2RY11−/− mutants relieved their suppression on the secretion of pro-inflammatory cytokines, resulting in an increased level of pro-inflammatory cytokines. Together, it can be inferred that P2RY11 is necessary for anti-inflammatory cytokines, thus playing a crucial role in sustaining the balance of the immune system.
Systemic inflammation has been linked to decreased HCRT levels in patients (Ogawa et al. 2022). Increased serum TNF-a levels were found in narcolepsy patients (Chen et al. 2013). Combined with the extremely high expression of il1b, il6 and tnfa found in zebrafish P2RY11−/− mutants, we speculate that decreased HCRT expression may be due to the increased pro-inflammatory cytokines in the P2RY11 mutant. This increase could trigger local inflammation in the brain (Fig. 7), ultimately leading to the cell death of HCRT neurons or the epigenetic silencing of HCRT (Seifinejad et al. 2023).
In addition to their important functions in innate immunity, macrophages and neutrophils contribute to wound healing and tissue regeneration (Li et al. 2012). Macrophages and neutrophils are recruited to sites of inflammation by gradients of chemoattractant (Kronlage et al. 2010). Here, we demonstrated that the slower migration and decreased accumulation of macrophages and neutrophils at the injury site, along with reduced expression of genes related with “leukocyte chemiotaxis” in the P2RY11−/− mutant, indicate that the participation of P2RY11 is crucial for neutrophils and macrophages to infiltrate the damaged tissue. Purinergic signaling plays important roles in regulating chemotaxis of neutrophils and macrophages along chemical gradients (Wang and Chen 2018; Desai and Leitinger 2014). Both cells can translate the sensing of a chemoattractant into gradient navigation by releasing ATP and the autocrinally stimulating purinergic receptors linked to their membrane protrusions (Wang and Chen 2018). P2Y receptors on macrophages and neutrophils play a critical role in ATP-responsive amplification of chemotaxis (Desai and Leitinger 2014). The relationship between P2RY11 and chemotactic navigation is completely unknown. It is worth further investigation as it might reveal the mechanism by which P2RY11 reduce the recruitment of macrophages and neutrophils. Additionally, we also observed that the numbers of neutrophils and macrophages in P2RY11−/− mutant CHT were significantly lower than wild-type larvae, suggesting a potential role of P2RY11 in myeloid hematopoiesis. On the other hand, it may also be a reason for the decreased recruitment of the two types of cells to the injury site.
In summary, we observed striking similarities in the expression of HCRT and daytime sleepiness between the NT1 patients and P2RY11-deficient zebrafish. Knockout of P2RY11 in zebrafish resulted in systemic inflammation, suggesting an essential role of P2RY11 in regulating the inflammatory response under normal physiological conditions. In addition, we revealed the vital function of P2RY11 in recruiting macrophages and neutrophils to damaged tissues. Our data expands understanding of the roles of P2RY11 in the occurrence of narcolepsy and inflammatory response. Therefore, our model provides an exciting novel tool to unravel the pathogenesis mechanisms of NT1, highlighting its typical traits, and to explore the function of P2RY11-mediated purinergic signaling in the infiltration of neutrophils and macrophages into damaged tissues.
Data availability
The data and materials of the study can be obtained from the corresponding author upon request. The RNA-seq data was submitted to the NCBI GEO repository with the accession number GSE264674.
References
Andersen LL, Huang Y, Urban C, Oubraham L, Winheim E, Stafford C, Nagl D, Oduill F, Ebert T, Engleitner T, Paludan SR, Krug A, Rad R, Hornung V, Pichlmair A. Systematic P2Y receptor survey identifies P2Y11 as modulator of immune responses and virus replication in macrophages. The EMBO Journal. 2023;42:e113279.
Chen Y-H, Huang Y-S, Chen C-H. Increased plasma level of tumor necrosis factor α in patients with narcolepsy in Taiwan. Sleep Med. 2013;14:1272–6.
Degn M, Dauvilliers Y, Dreisig K, Lopez R, Pfister C, Pradervand S, RahbekKornum B, Tafti M. Rare missense mutations in P2RY11 in narcolepsy with cataplexy. Brain : J Neurol. 2017;140:1657–68.
Desai BN, Leitinger N. Purinergic and calcium signaling in macrophage function and plasticity. Front Immunol. 2014;5:580.
di Virgilio F, Sarti AC, Coutinho-Silva R. Purinergic signaling, DAMPs, and inflammation. Am J Physiol Cell Physiol. 2020;318:C832–5.
Dreisig K, Kornum BR. A critical look at the function of the P2Y11 receptor. Purinergic Sign. 2016;12:427–37.
Gruenbacher G, Gander H, Dobler G, Rahm A, Klaver D, Thurnher M. The human G protein-coupled ATP receptor P2Y11 is a target for anti-inflammatory strategies. Br J Pharmacol. 2021;178:1541–55.
Kennedy C. P2Y11 receptors: properties, distribution and functions. Adv Exp Med Biol. 2017;1051(107):122.
Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dynam: Off Publ Am Assoc Anatom. 1995;203:253–310.
Klaver D, Gander H, Dobler G, Rahm A, Thurnher M. The P2Y11 receptor of human M2 macrophages activates canonical and IL-1 receptor signaling to translate the extracellular danger signal ATP into anti-inflammatory and pro-angiogenic responses. Cell Mol Life Sci: CMLS. 2022;79:519.
Klaver D, Thurnher M. Control of macrophage inflammation by P2Y purinergic receptors. Cells. 2021;10(5):1098.
