Abstract
The primary symptom of diabetic encephalopathy (DE), a kind of central diabetic neuropathy caused by diabetes mellitus (DM), is cognitive impairment. In addition, the tetracyclic oxindole alkaloid isorhynchophylline (IRN) helps lessen cognitive impairment. However, it is still unclear how IRN affects DM and DE and what mechanisms are involved. The effectiveness of IRN on brain insulin resistance was carefully examined in this work, both in vitro and in vivo. We found that IRN accelerates spliced form of X-box binding protein 1 (sXBP1) translocation into the nucleus under high glucose conditions in vitro. IRN also facilitates the nuclear association of pCREB with sXBP1 and the binding of regulatory subunits of phosphatidylinositol 3-kinase (PI3K) p85α or p85β with XBP1 to restore high glucose impairment. Also, IRN treatment improves high glucose–mediated impairment of insulin signaling, endoplasmic reticulum stress, and pyroptosis/apoptosis by depending on sXBP1 in vitro. In vivo studies suggested that IRN attenuates cognitive impairment, ameliorating peripheral insulin resistance, activating insulin signaling, inactivating activating transcription factor 6 (ATF6) and C/EBP homology protein (CHOP), and mitigating pyroptosis/apoptosis by stimulation of sXBP1 nuclear translocation in the brain. In summary, these data indicate that IRN contributes to maintaining insulin homeostasis by activating sXBP1 in the brain. Thus, IRN is a potent antidiabetic agent as well as an sXBP1 activator that has promising potential for the prevention or treatment of DE.








Similar content being viewed by others
Data availability
All data of the present study are available from the corresponding author upon reasonable requests.
Abbreviations
- AlCl 3 :
-
aluminum chloride
- AMPA :
-
alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid
- ANOVA :
-
one-way analysis of variance
- ASC :
-
apoptosis-associated speck-like protein containing a caspase recruitment domain
- ATF4 :
-
activating transcription factor 4
- ATF6 :
-
activating transcription factor 6
- BBB :
-
blood-brain barrier
- BCL2 :
-
B-cell lymphoma 2
- BDNF :
-
brain-derived neurotrophic factor
- BSA :
-
bovine serum albumin
- C/EBP :
-
CCAAT/enhancer binding protein
- caspase-1 :
-
cysteinyl aspartate–specific proteinase 1
- CCK‐8 :
-
Cell Counting Kit-8
- CHOP :
-
C/EBP homologous protein
- CL :
-
contacting latency
- CNS :
-
central nervous system
- Co-IP :
-
co-immunoprecipitation
- CREB :
-
cAMP response element binding protein
- DAPI :
-
2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride
- DCFH-DA :
-
2,7-dichlorodihydrofluorescein diacetate
- DE :
-
diabetic encephalopathy
- D-gal :
-
d-galactosamine
- DL :
-
drinking latency
- DM :
-
diabetes mellitus
- DMEM :
-
Dulbecco’s modified Eagle’s medium
- DNA :
-
deoxyribonucleic acid
- DNase :
-
deoxyribonuclease
- ECL :
-
efficient chemiluminescence
- EL :
-
escape latency
- ELISA :
-
enzyme-linked immunosorbent assay
- ERAD :
-
ER-associated degradation
- ERS :
-
endoplasmic reticulum stress
- ERSE :
-
ERS response element
- FACS :
-
fluorescent-activated cell sorting
- FBG :
-
fasting blood glucose
- FITC :
-
fluorescein isothiocyanate
- GABA :
-
gamma-aminobutyric acid
- GABRA1 :
-
gamma-aminobutyric receptor subunit α1
- GAP43 :
-
growth-associated protein 43
- GAPDH :
-
glyceraldehyde-3-phosphate dehydrogenase
- GSDMD :
-
recombinant gasdermin D
- HEK :
-
human embryonic kidney
- HEPES :
-
2-[4-(2-hydroxyethyl)-1-piperazinyl]ethanesulfonic acid
- HOMA‐IR :
-
homeostasis model assessment of insulin resistance
- i.p. :
-
intraperitoneally
- IL-1β :
-
interleukin-1 beta
- IR :
-
insulin resistance
- IRE1α :
-
inositol-requiring enzyme 1 alpha
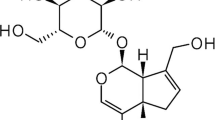
- IRN :
-
isorhynchophylline
- IRS1 :
-
insulin receptor substrate 1
- KRP :
-
Krebs‐Ringer phosphate
- m-IL-1β :
-
mature IL-1β
