Abstract
Bacground
Sodium–glucose cotransporter 2 (SGLT2) inhibitors and dipeptidyl peptidase-4 inhibitors (DPP4I) are used to treat type 2 diabetes (T2DM). DPP4 inhibitors (DPP4) attenuate Nlrp3 inflammasome activation in the kidney. SGLT2 inhibition reduces inflammation and attenuates the progression of diabetic nephropathy (DN). The effects of dapagliflozin (Dapa) on the activation of the Nlrp3 inflammasome and the combined effect of SGLT2 and DPP4 on T2DM-induced inflammasome activation and progression of DN have not been previously studied. We assessed whether Dapa attenuates the inflammasome activation and progression of DN in T2DM mice and whether these effects can be augmented by adding DPP4I saxagliptin (Saxa).
Methods and Results
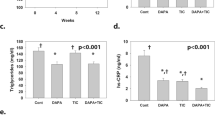
Male BTBR ob/ob and wild-type (WT) mice received vehicle, Dapa, or Dapa+Saxa for 8 weeks. Serum BUN in the WT mice was 16.9 ± 0.8 mg/dl. It increased to 55.7 ± 2.8 mg/dl in the BTBR mice. Dapa alone reduced BUN to 31.4 ± 1.2 mg/dl. A greater effect was seen in the Dapa+Saxa combination (24.8 ± 0.8 mg/dl). Serum creatinine was 0.16 ± 0.02 and 1.01 ± 0.04 mg/dl in the WT and BTBR mice, respectively. Dapa and Dapa+Saxa attenuated the increase of creatinine to 0.65 ± 0.02 and 0.40 ± 0.03 mg/dl, respectively. Serum cystatin C was elevated in the BTBR mice (3.9 ± 0.1 vs. 0.6 ± 0.2 ng/ml) as compared to WT mice. Dapa (2.4 ± 0.1) and Dapa+Saxa (1.4 ± 0.1) attenuated this increase. Kidney weight was higher in the BTBR than that of WT mice. Dapa reduced the kidney/body weight ratio in the BTBR mice. Dapa+Saxa tended to have greater effect, but the difference was not significant. mRNA levels of NALP3, ASC, IL-1β, IL-6, caspase-1, TNF-α, collagen-1, and collagen-3 significantly increased in the kidneys of the BTBR compared to the WT mice. Dapa alone and to a greater extent, Dapa+Saxa, attenuated the activation of the inflammasome. Yet, the combination did not result in greater attenuation of the collagen-1 and collagen-3 mRNA levels. The P-AMPK/total AMPK ratio was lower in the BTBR mice than in the WT mice. Dapa and Dapa+ Saxa equally increased the ratio. Conclusions: Dapa attenuates T2DM-induced activation of the inflammasome and progression of DN in BTBR ob/ob mice. Adding Saxa to Dapa augmented attenuation of the inflammasome, but had no significant effect on kidney weight or collagen-1 and collagen-3 mRNA levels. Future clinical trials are necessary to study the effect of combined SGLT2 inhibitor and incretin therapy on renal outcomes in patients with T2DM.







Similar content being viewed by others
References
Wanner C, Inzucchi SE, Zinman B. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:1801–2.
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57.
Buse JB. The LSCa. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:1798–9.
Mann JFE, Orsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377:839–48.
Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–44.
Mosenzon O, Leibowitz G, Bhatt DL, Cahn A, Hirshberg B, Wei C, et al. Effect of saxagliptin on renal outcomes in the SAVOR-TIMI 53 trial. Diabetes Care. 2017;40:69–76.
Birnbaum Y, Bajaj M, Qian J, Ye Y. Dipeptidyl peptidase-4 inhibition by saxagliptin prevents inflammation and renal injury by targeting the Nlrp3/ASC inflammasome. BMJ Open Diabetes Res Care. 2016;4:e000227.
Ye Y, Bajaj M, Yang HC, Perez-Polo JR, Birnbaum Y. SGLT-2 inhibition with dapagliflozin reduces the activation of the Nlrp3/ASC inflammasome and attenuates the development of diabetic cardiomyopathy in mice with type 2 diabetes. Further augmentation of the effects with saxagliptin, a DPP4 inhibitor. Cardiovasc Drugs Ther. 2017;31:119–32.