Kornum BR, Kawashima M, Faraco J, Lin L, Rico TJ, Hesselson S, Axtell RC, Kuipers H, Weiner K, Hamacher A, Kassack MU, Han F, Knudsen S, Li J, Dong X, Winkelmann J, Plazzi G, Nevsimalova S, Hong S-C, Honda Y, Honda M, HöGL B, Ton TGN, Montplaisir J, Bourgin P, Kemlink D, Huang Y-S, Warby S, Einen M, Eshragh JL, Miyagawa T, Desautels A, Ruppert E, Hesla PE, Poli F, Pizza F, Frauscher B, Jeong J-H, Lee S-P, Strohl KP, Longstreth WT, Kvale M, Dobrovolna M, Ohayon MM, Nepom GT, Wichmann HE, Rouleau GA, Gieger C, Levinson DF, Gejman PV, Meitinger T, Peppard P, Young T, Jennum P, Steinman L, Tokunaga K, Kwok P-Y, Risch N, Hallmayer J, Mignot E. Common variants in P2RY11 are associated with narcolepsy. Nat Genet. 2011;43:66–71.
Kronlage M, Song J, Sorokin L, Isfort K, Schwerdtle T, Leipziger J, Robaye B, Conley PB, Kim H-C, Sargin S, Schön P, Schwab A, Hanley PJ. Autocrine purinergic receptor signaling is essential for macrophage chemotaxis. Sci Sign. 2010;3:ra55.
Li L, Yan B, Shi Y-Q, Zhang W-Q, Wen Z-L. Live imaging reveals differing roles of macrophages and neutrophils during zebrafish tail fin regeneration. J Biol Chem. 2012;287:25353–60.
Novoa B, Figueras A. Zebrafish: model for the study of inflammation and the innate immune response to infectious diseases. Adv Exp Med Biol. 2012;946:253–75.
Ogawa Y, Shimojo N, Ishii A, Tamaoka A, Kawano S, Inoue Y. Reduced CSF orexin levels in rats and patients with systemic inflammation: a preliminary study. BMC Res Notes. 2022;15:221.
Rihel J, Prober DA, Arvanites A, Lam K, Zimmerman S, Jang S, Haggarty SJ, Kokel D, Rubin LL, Peterson RT, Schier AF. Zebrafish behavioral profiling links drugs to biological targets and rest/wake regulation. Science (New York, NY). 2010;327:348–51.
Sakaki H, Tsukimoto M, Harada H, Moriyama Y, Kojima S. Autocrine regulation of macrophage activation via exocytosis of ATP and activation of P2Y11 receptor. PLoS ONE. 2013;8:e59778.
Seifinejad A, Ramosaj M, Shan L, Li S, Possovre M-L, Pfister C, Fronczek R, Garrett-Sinha LA, Frieser D, Honda M, Arribat Y, Grepper D, Amati F, Picot M, Agnoletto A, Iseli C, Chartrel N, Liblau R, Lammers GJ, Vassalli A, Tafti M. Epigenetic silencing of selected hypothalamic neuropeptides in narcolepsy with cataplexy. Proc Natl Acad Sci USA. 2023;120:e2220911120.
Swennen ELR, Dagnelie PC, van den Beucken T, Bast A. Radioprotective effects of ATP in human blood ex vivo. Biochem Biophys Res Commun. 2008;367:383–7.
te Velde AA, Huijbens RJ, Heije K, de Vries JE, Figdor CG. Interleukin-4 (IL-4) inhibits secretion of IL-1 beta, tumor necrosis factor alpha, and IL-6 by human monocytes. Blood. 1990;76:1392–7.
Vejnar CE, Moreno-Mateos MA, Cifuentes D, Bazzini AA, Giraldez AJ. Optimized CRISPR-Cas9 system for genome editing in zebrafish. Cold Spring Harbor Protocols. 2016.
Wang X, Chen D. Purinergic regulation of neutrophil function. Front Immunol. 2018;9:399.
Zhang J-M, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 2007;45(27):37.
Acknowledgments
The authors thank Dr. Shao Ming for sharing CRISPR/Cas9-related material (zCas9 plasmid). We thank the generous help for the animal facilities maintenance from Mr. Cui Jianlin
Funding
This work was financially supported by the National Natural Science Foundation of China (62027812, 81901346).
Author information
Authors and Affiliations
Contributions
C.D. conceived the project and designed the experiments. Z.L. performed most experiments. W.L. performed analysis of the RNA sequencing; W.Y., L.A., X.Q., and H.M. generated the P2RY11−/− mutants; G.Q. generated the Tg(hcrt:GFP); H.H. assisted in the quantitative analyses. S.M. and Z.X. provided important contributions to the behavior analysis. C.D. wrote the manuscript. All authors read and approved the final paper.
Corresponding authors
Ethics declarations
Ethical approval
This study was approved by the Ethics Committee of NanKai University. Animal experiments were conducted conforming to the approval from the Institutional Animal Care and Use Committee of Nankai University.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The P2RY11 mutation decreases the expression of HCRT and increases daytime sleepiness in zebrafish.
The P2RY11 is essential for the production of anti-inflammatory cytokines.
The P2RY11 mutation reduces the recruitment of macrophages and neutrophils to the injury site.
Zebrafish P2RY11 mutant is a reliable model to unravel the pathogenesis mechanisms of NT1.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, L., Wang, Lf., Wang, Yc. et al. Deficiency of P2RY11 causes narcolepsy and attenuates the recruitment of neutrophils and macrophages in the inflammatory response in zebrafish. Cell Biol Toxicol 40, 36 (2024). https://doi.org/10.1007/s10565-024-09882-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10565-024-09882-5