- mRNA :
-
messenger RNA
- MWM :
-
Morris water maze
- N2A :
-
mouse neuroblastoma N2a cells
- 2‐NBDG :
-
2-[N‐(7‐nitrobenz‐2‐oxa‐1,3‐diazol‐4‐yl)-amino)-2-deoxy-d-glucose
- NLRP3 :
-
NOD-like receptor protein 3
- NRF2 :
-
nuclear factor erythroid 2–related factor 2
- OS :
-
oxidative stress
- PBS :
-
phosphate buffered saline
- pCREB :
-
phosphorylation of cAMP response element binding protein
- pGAP43 :
-
phosphorylation of growth-associated protein 43
- PHAs :
-
primary hippocampal astrocytes
- PI :
-
propidium iodide
- PI3K :
-
phosphatidylinositol 3-kinase
- pTrkB :
-
phosphorylation of tropomyosin receptor kinase B
- PVDF :
-
polyvinylidene fluoride
- ROS :
-
reactive oxygen species
- SA% :
-
spontaneous alternation percentage
- SD :
-
Sprague Dawley
- SDS-PAGE :
-
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- shRNA :
-
short hairpin RNA
- shXBP1 :
-
short hairpin RNA of XBP1
- SPSS :
-
Statistical Product and Service Solutions
- STZ :
-
streptozotocin
- T1DM :
-
type 1 diabetes mellitus
- TrkB :
-
tropomyosin receptor kinase B
- TUNEL :
-
terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling
- UPR :
-
unfolded protein response
- XBP1 :
-
X-box binding protein 1
- YM :
-
Y-maze
References
Acosta-Alvear D, et al. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol Cell. 2007;27(1):53–66.
Akiyama M, et al. X-box binding protein 1 is essential for insulin regulation of pancreatic α-cell function. Diabetes. 2013;62(7):2439–49.
Albert ML. Death-defying immunity: do apoptotic cells influence antigen processing and presentation? Nat Rev Immunol. 2004;4(3):223–31.
Alonso M, Medina JH, Pozzo-Miller L. ERK1/2 activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons. Learn Mem. 2004;11(2):172–8.
Andrisani OM. CREB-mediated transcriptional control. Crit Rev Eukaryot Gene Expr. 1999;9(1):19–32.
Bandopadhyay R, de Belleroche J. Pathogenesis of Parkinson’s disease: emerging role of molecular chaperones. Trends Mol Med. 2010;16(1):27–36.
Bellezza I, et al. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta, Mol Cell Res. 2018;1865(5):721–33.
Bence NF, Sampat RM, Kopito RR. Impairment of the ubiquitin-proteasome system by protein aggregation. Science. 2001;292(5521):1552–5.
Bergsbaken T, Cookson BT. Macrophage activation redirects yersinia-infected host cell death from apoptosis to caspase-1-dependent pyroptosis. PLoS Pathog. 2007;3(11):09.
Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7(2):99–109.
Birnbaum Y, et al. Combined SGLT2 and DPP4 inhibition reduces the activation of the Nlrp3/ASC inflammasome and attenuates the development of diabetic nephropathy in mice with type 2 diabetes. Cardiovasc Drugs Ther. 2018;32(2):135–45.
Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011;18(2):139–43.
Cai XJ, Xu HQ, Lu Y. C-peptide and diabetic encephalopathy. Chin Med Sci J. 2011;26(2):119–25.
Chen Y, et al. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J. 1996;15(15):3853–60.
Czabotar PE, et al. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15(1):49–63.
Dey S, et al. Both transcriptional regulation and translational control of ATF4 are central to the integrated stress response. J Biol Chem. 2010;285(43):33165–74.
Díaz-Gerevini GT, et al. Diabetic encephalopathy: beneficial effects of supplementation with fatty acids ω3 and nordihydroguaiaretic acid in a spontaneous diabetes rat model. Lipids Health Dis. 2019;18(1):43.
Fang Y, et al. Pyroptosis: a new frontier in cancer. Biomed Pharmacother. 2020;121:109595.
Figurov A, et al. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381(6584):706–9.
Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8(11):1812–25.
Fink SL, Bergsbaken T, Cookson BT. Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proc Natl Acad Sci USA. 2008;105(11):4312–7.
Freed-Pastor WA, Prives C. Mutant p53: one name, many proteins. Genes Dev. 2012;26(12):1268–86.
