Abstract
One-pot synthesis of menthol from citronellal or citral was summarized. Both batch and continuous reactors have been recently applied. This reaction is very complex and a bifunctional catalyst exhibiting especially Lewis acid sites for cyclisation of citronellal to isopulegol are needed, while metal particles are required for its hydrogenation to menthols. Typically, too mild acidity of the catalyst and small particles do not catalyze menthol formation. Furthermore, too high acidity causes catalyst deactivation and dehydration of menthol. Very high menthol yields have been obtained in batch reactor over nobel and transition metal supported bifunctional catalysts. Shape selectivity was demonstrated for Ni-supported on Zr-modified beta zeolite, which gave high diastereoselectivity to the desired L-menthol. Recently one-pot synthesis of menthol in a trickle bed reactor has been investigated. Catalyst suffers only minor deactivation in transformation of citronellal to menthol, while more severe catalyst deactivation occurred in transforming citral to menthols. Noteworthy from the industrial point of view is that the product distribution obtained with the same catalyst under kinetic regime or under diffusional limitations differs from each other. The metal location and synthesis method of extrudates can have a major effect on the catalyst performance. Kinetic modelling of the data obtained from the trickle bed reactor considering the effectiveness factor is discussed.
Graphical Abstract
The results from one-pot synthesis of menthol finding applications in pharmaceuticals and fragrances from citral and its hydrogenated product, citronellal over bifunctional catalysts metal–acid are summarized. The relationship between the catalyst properties and the performance is discussed. In the continuous mode catalyst deactivation becomes apparent and in such mode of operation the product distribution might differ from those obtained in a batch reactor.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Menthol is an important chemical, which is used in pharmaceutical, cosmetic and flavoring applications [1]. The desired menthol stereoisomer with the most effective coolant sensation is (−)-menthol among its eight possible stereoisomers including: (±)-neomenthol, (±)-menthol, (±)-isomenthol and (±)-neoisomenthol [2]. Natural menthol, obtained from peppermint essential oil separation, does not correspond to its increasing demand, and thus synthetic menthol process has been developed by several companies. In the Haarmann & Reimer process, m-cresol is first propylated to thymol and the product is thereafter hydrogenated to racemic (±)-menthols over Ru/Al2O3 catalyst. The desired product (−)-menthol is obtained from racemic (±)-menthols by separation crystallization process [3]. In the Takasago process myrcene is transformed to (−)-menthol with a Rh-BINAP catalyst [4, 5]. In addition, BASF is also producing (−)-menthol starting from enantioselective hydrogenation of nerol to (+)-citronellal by its cyclization to (−)-isopulegol and further of (−)-menthol [6].
Currently a lot of research efforts are devoted to develop new processes based on natural feedstock. A recent review summarizes industrial synthesis routes for (−)-menthol [6]. One possible feedstock is citral [7,8,9,10,11,12,13,14,15,16,17,18] and its derivative citronellal [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36] used as starting materials for production of menthol in a one-pot fashion. The reaction scheme for citral transformation to menthols is shown in Fig. 1. In addition to menthols formed through cyclisation of citronellal to isopulegol and subsequent hydrogenation, citral can be hydrogenated to acyclic products. Other possible size reactions are dehydration of menthols [14, 37] and dimerization of citronellal [20] over acidic catalysts (Scheme 1).
The maximum yield of menthol as a function of metal particle size in citronellal transformation to menthol in a batch reactor. Notation: entries 1–4: conditions: 1,4-dioxane, 2.9 MPa H2, 80 °C, c0 = 2.6 mol/l, mcal/mcat = 40 wt/wt, 12 h, 1: 1 wt% Pt/H-Beta, 2: 2 wt% Pt/H-Beta, 3 wt% Pt/H-Beta, 4 wt% Pt/H-Beta [24], entries 5–13: conditions: hexane, 2.5 MPa H2, 100 °C, c0 = 0.22 mol/l, mcal/mcat = 10 wt/wt, yields at 40 min, entry 5: 4 wt% Ru/H-Beta-25, 6: 4 wt% Ru/H-Beta-35, 7: 4 wt% Ru/H-Beta-150, 8: 2 wt% Ru/H-Beta-25, 9: 2 wt% Ru/H-Beta-35, 10: 2 wt% Ru/H-Beta-150, 11:1 wt% Ru/H-Beta-25, 12: 1 wt% Ru/H-Beta-35, 13: 1 wt% Ru/H-Beta-150 [28]
A simplified reaction scheme for citral transformation to menthols [15]
The key step in one-pot synthesis of menthol from citral and citronellal is the cyclisation of citronellal, which is the rate determining step [30]. Cyclisation of citronellal occurs on Lewis acid sites, e.g. ZnCl2 can coordinate citronellal through its carbonyl group [38, 39], while for example Zr ion can activate the electron rich double bond facilitating favourable orientation of citronellal for the ring closure. Alternatively, Fe2+ or Fe3+ species [40] or Al species [41] act as Lewis acid sites. Thereafter isopulegol hydrogenation is straightforward. The challenge is, however, in citral transformations that the amount of Brønsted acid sites should not be too high, because otherwise dehydration of menthol to menthenes and menthanes is observed. Dehydration of menthol occurs in the presence of an acidic catalyst, when a carbocation is formed via a hybrid shift and the formed water is cleaved [14].