Hudkins KL, Pichaiwong W, Wietecha T, Kowalewska J, Banas MC, Spencer MW, et al. BTBR Ob/Ob mutant mice model progressive diabetic nephropathy. J Am Soc Nephrol: JASN. 2010;21:1533–42.
De Nicola L, Gabbai FB, Liberti ME, Sagliocca A, Conte G, Minutolo R. Sodium/glucose cotransporter 2 inhibitors and prevention of diabetic nephropathy: targeting the renal tubule in diabetes. Am J Kidney Dis. 2014;64:16–24.
Tahara A, Kurosaki E, Yokono M, Yamajuku D, Kihara R, Hayashizaki Y, et al. Effects of SGLT2 selective inhibitor ipragliflozin on hyperglycemia, hyperlipidemia, hepatic steatosis, oxidative stress, inflammation, and obesity in type 2 diabetic mice. Eur J Pharmacol. 2013;715:246–55.
Benetti E, Mastrocola R, Vitarelli G, Cutrin JC, Nigro D, Chiazza F, et al. Empagliflozin protects against diet-induced NLRP-3 inflammasome activation and lipid accumulation. J Pharmacol Exp Ther. 2016;359:45–53.
Tahara A, Kurosaki E, Yokono M, Yamajuku D, Kihara R, Hayashizaki Y, et al. Effects of sodium-glucose cotransporter 2 selective inhibitor ipragliflozin on hyperglycaemia, oxidative stress, inflammation and liver injury in streptozotocin-induced type 1 diabetic rats. J Pharm Pharmacol. 2014;66:975–87.
Vallon V, Rose M, Gerasimova M, Satriano J, Platt KA, Koepsell H, et al. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Renal Physiol. 2013;304:F156–67.
Gangadharan KM, Gross S, Mudaliar H, Huang C, Pegg K, Mather A, et al. Inhibition of kidney proximal tubular glucose reabsorption does not prevent against diabetic nephropathy in type 1 diabetic eNOS knockout mice. PLoS One. 2014;9:e108994.
Ojima A, Matsui T, Nishino Y, Nakamura N, Yamagishi S. Empagliflozin, an inhibitor of sodium-glucose cotransporter 2 exerts anti-inflammatory and antifibrotic effects on experimental diabetic nephropathy partly by suppressing AGEs-receptor axis. Horm Metab Res. 2015;47:686–92.
Vallon V, Gerasimova M, Rose MA, Masuda T, Satriano J, Mayoux E, et al. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am J Physiol Renal Physiol. 2014;306:F194–204.
Kojima N, Williams JM, Takahashi T, Miyata N, Roman RJ. Effects of a new SGLT2 inhibitor, luseogliflozin, on diabetic nephropathy in T2DN rats. J Pharmacol Exp Ther. 2013;345:464–72.
Gembardt F, Bartaun C, Jarzebska N, Mayoux E, Todorov VT, Hohenstein B, et al. The SGLT2 inhibitor empagliflozin ameliorates early features of diabetic nephropathy in BTBR ob/ob type 2 diabetic mice with and without hypertension. Am J Physiol Renal Physiol. 2014;307:F317–25.
Terami N, Ogawa D, Tachibana H, Hatanaka T, Wada J, Nakatsuka A, et al. Long-term treatment with the sodium glucose cotransporter 2 inhibitor, dapagliflozin, ameliorates glucose homeostasis and diabetic nephropathy in db/db mice. PLoS One. 2014;9:e100777.
Ishibashi Y, Matsui T, Yamagishi SI. Tofogliflozin, a selective inhibitor of sodium-glucose cotransporter 2, suppresses renal damage in KKAy/Ta mice, obese and type 2 diabetic animals. Diab Vasc Dis Res. 2016;13:438–41.
Gallo LA, Ward MS, Fotheringham AK, Zhuang A, Borg DJ, Flemming NB, et al. Once daily administration of the SGLT2 inhibitor, empagliflozin, attenuates markers of renal fibrosis without improving albuminuria in diabetic db/db mice. Sci Rep. 2016;6:26428.
Tang L, Wu Y, Tian M, Sjostrom CD, Johansson U, Peng XR, et al. Dapagliflozin slows the progression of the renal and liver fibrosis associated with type 2 diabetes. Am J Physiol Endocrinol Metab. 2017;313(5):E563–76.