Giordano A, et al. Obese adipocytes show ultrastructural features of stressed cells and die of pyroptosis. J Lipid Res. 2013;54(9):2423–36.
Guo Y, et al. Thioredoxin-1 is a target to attenuate Alzheimer-like pathology in diabetic encephalopathy by alleviating endoplasmic reticulum stress and oxidative stress. Front Physiol. 2021;12:651105.
He F, Ru X, Wen T. NRF2, a transcription factor for stress response and beyond. Int J Mol Sci. 2020;21(13):4777.
Hersh D, et al. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci USA. 1999;96(5):2396–401.
Hetz C, Papa FR. The unfolded protein response and cell fate control. Mol Cell. 2018;69(2):169–81.
Hetz C, et al. The unfolded protein response: integrating stress signals through the stress sensor IRE1α. Physiol Rev. 2011;91(4):1219–43.
Hillary RF, FitzGerald U. A lifetime of stress: ATF6 in development and homeostasis. J Biomed Sci. 2018;25(1):48.
Hu H, et al. The C/EBP homologous protein (CHOP) transcription factor functions in endoplasmic reticulum stress-induced apoptosis and microbial infection. Front Immunol. 2018;9:3083.
Jiang Y, et al. GLP-1 improves adipocyte insulin sensitivity following induction of endoplasmic reticulum stress. Front Pharmacol. 2018;9:1168.
Jin W. Regulation of BDNF-TrkB signaling and potential therapeutic strategies for Parkinson’s disease. J Clin Med. 2020;9(1):257.
Jurczak MJ, et al. Dissociation of inositol-requiring enzyme (IRE1α)-mediated c-Jun N-terminal kinase activation from hepatic insulin resistance in conditional X-box-binding protein-1 (XBP1) knock-out mice. J Biol Chem. 2012;287(4):2558–67.
Kanda N, Watanabe S. 17beta-estradiol inhibits oxidative stress-induced apoptosis in keratinocytes by promoting Bcl-2 expression. J Invest Dermatol. 2003;121(6):1500–9.
Kaser A, et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134(5):743–56.
Kawasumi M, et al. Targeted introduction of V642I mutation in amyloid precursor protein gene causes functional abnormality resembling early stage of Alzheimer’s disease in aged mice. Eur J Neurosci. 2004;19(10):2826–38.
Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7(12):1013–30.
Kraeuter AK, Guest PC, Sarnyai Z. The Y-maze for assessment of spatial working and reference memory in mice. Methods Mol Biol. 2019;1916:105–11.
Kumar V, et al. Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways. J Neurosci. 2005;25(49):11288–99.
Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23(21):7448–59.
Lee J, et al. p38 MAPK-mediated regulation of Xbp1s is crucial for glucose homeostasis. Nat Med. 2011;17(10):1251–60.
Lee HM, et al. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes. 2013;62(1):194–204.
Lee H, Baek SH, Lee JH, Kim C, Ko JH, Lee SG, Chinnathambi A, Alharbi SA, Yang WM, Um JY, Sethi G, Ahn KS. Isorhynchophylline, a potent plant alkaloid, induces apoptotic and anti-metastatic effects in human hepatocellular carcinoma cells through the modulation of diverse cell signaling cascades. Int J Mol Sci. 2017;18(5):1095.
Lepretti M, Martucciello S, Aceves MAB, Putti R, Lionetti L. Omega-3 fatty acids and insulin resistance: focus on the regulation of mitochondria and endoplasmic reticulum stress. Nutrients. 2018;10(3):350.
Li HQ, et al. Isorhynchophylline alleviates learning and memory impairments induced by aluminum chloride in mice. Chin Med. 2018;13:29.
Li HQ, et al. Isorhynchophylline ameliorates cognitive impairment via modulating amyloid pathology, tau hyperphosphorylation and neuroinflammation: studies in a transgenic mouse model of Alzheimer’s disease. Brain Behav Immun. 2019;82:264–78.
Li DX, et al. NLRP3 inflammasome-dependent pyroptosis and apoptosis in hippocampus neurons mediates depressive-like behavior in diabetic mice. Behav Brain Res. 2020;391:112684.
Lin Y, et al. Ginsenoside Rb2 improves insulin resistance by inhibiting adipocyte pyroptosis. Adipocyte. 2020a;9(1):302–12.
Lin J, Cheng A, Cheng K, Deng Q, Zhang S, Lan Z, Wang W, Chen J. New insights into the mechanisms of pyroptosis and implications for diabetic kidney disease. Int J Mol Sci. 2020b;21(19):7057.