Furthermore, some two-step concepts have been developed [40, 42]. Citral is present in essential oil and can be separated by distillation of lemongrass oil [18]. Furthermore, a continuous industrial process for production of citral was established in 2004 by BASF in Ludwigshafen, with the annual capacity of 40,000 metric tons [43] Citral can be further selectively hydrogenated to citronellal using metal-supported catalysts.
The aim in this work is to summarize the recent developments in one-pot synthesis of menthol from citronellal and citral over heterogeneous catalysts including the work of the authors. The main emphasis is put on catalyst properties such as acidity and metal particles size as well as on reaction conditions in one-pot synthesis of menthols in a batch reactor, when the reaction is performed under kinetic regime. In addition, the results from the continuous reactor will be summarized and compared with those performed in a batch operation in the absence of mass transfer limitations.
2 Catalyst Selection for Menthol Production
2.1 Metal Selection, Particle Size and Loading
One-pot transformations of citronellal have been intensively investigated in batch reactors with high menthol yields obtained over several bifunctional catalysts as summarized for the highest menthol yields in Table 1. Among very selective catalysts are 3 wt% Ir-Beta [34, 36], 3 wt% Ni/S-ZrO2 [23], 1.5 wt% Pd supported on heteropolyacid-SiO2 [31], 2 wt% Pt/H-Beta and 4 wt% Au/MgF2 [33]. Obviously, these catalysts differ much from each other. As can be seen from this list, both noble metals, Ir, Pt and Pd as well as transition metals, e.g. Ni have given excellent results at low metal loadings (Table 1). Pd supported on a heteropolyacid [31] and on a perfluorinated polymer [35] was very selective in menthol formation (Table 1, entry 2, 3). The latter catalyst gave also very high stereoselectivity. XPS measurements revealed that Pd was partially also in the ionic form, which might increase its Lewis acidity. Unfortunately acidity was not determined in this work. Noteworthy is also that Pd/PFSA catalyst with a small Pd particle size gave nearly no other products as menthols. This might be due to a low metal loading [35]. This catalyst has also strong acidity (see Sect. 2.2). In general the metal particle size has, however, a large effect on menthol selectivity, namely when comparing the performance and properties of 3 wt% Ir/H-Beta (Table 1, entry 4 and 6), it can be observed that hydrogenation to 3,7-dimethyloctanol is promoted more on small Ir particles. Analogously hydrogenation of citronellal occurred also to some extent with Ru/H-Beta-25, Zr-Beta/15 wt% Ni-MCM-41 and 2 wt% Pt/H-Beta (Table 1, entries, 5, 8, 10) [11, 24, 28]. On the other hand, no acyclic hydrogenation products were formed over 4 wt% Au/MgF2 (Table 1, entry 7). This catalyst was prepared by the incipient wetness method from tetrachloroaurate precursor being calcined at 100 °C. Au was in the ionic state in this catalyst, which was less active for citronellal hydrogenation, while when its counterpart was calcined at 150 °C, gold was partially in the metallic state giving a very low activity. Furthermore, the support contains both Lewis and acidic sites, although their content was not quantified. A direct comparison of different metals with the same metal loading supported on beta zeolite was performed in citronellal transformations under 1.5 MPa at 80 °C in cyclohexane in [34]. The results showed the following decreasing order for the menthol yield: Ir >> Pt \(\approx\) Rh > Pd > Ru. Furthermore, in [34] the highest yield of 3,7-dimethyloctanol was obtained over Pd/H-Beta, while 3 wt% Ru-Beta catalyst promoted formation of citronellol. It is, however, also visible from Table 1 that the highest yields of menthols obtained with 15 wt% Ru/ZnBr2/SiO2 and 1 wt% Ru/H-Beta catalysts were 83% [25] and 90% [29], respectively. For the former catalyst neither the metal particle size nor acidity was described. A comparative study on the performance of Pt/Ga-MCM-41 and Cu/Ga-MCM-41 in citronellal transformations showed that the former catalyst exhibited better performance than the latter one [21]. It was stated that an optimum catalyst, Pt/Ga-MCM-41 for one-pot transformation of citronellal to menthols exhibits high hydrogenation activity, strong Lewis acidity and mild Brønsted acidity [21].