Tahara A, Takasu T, Yokono M, Imamura M, Kurosaki E. Characterization and comparison of SGLT2 inhibitors: part 3. Effects on diabetic complications in type 2 diabetic mice. Eur J Pharmacol. 2017;809:163–71.
Shi S, Srivastava SP, Kanasaki M, He J, Kitada M, Nagai T, et al. Interactions of DPP-4 and integrin beta1 influences endothelial-to-mesenchymal transition. Kidney Int. 2015;88:479–89.
De Nardo D, Latz E. NLRP3 inflammasomes link inflammation and metabolic disease. Trends Immunol. 2011;32:373–9.
Dixit VD. Nlrp3 inflammasome activation in type 2 diabetes: is it clinically relevant? Diabetes. 2013;62:22–4.
Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat Immunol. 2010;11:897–904.
Anders HJ, Lech M. NOD-like and Toll-like receptors or inflammasomes contribute to kidney disease in a canonical and a non-canonical manner. Kidney Int. 2013;84:225–8.
Anders HJ, Muruve DA. The inflammasomes in kidney disease. J Am Soc Nephrol: JASN. 2011;22:1007–18.
Hu QH, Zhang X, Pan Y, Li YC, Kong LD. Allopurinol, quercetin and rutin ameliorate renal NLRP3 inflammasome activation and lipid accumulation in fructose-fed rats. Biochem Pharmacol. 2012;84:113–25.
Wang S, Li Y, Fan J, Zhang X, Luan J, Bian Q, et al. Interleukin-22 ameliorated renal injury and fibrosis in diabetic nephropathy through inhibition of NLRP3 inflammasome activation. Cell Death Dis. 2017;8:e2937.
Bae HR, Kim DH, Park MH, Lee B, Kim MJ, Lee EK, et al. beta-Hydroxybutyrate suppresses inflammasome formation by ameliorating endoplasmic reticulum stress via AMPK activation. Oncotarget. 2016;7(41):66444–54.
Lee HM, Kim JJ, Kim HJ, Shong M, Ku BJ, Jo EK. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes. 2013;62:194–204.
Lee MJ, Feliers D, Mariappan MM, Sataranatarajan K, Mahimainathan L, Musi N, et al. A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am J Physiol Renal Physiol. 2007;292:F617–27.
Zhang B, Shi YQ, Zou JJ, Chen XF, Tang W, Ye F, et al. High glucose stimulates cell proliferation and collagen IV production in rat mesangial cells through inhibiting AMPK-KATP signaling. Int Urol Nephrol. 2017;49:2079–86.
Hawley SA, Ford RJ, Smith BK, Gowans GJ, Mancini SJ, Pitt RD, et al. The Na+/glucose cotransporter inhibitor canagliflozin activates AMPK by inhibiting mitochondrial function and increasing cellular AMP levels. Diabetes. 2016;65:2784–94.
Wu C, Hu S, Wang N, Tian J. Dipeptidyl peptidase-4 inhibitor sitagliptin prevents high glucose-induced apoptosis via activation of AMP-activated protein kinase in endothelial cells. Mol Med Rep. 2017;15:4346–51.
Funding
The study was funded by an investigator initiated grant from AstraZeneca and the John S. Dunn Chair in Cardiology Research and Education.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Ye received research grants from AstraZeneca and Boehringer Ingelheim. Dr. Bajaj received research grants from AstraZeneca, Boehringer Ingelheim, Eli-Lilly, Sanofi-Aventis and Novo Nordisk. Dr. Yang is an employee of AstraZeneca. Dr. Yochai Birnbaum receives research grants from AstraZeneca, and he is a speaker for AstraZeneca.
Research Involving Animals
Mice received humane care in compliance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85-23, revised 1996). The protocol was approved by the University of Texas Medical Branch IACUC, Galveston, Texas, USA.
Rights and permissions
About this article
Cite this article
Birnbaum, Y., Bajaj, M., Yang, HC. et al. Combined SGLT2 and DPP4 Inhibition Reduces the Activation of the Nlrp3/ASC Inflammasome and Attenuates the Development of Diabetic Nephropathy in Mice with Type 2 Diabetes. Cardiovasc Drugs Ther 32, 135–145 (2018). https://doi.org/10.1007/s10557-018-6778-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-018-6778-x