Liu CL, et al. High-content screening identifies inhibitors of the nuclear translocation of ATF6. Int J Mol Med. 2016;37(2):407–14.
Lu J, Pang L, Zhang B, Gong Z, Song C. Silencing circANKRD36 inhibits streptozotocin-induced insulin resistance and inflammation in diabetic rats by targeting miR-145 via XBP1. Inflamm Res. 2021;70(6):695–704.
Luan B, et al. CREB pathway links PGE2 signaling with macrophage polarization. Proc Natl Acad Sci USA. 2015;112(51):15642–7.
Luc K, Schramm-Luc A, Guzik TJ, Mikolajczyk TP. Oxidative stress and inflammatory markers in prediabetes and diabetes. J Physiol Pharmacol. 2019;70(6). https://doi.org/10.26402/jpp.2019.6.01
Ma YL, et al. Brain-derived neurotrophic factor antisense oligonucleotide impairs memory retention and inhibits long-term potentiation in rats. Neuroscience. 1998;82(4):957–67.
Madhusudhan T, et al. Defective podocyte insulin signalling through p85-XBP1 promotes ATF6-dependent maladaptive ER-stress response in diabetic nephropathy. Nat Commun. 2015;6:6496.
Madhusudhan T, et al. Signal integration at the PI3K-p85-XBP1 hub endows coagulation protease activated protein C with insulin-like function. Blood. 2017;130(12):1445–55.
Mamiya T, et al. Enhancement of spatial attention in nociceptin/orphanin FQ receptor-knockout mice. Brain Res. 1998;783(2):236–40.
Mariathasan S, et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430(6996):213–8.
Mariathasan S, et al. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J Exp Med. 2005;202(8):1043–9.
Mariathasan S, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440(7081):228–32.
Maris M, et al. Deletion of C/EBP homologous protein (Chop) in C57Bl/6 mice dissociates obesity from insulin resistance. Diabetologia. 2012;55(4):1167–78.
Martinon F, et al. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol. 2010;11(5):411–8.
Matzinger M, Fischhuber K, Heiss EH. Activation of Nrf2 signaling by natural products-can it alleviate diabetes? Biotechnol Adv. 2018;36(6):1738–67.
May P, May E. Twenty years of p53 research: structural and functional aspects of the p53 protein. Oncogene. 1999;18(53):7621–36.
McLaughlin T, et al. Loss of XBP1 accelerates age-related decline in retinal function and neurodegeneration. Mol Neurodegener. 2018;13(1):16.
McLaughlin T, Siddiqi M, Wang JJ, Zhang SX. Loss of XBP1 leads to early-onset retinal neurodegeneration in a mouse model of type I diabetes. J Clin Med. 2019;8(6):906.
Mijnhout GS, et al. Diabetic encephalopathy: a concept in need of a definition. Diabetologia. 2006;49(6):1447–8.
Nakanishi K, Sudo T, Morishima N. Endoplasmic reticulum stress signaling transmitted by ATF6 mediates apoptosis during muscle development. J Cell Biol. 2005;169(4):555–60.
Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11(4):381–9.
Ozcan U, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306(5695):457–61.
Park SW, et al. The regulatory subunits of PI3K, p85alpha and p85beta, interact with XBP-1 and increase its nuclear translocation. Nat Med. 2010;16(4):429–37.
Raciti GA, et al. Glucosamine-induced endoplasmic reticulum stress affects GLUT4 expression via activating transcription factor 6 in rat and human skeletal muscle cells. Diabetologia. 2010;53(5):955–65.
Ramqvist T, et al. Wild-type p53 induces apoptosis in a Burkitt lymphoma (BL) line that carries mutant p53. Oncogene. 1993;8(6):1495–500.
Rojo de la Vega M, Chapman E, Zhang DD. NRF2 and the hallmarks of cancer. Cancer Cell. 2018;34(1):21–43.
Sage AT, et al. Metabolic syndrome and acute hyperglycemia are associated with endoplasmic reticulum stress in human mononuclear cells. Obesity (Silver Spring). 2012;20(4):748–55.
Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833(12):3460–70.
Schönthal AH. Endoplasmic reticulum stress: its role in disease and novel prospects for therapy. Scientifica (Cairo). 2012;2012:857516.
Seo HA, Lee IK. The role of Nrf2: adipocyte differentiation, obesity, and insulin resistance. Oxidative Med Cell Longev. 2013;2013:184598.