The effect of the metal particle size in one-pot synthesis of menthol from citronellal has been scarcely investigated in a systematic way reporting just few data points [24, 28]. The menthol yields from these studies [24, 28] with different Pt and Ru supported catalysts are shown as a function of the metal particle size (Fig. 1). These results clearly illustrate that there is an optimum Pt particle size giving the highest menthol yield [24]. This trend was not very clear for Ru catalysts and the highest menthol yields were obtained for 2 wt% Ru/H-Beta with 2 nm particles [28], while particles in 2 wt% Pt–H-Beta of the average size of ca. 7 nm were the best ones in [24] (Fig. 1).
In citral transformation to menthols both noble [8, 9, 12, 17, 33] and transition metal [9,10,11] have been reported. Citral transformations to menthol were also investigated over different metal (Pd, Ru, Rh, Ir, Pt) modified on Beta zeolite catalysts, without reporting the metal particle sizes [8]. The menthol yields obtained at 100 °C under 1.0 MPa hydrogen in 24 h using toluene as a solvent decreased in the following order: Pd > Ir >> Ru with the highest menthol yield obtained with Pd/H-Beta was 78.6% (Table 1, entry 18) [8] while no menthols were formed over Rh/H-Beta and Pt/H-Beta. Pt/H-Beta was active in hydrogenation promoting formation of citronellol because Pt exhibits a wider d-band width in comparison to Pd and thus it has stronger interactions with the C=O bond and weaker interactions with the C=C bond [8]. A relatively high menthol yield formed from citral in [8] is different than the one reported in [9], when the highest amount of hydrogenolysis products was formed over 5 wt% Ir-H-MCM-41 reason for high menthol yields in [8] over 3 wt% Ir-MCM-41 might be lower Ir loading in comparison to [9]. In addition, high amounts of dehydration products were formed over Pt and Rh supported on Beta zeolite [8]. In [17] Pd was supported together with ZnCl2 and N-butyl-4-methylpyridinium tetrafluoroborate ionic liquid on active carbon cloth and tested in one-pot citral transformation to menthol (Table 1, entry 21). The results showed that both 40% menthol and ca. 35% 3,7-dimethyloctanal were formed as the main products indicating that this catalyst exhibited too high hydrogenation activity. Moreover, Pd agglomeration occurred to a certain extent.
Supported nickel catalysts have been also very selective to menthols [10, 12, 27]. For example 5 wt% Ni/Zr-Beta gave a high menthol yield and also high stereoselectivity (Table 1, entry 14). High stereoselectivity can be due to the shape selectivity effect in Zr-modified beta zeolite (see Sect. 2.3). It was also demonstrated in [27] that with a higher Ni loading more acyclic hydrogenation products were formed. Another efficient catalyst was 8 wt% Ni–Al-H-MCM-41 with a mild acidity (Table 1, entry 17) [10]. This catalyst exhibiting a very low metal dispersion (3.8%) which indicates the presence of large Ni particles, was able to produce menthol also due to its low acidity. The initial citral transformation rate was also increased when increasing the metal loading, i.e. when the metal loading was increased from 3 to 8 wt%, the initial citral transformation rate increased by a factor of 1.6 [10]. When comparing the performance of 8 wt% Ni–Al-MCM-41 [10] with 5 wt% Ni–H-MCM-41 [9], it can also be observed that the latter one generated more defunctionalized dehydration products (menthanes, menthenes) (Table 1, entry 17, 21). A direct comparison of acidity of these catalysts is not straightforward, because in the former catalyst the amount of acid sites was reported only on the basis of the peak areas, not being