Sha H, et al. Stressed out about obesity: IRE1α-XBP1 in metabolic disorders. Trends Endocrinol Metab. 2011;22(9):374–81.
Shao D, et al. CHOP mediates XBP1S-induced renal mesangial cell necrosis following high glucose treatment. Eur J Pharmacol. 2015;758:89–96.
Shen X, et al. Genetic interactions due to constitutive and inducible gene regulation mediated by the unfolded protein response in C. elegans. PLoS Genet. 2005;1(3):e37.
Soares JLS, et al. Gain-of-function variants in NLRP1 protect against the development of diabetic kidney disease: NLRP1 inflammasome role in metabolic stress sensing? Clin Immunol. 2018;187:46–9.
Soppet D, et al. The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell. 1991;65(5):895–903.
Stienstra R, et al. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci U S A. 2011;108(37):15324–9.
Takatani T, et al. IRS1 deficiency protects β-cells against ER stress-induced apoptosis by modulating sXBP-1 stability and protein translation. Sci Rep. 2016;6:28177.
Takechi R, et al. Blood-brain barrier dysfunction precedes cognitive decline and neurodegeneration in diabetic insulin resistant mouse model: an implication for causal link. Front Aging Neurosci. 2017;9:399.
Tan Z, et al. ZIKV infection activates the IRE1-XBP1 and ATF6 pathways of unfolded protein response in neural cells. J Neuroinflammation. 2018;15(1):275.
Thumbikat P, et al. Mechanisms underlying Mannheimia haemolytica leukotoxin-induced oncosis and apoptosis of bovine alveolar macrophages. Microb Pathog. 2005;38(4):161–72.
Wang P, Yan H, Li JC. CREB-mediated Bcl-2 expression in trichosanthin-induced Hela cell apoptosis. Biochem Biophys Res Commun. 2007;363(1):101–5.
Wang Z, et al. Endoplasmic reticulum stress-induced neuronal inflammatory response and apoptosis likely plays a key role in the development of diabetic encephalopathy. Oncotarget. 2016;7(48):78455–72.
Wang L, et al. Knockout of TRPC6 promotes insulin resistance and exacerbates glomerular injury in Akita mice. Kidney Int. 2019;95(2):321–32.
Wang C, et al. Diabetic encephalopathy causes the imbalance of neural activities between hippocampal glutamatergic neurons and GABAergic neurons in mice. Brain Res. 2020;1742:146863.
Winnay JN, et al. A regulatory subunit of phosphoinositide 3-kinase increases the nuclear accumulation of X-box-binding protein-1 to modulate the unfolded protein response. Nat Med. 2010;16(4):438–45.
Xian YF, et al. Protective effect of isorhynchophylline against β-amyloid-induced neurotoxicity in PC12 cells. Cell Mol Neurobiol. 2012;32(3):353–60.
Xian YF, et al. Isorhynchophylline protects PC12 cells against beta-amyloid-induced apoptosis via PI3K/Akt signaling pathway. Evid Based Complement Alternat Med. 2013;2013:163057.
Xian YF, et al. Isorhynchophylline improves learning and memory impairments induced by D-galactose in mice. Neurochem Int. 2014a;76:42–9.
Xian YF, et al. Isorhynchophylline treatment improves the amyloid-β-induced cognitive impairment in rats via inhibition of neuronal apoptosis and tau protein hyperphosphorylation. J Alzheimers Dis. 2014b;39(2):331–46.
Xiang W, et al. CREB down-regulation in the laterodorsal thalamic nucleus deteriorates memory consolidation in rats. Learn Mem. 2019;26(6):182–6.
Xie C, et al. lncRNA GAS5/miR-452-5p reduces oxidative stress and pyroptosis of high-dlucose-stimulated renal tubular cells. Diabetes Metab Syndr Obes. 2019;12:2609–17.
Xiong W, et al. Atorvastatin inhibits endoplasmic reticulum stress through AMPK signaling pathway in atherosclerosis in mice. Exp Ther Med. 2020;19(3):2266–72.
Xu J, et al. Advances in the relationship between pyroptosis and diabetic neuropathy. Front Cell Dev Biol. 2021;9:753660.
Xue HY, et al. Neuroprotective properties of aucubin in diabetic rats and diabetic encephalopathy rats. Mol Biol Rep. 2012;39(10):9311–8.
Yamamoto K, et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell. 2007;13(3):365–76.
Yang Y, et al. Transcription factor C/EBP homologous protein in health and diseases. Front Immunol. 2017;8:1612.