quantified as μmol/gcat. As a comparison Pd-H-MCM-41 was also used in [9]. This catalyst was not very selective towards menthol formation giving the selectivity of 44% to menthols, while NI–H-MCM-41 resulted in 54% selectivity. Furthermore, a direct comparison of different metals supported on H-MCM-41 was made in the case of citral transformations [9]. It was confirmed that Pd catalyst exhibited initially nine fold higher hydrogenation rate than the corresponding Ni catalyst, while over Ir-H-MCM-41 even 22 fold higher initial hydrogenolysis rate was obtained compared to Ni–H-MCM-41 [9]. These results are in accordance with high hydrogenolysis activity of Ir catalyst leading to formation of hydrocarbons from isomenthone and menthone [44]. High menthol selectivity was also obtained over 15 wt% Ni-MCM-41/Zr-Beta (Table 1, entry 10) [11]. The results from two catalyst screening studies with both of them having four different catalysts showed that the highest menthol yields were obtained from citral transformations at 70 °C under 0.5 MPa hydrogen over 3 wt% Ni/Al-H-MCM-41 and 3 wt% Ni/Beta exhibiting quite large metal particle sizes, i.e. the Ni particle size of 17 nm and 22 nm, respectively. The catalysts with smaller metal particle sizes, i.e. 1 wt% Pd/H-Beta (1.8 nm) and 3 wt% Ni/SiO2-Al2O3 (8 nm) gave 22% and 68% yields of menthol, respectively [18]. Over Pd/H-Beta high amounts of acyclic hydrogenation product, 3,7-dimethyloctanal were also formed [18]. Furthermore, acidity of 3 wt% Ni/SiO2-Al2O3 was 8.5 fold that of 3 wt% Ni/Al-MCM-41. On the other hand, an optimum metal particle size, 5.8 nm for 5 wt% Ni–H-MCM-41, was found for citral transformation (Table 1, entry 21, Fig. 2). It should, however, be noted that in [9] Ru and Ir with the metal particle size of 2 nm and 11 nm, respectively, gave very low menthol yields (Fig. 2). Ir is known to enhance hydrogenolysis, which was also very high in their work (82% yield). Higher Ir loading in Ir/Beta favoured also hydrogenation, i.e. formation of 3,7-dimethyloctanol in citronellal transformations [33, 36], analogously to reported in [12] for increased Pd loading in Pd-HPA-MM (phosphotungstic acid containing acid treated montmorillonite). This result indicates that the electronic properties of the metal have also a major effect on menthol formation from citral.
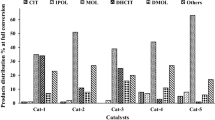
Menthol yield in citral transformation. Notation: 1.1 wt% Pd/H-Beta, 5. 3 wt% Ni/SiO2-Al2O3, 7. 3 wt% Ni/H-Beta and 8. 3 wt% Ni/Al-MCM-41, conditions: toluene, 0.5 MPa H2, 70 °C, c0 = 0.18 mol/l, mcit/mcat = 1.7 wt/wt, 6 h [10] and 2. 5 wt% Ru–H-MCM-41, 3. 5 wt% Ni–H-MCM-41, 4. 5 wt% Pd-H-MCM-41 and 6. 5 wt% Ir-H-MCM-41, conditions: batch reactor, cyclohexane, 0.1 MPa H2, 70 °C, c0 = 0.01 mol/l, mcit/mcat = 1.0 wt/wt, 6.5 h [9]
The effect of metal loading for Ni catalysts supported on heteropolyacid (HPA)-montmorillonite was investigated in citral transformation in [12], without reporting Ni particle sizes. In [12] a higher Ni loading in 8 wt% Ni-HPA-MM (montmorillonite) was better for producing menthol in comparison to 5 wt% Ni-HPA-MM. It was also reported that only small amounts of acyclic hydrogenation products were formed over 8 wt% Ni-HPA-MM. A high menthol yield over 8 wt% Ni-HPA-MM was, however, related to its low Brønsted to Lewis acid site (BAS/LAS) ratio [12]. In [11], citronellal transformations were performed over Zr-Beta/3 wt% Ni-MCM-41 catalyst, however, using inert atmosphere during the first hour and then switching to hydrogen. This approach gave a high menthol yield of 92% (Table 1, entry 10). Noteworthy is also very high stereoselectivity to the desired (−)-menthol.
2.2 Catalyst Acidity
Catalyst acidity is crucial in one-pot menthol synthesis starting from citronellal as shown for example in [23], where only 10% yield of menthols was obtained over 3 wt% Ni/SiO2, which did not exhibit enough acidity. On the other hand, a very promising catalyst for menthol synthesis form citronellal was Pd supported on a perfluorinated polymer (Table 1, entry 3) [35]. The latter exhibited an acidic strength comparable to that of sulphuric acid with the Hammett acidity of ca. − 12. The role of ionic metal species promoting isopulegol and menthol formation was also emphasized in increasing Lewis acidity of the catalyst in [36], where the presence of ionic iridium species was confirmed by XPS and temperature programmed reduction, i.e. 25% of iridium was in the ionic form. Analogous results were also obtained in [34] with 3 wt% Ir/H-Beta zeolite catalyst. The effect of catalyst acidity has also been systematically investigated with several catalysts in citronellal transformation to menthols [21] while in other publications acidity has not been determined or reported [11, 22, 24, 25, 27, 30, 31, 33, 35]. The menthol yield decreased also with increasing total acidity in citronellal transformations to menthols for different Pd and Pt supported catalysts (Fig. 3) [21], while for Cu catalyst with a low hydrogenation ability this trend was not observed. In [21] the best performing catalyst was Pd/Ga-MCM-41 with a low amount of total acid sites. In citral transformations to menthol mild catalyst acidity was preferred as stated in [10, 12], while when the catalyst acidity was too high, as in case with Ni/H-Y [9], catalyst deactivation and formation of hydrogenolysis products was very prominent giving only 42% conversion and less than 1% menthols. On the other hand, mildly acidic Ni–H-MCM-41 under the same conditions menthol selectivity was 54% at complete citral conversion (Table 1, entry 21). A low Brønsted to Lewis acid ratio in 8 wt% Ni-HPA-MM (montmorillonite) was beneficial giving menthol yield higher than with 5 wt% Ni [12]. Analogously Ni/Al-MCM-41 with much lower acidity than in Ni/Al2O3-SiO2 gave 94% menthol yield (Table 1, entry 17) in comparison to 68% obtained with the latter catalyst [10]. The bifunctional 15 wt% Ni-MCM-41/Zr-Beta exhibiting both Brønsted and Lewis acid sites and relatively large particles was also a promising catalyst for one-pot synthesis of menthol from citral (Table 1, entry 10) [11]. It is known that Zr-beta exhibited predominantly Lewis acid sites in addition to a low amount of Brønsted acid sites [11]. Based on the above results it can be concluded that mild catalyst acidity is required for one-pot transformation of citronellal and citral to menthol.
Menthol yield as a function of catalyst acidity in citronellal transformation to menthols over the following catalysts: 1–4 (●) Pd, 1. Pd/Ga-MCM-41, 2. Pd/Ga-SBA-15, 3. Pd/Al-SBA-15, 4. Pd/Al-MCM-41, 5–9 (■) Pt, 5. Pt/Ga-MCM-41, 6. Pt/Ga-SBA-15, 7. Pt/Al-SBA-15, 8. Pt/Al-MCM-41, 9–12 (▲) Cu, 9. Cu/Ga-MCM-41, 10. Cu/Ga-SBA-15, 11. Cu/Al-MCM-41, and 12. Cu/Al-SBA-15. Th e metal content in all catalystswas ca. 2 wt%. Conditions: batch reactor, solvent 2-propanol, 120–130 °C, microwave heating, c0 = 0.005 mol/l, mcal/mcat = 30.8 wt/wt, 15 min reaction time, in the presence of 1 mmol K2CO3 [21]
2.3 Support Properties
The main role of the support in a bifunctional catalyst is to provide both high surface area for dispersion of a metal as well as to be a source of acidity. In addition, a large enough pore size facilitates diffusion of the substrate to the active sites [33], while an optimized pore size facilitates high diastereoselectivity to the desired L-menthol [11, 27]. One comparative example of zeolites as supports is related to Ir supported on modenite or beta. The latter one gave a high menthol yield in citronellal transformations [33]. An easy penetration of 2,6-di-tert-pyridine used as a probe molecule to study acidity into beta zeolite indicated that this material is also suitable for citronellal transformations of menthols [33]. As a comparison to Ir/Beta zeolite, Ir supported on mordenite gave mainly isopulegol as a product in citronellal transformation in cyclohexane at 80 °C under 0.8 MPa hydrogen [33].