Ye Z, et al. Parecoxib suppresses CHOP and Foxo1 nuclear translocation, but increases GRP78 levels in a rat model of focal ischemia. Neurochem Res. 2013;38(4):686–93.
Yoon SB, et al. Developmental competence of bovine early embryos depends on the coupled response between oxidative and endoplasmic reticulum stress. Biol Reprod. 2014;90(5):104.
Yoshida H, et al. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107(7):881–91.
Zeinivand M, Nahavandi A, Zare M. Deferoxamine regulates neuroinflammation and oxidative stress in rats with diabetes-induced cognitive dysfunction. Inflammopharmacology. 2020;28(2):575–83.
Zhai Y, Meng X, Ye T, Xie W, Sun G, Sun X. Inhibiting the NLRP3 inflammasome activation with MCC950 ameliorates diabetic encephalopathy in db/db mice. Molecules. 2018;23(3):522.
Zhang Y, et al. Amino acid deprivation induces CREBZF/Zhangfei expression via an AARE-like element in the promoter. Biochem Biophys Res Commun. 2010;391(3):1352–7.
Zhang YN, Yang YF, Xu W, Yang XW. The blood-brain barrier permeability of six indole alkaloids from Uncariae Ramulus Cum Uncis in the MDCK-pHaMDR cell monolayer model. Molecules. 2017;22(11):1944.
Zhang C, et al. Evidence on integrating pharmacokinetics to find truly therapeutic agent for Alzheimer’s disease: comparative pharmacokinetics and disposition kinetics profiles of stereoisomers isorhynchophylline and thynchophylline in rats. Evid Based Complement Alternat Med. 2019a;2019:4016323.
Zhang T, et al. Transcription factor p53 suppresses tumor growth by prompting pyroptosis in non-small-cell lung cancer. Oxidative Med Cell Longev. 2019b;2019:8746895.
Zhao Y, et al. The dynamic changes of endoplasmic reticulum stress pathway markers GRP78 and CHOP in the hippocampus of diabetic mice. Brain Res Bull. 2015;111:27–35.
Zheng Q, et al. Isorhynchophylline ameliorates paraquat-induced acute kidney injury by attenuating oxidative stress and mitochondrial damage via regulating toll-interacting expression. Toxicol Appl Pharmacol. 2021;420:115521.
Zhou JY, Zhou SW. Isorhynchophylline: a plant alkaloid with therapeutic potential for cardiovascular and central nervous system diseases. Fitoterapia. 2012;83(4):617–26.
Zhou Y, et al. Regulation of glucose homeostasis through a XBP-1-FoxO1 interaction. Nat Med. 2011;17(3):356–65.
Zhou Z, Su Y, Fa XE. Isorhynchophylline exerts anti-inflammatory and anti-oxidative activities in LPS-stimulated murine alveolar macrophages. Life Sci. 2019;223:137–45.
Acknowledgements
We thank Prof. Yuqiang Ding for the critical reading of the manuscript. We thank Dr. Haoqi Ni for optimizing the image analysis method and technical supports.
Funding
This study was supported by the Basic Scientific Research Projects of Wenzhou City (Y20180076), the Natural Science Foundation of Zhejiang province (LY21H030012), and the Natural Science Foundation of China (81671042, 81300308).
Author information
Authors and Affiliations
Contributions
Saidan Ding substantially contributed to the study conception and design, data interpretation, and manuscript revision. Jian Wang and Xuebao Wang performed all the in vitro assays and data analysis. Yongheng Bai, Yiru Ye, and Baihui Chen performed the in vivo experiments and data analysis. Yan Lang and Minxue Zhang contributed to the manuscript preparation. All the authors contributed to the manuscript revision and read and approved the final article.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The study was approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University. The study was approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Fig. S1
insulin-resistant PHAs and insulin-resistant CTX TNA2 cells are successfully established. (a,b) 2-NBDG uptake assay of PHAs (a) and CTX TNA2 cells (b) stimulated with 100 nM insulin for 30 min in preincubation of various concentrations of glucose (20, 40 or 80 mM ) for 24hr by using a fluorometric plate reader. Data are shown as mean ± SD.* P <0.05, ** P <0.01. ns, nonsignificant. (JPG 80 kb)

Fig. S2
2-NBDG uptake assay of PHAs. (a) PHAs were stimulated with 100 nM insulin for 30 min in pretreatment of various concentrations of IRN (1, 10 or 50μM) for 12h after preincubation of 40 mM glucose for 24hr. 2-NBDG uptake assay of PHAs by using a fluorescence microscope. (PNG 211 kb)
Fig. S3
IRN increases glucose uptake in insulin-resistant CTX TNA2 cells. (a) 2-NBDG uptake assay of CTX TNA2 cells stimulated with 100nM insulin for 30 min in pretreatment of various concentrations of IRN (1, 10 or 50μM) for 12hr after preincubation of 40 mM glucose for 24hr by using a fluorometric plate reader. Data are shown as mean ± SD.* P <0.05, ** P <0.01. ns, nonsignificant. (JPG 80 kb)
Fig. S4
IRN promotes sXBP1 nuclear translocation in insulin-resistant astrocytes. (a) CTX TNA2 cells were treated with IRN (10μM) for 12h after preincubation of 40 mM glucose for 24hr. Immunoblot analysis of total/nuclear/cytoplasmic lysates of CTX TNA2 cells using anti-sXBP1/GAPDH/laminB1 antibodies and subsequent quantification (b). Data are shown as mean ± SD. * P <0.05, ** P <0.01. ns, nonsignificant. (JPG 161 kb)
Fig. S5
The expression of XBP1 and sXBP1 nuclear translocation are decreased after shXBP1 knockdown in PHAs. (a,b) Immunoblot analysis of total/nuclear lysates of PHAs after shXBP1 infection using anti‐sXBP1/ GAPDH/laminB1 antibodies (a) and subsequent densitometry (b). Data are shown as mean ± SD.* P <0.05, ** P <0.01. ns, nonsignificant. (JPG 108 kb)
Fig. S6
The expression of XBP1 and sXBP1 nuclear translocation are decreased after shXBP1 knockdown in CTX TNA2 cells. (a,b) Immunoblot analysis of total/nuclear lysates of CTX TNA2 cells after shXBP1 infection using anti‐sXBP1/ GAPDH/laminB1 antibodies (a) and subsequent densitometry (b). Data are shown as mean ± SD.* P <0.05, ** P <0.01. ns, nonsignificant. (JPG 115 kb)
Fig. S7
The expression of XBP1 and sXBP1 nuclear translocation are increased after XBP1 overexpression in PHAs. (a,b) Immunoblot analysis of total/nuclear lysates of PHAs after XBP1 infection using anti‐sXBP1/ GAPDH/laminB1 antibodies (a) and subsequent densitometry (b). Data are shown as mean ± SD.* P <0.05, ** P <0.01. ns, nonsignificant. (JPG 113 kb)
Fig. S8
IRN activates insulin signaling via XBP1 activation in insulin-resistant astrocytes. (a) PHAs with or without transfection of XBP1 shRNA were stimulated with 100 nM insulin for 30 min in pretreatment of 50μM IRN for 12h after preincubation of 40 mM glucose for 24hr. 2-NBDG uptake assay of PHAs by using a fluorometric plate reader. (b) Immunoblot analysis of lysates of PHAs using anti-pInsR-Tyr1361/InsR/pIRS1-Tyr896/ pIRS1-Ser312/IRS1 antibodies and subsequent densitometry (c). (d) PHAs with or without transfection of XBP1 construct, were stimulated with 100 nM insulin for 30 min in pretreatment of 50μM IRN for 12h after preincubation of 40 mM glucose for 24hr. Immunoblot analysis of lysates of PHAs using anti-pInsR-Tyr1361/InsR/pIRS1-Tyr896/pIRS1-Ser312/IRS1 antibodies and subsequent densitometry (e). (f) CTX TNA2 cells with or without transfection of XBP1 shRNA, were stimulated with 100 nM insulin for 30 min in pretreatment of 50μM IRN for 12h after preincubation of 40 mM glucose for 24hr. 2-NBDG uptake assay of CTX TNA2 cells by using a fluorometric plate reader. Data are shown as mean ± SD.* P <0.05, ** P <0.01. ns, nonsignificant. (JPG 150 kb)

Fig. S9
IRN accelerates CREB-sensitive gene transcription via sXBP1 in insulin-resistant PHAs. (a) PHAs with cotransfection of a luciferase reporter construct driven by CREB and a shRNA construct against XBP1, were stimulated with 100 nM insulin for 30 min in pretreatment of 50μM IRN for 12h after preincubation of 40 mM glucose for 24hr. Assay for CREB transcriptional activation of PHAs via the luminometry. Data are shown as mean ± SD. * P <0.05, ** P <0.01. ns, nonsignificant. (PNG 145 kb)
Fig. S10
Analyses of production of reactive oxygen species (ROS). (a,b) Flow cytometric analyses of production of ROS of PHAs (a) and relative median fluorescence intensity (MFI) (b). (JPG 45 kb)
Fig. S11
Analyses of apoptosis of N2A cells. (a,b) Flow cytometric analyses of apoptosis of N2A cells (a) and relative MFI (b). (JPG 48 kb)
Fig. S12
IRN increases p85β -sXBP1 interaction in brain in STZ-induced diabetic mice. (a) STZ-induced diabetic mice were intraperitoneally treated with various concentrations of IRN (20,40 or 80mg/kg) or 40mg/kg IRN for 6 weeks. Immunoblot analysis of p85β/sXBP1 proteins in hippocampal lysates immunoprecipitated with anti- p85β antibody. (JPG 74 kb)
Fig. S13
The expression of XBP1 and sXBP1 nuclear translocation are decreased after shXBP1 knockdown in brain in STZ-induced diabetic mice. (a) Immunoblot analysis of total/nuclear lysates of brain of WT mice or diabetic mice after shXBP1 infection using anti‐sXBP1/ GAPDH/laminB1 antibodies (a) and subsequent densitometry (b). Data are shown as mean ± SD. * P <.05, ** P <.01. ns, nonsignificant. WT, wild type. (JPG 63 kb)
Fig. S14
IRN protects against ERS in STZ-induced diabetic mice. (a) STZ-induced diabetic mice were intraperitoneally treated with IRN (40 mg/kg) for 6 weeks. Immunoblot analysis of total/nuclear hippocampal lysates using anti-ATF6/CHOP/laminB1/GAPDH antibodies and subsequent quantification (b). Data are shown as mean ± SD. * P <0.05, ** P <0.01. ns, nonsignificant. (JPG 299 kb)
Fig. S15
IRN attenuates insulin resistance via sXBP1 in brain in STZ-induced diabetic mice. (a-c) STZ-induced diabetic mice with or without intracerebroventricular XBP1 shRNA transfection for 24hr were intraperitoneally treated with various concentrations of IRN (20, 40 or 80mg/kg) or 40mg/kg IRN for 6 weeks. Assay for fasting plasma insulin levels (FINS, a) and fasting plasma insulin levels (FBG, b) in each group. (c) HOMA -IR was calculated from fasting glucose and insulin levels in panels (a) and (b). Data are shown as mean ± SD. * P <0.05, ** P <0.01. ns, nonsignificant. (JPG 86 kb)

Fig. S16
IRN attenuates cognitive impairment and insulin resistance in db/db mice. (a-d) db/db mice were intraperitoneally injected with various concentrations of IRN (20,40 or 80mg/kg) or curcumin (50mg/kg) for 6 weeks. Morris water maze in each group. Representative moving patterns in the probe trials (a). Quantification of the spent time of platform (b). Quantification of the average speed (c). YM in each group. Quantification of spontaneous alternation percentage (SA%) (d). (e-g) Assay for fasting plasma insulin levels (FINS, e) and fasting blood glucose levels (FBG, f) in each group. (g) HOMA -IR was calculated from fasting glucose and insulin levels in panels (e) and (f). Data are shown as mean ± SD. * P <0.05, ** P <0.01. ns, nonsignificant. (PNG 1571 kb)
Fig. S17
IRN increases sXBP1 nuclear translocation in brain in db/db mice. (a) db/db mice were intraperitoneally treated with various concentrations of IRN (20,40 or 80mg/kg) for 6 weeks. Immunoblot analysis of total/nuclear hippocampal lysates using anti-sXBP1/pIRE1α/IRE1α/laminB1/GAPDH antibodies (a) and subsequent densitometry (b). Data are shown as mean ± SD. * P <0.05, ** P <0.01. ns, nonsignificant. (JPG 77 kb)
Fig. S18
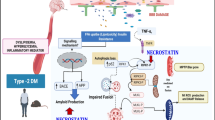
Graphical abstract. IRN attenuates cognitive impairment, ameliorating peripheral insulin resistance, activating insulin signaling, inhibiting ERS, and mitigating pyroptosis/apoptosis by depending on sXBP1 activation in diabetic encephalopathy. (JPG 559 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, J., Wang, X., Zhang, M. et al. The activation of spliced X-box binding protein 1 by isorhynchophylline therapy improves diabetic encephalopathy. Cell Biol Toxicol 39, 2587–2613 (2023). https://doi.org/10.1007/s10565-022-09789-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-022-09789-z