High stereoselectivity to the desired menthol isomer is one aim, which can diminish the efforts in down-stream processing. A promising example is Zr-beta zeolite as a support, as Zr exhibiting Lewis acidity can also be an active site in Zr-beta, i.e. as a form of partially hydrolyzed framework heteroatom in beta [45]. Because the size of Zr4+ is larger than that of Al3+ the shape selectivity effect is observed. In that case, Zr-beta promotes formation of (−)-isopulegol, which has methyl, hydroxyl and propenyl in equatorial positions and enhances diastereoselectivity of L-menthol [11, 27]. Formation of other pulegols was thus hindered due to a small pore size of the support. On the other hand, when a mesoporous support is used for a citronellal cyclisation step, such as WO3/TUD-1 [42], it was pointed out that stereoselectivity in pulegols is determined by thermodynamics due to a large pore size.
3 Effect of Reaction Conditions on Menthol Production
The effects of rection conditions, such as a solvent selection, temperature and pressure have been intensively studied in batch reactors and are discussed below. The results from continuous operation regarding reaction conditions are summarized in Sect. 4.
3.1 Solvent Selection
Solvent polarity has a large effect on menthol selectivity in both citronellal [24, 28, 33] and citral [8] transformations. In citronellal transformations hydrophobic solvents are preferable (Fig. 4), while menthol yields decreased in hydrophilic solvents [33]. Noteworthy is also that in acetonitrile the solvent is protonated leading to a loss of Brønsted acidity preventing formation of menthols [28]. It was also pointed out in [28] that 1,4-dioxane and tetrahydrofuran were the best solvents giving also high stereoselectivity. The highest yield of menthols was obtained over 2 wt% Pt/H-Beta in 1,4-dioxane [24]. On the other hand, very hydrophobic solvents, e.g. hexane gave various hydrogenation product, while defunctionalized products were formed in toluene [28]. The high amount of hydrogenation products in hexane can be explained by high hydrogen solubility in this solvent [28]. Analogous observations were reported in [24, 33], as toluene gave high yields of 3,7-dimethyloctanol in comparison to 1,4-dioxane. Interestingly water was used as a solvent in one-pot transformations of citronellal to menthols over Pd supported on perfluorinated polymer [35]. The results showed a high menthol yield, while the reaction time was very long. Furthermore, it was reported that productivity for menthol formation was only 0.047 mol/gmetal/h−1, which was very low in comparison to 1 wt% Ru/H-Beta [28] for which the corresponding value was 16 fold higher. The authors claimed that the menthol synthesis using Pd on a perfluorinated polymer is still very environmentally benign and that the reaction can be performed under mild conditions.
Menthol yield in citronellal transformation as a function of the dielectric constant of the solvent in a batch reactor. Notation: citronellal transformation in different solvents (+) under 0.8 MPa H2, 80 °C, c0 = 0.79 mol/l, mcat/mcat = 17.1 wt/wt, 24 h over 3 wt% Ir/H-Beta [33], (solid ball) under 2.0 MPa H2, 80 °C, c0 = 2.6 mol/l, mcal/mcat = 40 wt/wt, 12 h over 2 wt% Pt/H-Beta [24] and (o) under 2.5 MPa H2, 100 °C, c0 = 0.22 mol/l, mcal/mcat = 10 wt/wt in 60 min over 1 wt% Ru/H-Beta-25 [29]
3.2 Temperature
High temperature has a positive effect on menthol formation from citronellal [24, 28, 33], which is apparently related to different activation energy along different reaction routes. High menthol yields (93%) from citronellal can be obtained at high temperatures, i.e. 100 °C, 2 MPa over 2 wt% Pt/H-Beta with Si/Al ratio of 14 [24], while at 25 °C the yield was only 81%. At the same time the yields of hydrogenation products slightly increased. An opposite trend was observed over Ru/H-Beta-150, i.e. the selectivity to hydrogenation products decreased with increasing temperature, although selectivity to menthols increased. Furthermore, it was observed in [28] that the turnover frequency for hydrogenation of pulegols to menthols (calculated as moles hydrogenated per mole of exposed Ru by CO chemisorption, given in min−1) increased six fold from 60 to 100 °C. These results indicate that the relative ratio of hydrogenation vs cyclisation and menthol formation is catalyst specific. It can, however, be concluded that menthol formation in general is enhanced at higher temperatures.
Quantitative analysis of kinetics was performed for citronellal transformations to menthols over 2 wt% Ru/H-Beta-150 assuming dissociative adsorption for hydrogen on the same sites with the organic compounds [29]. The activation energy of 104 kJ/mol was determined for citronellal cyclisation [29], was higher than the activation energies for its hydrogenation to citronellol and dihydrocitronellal, being 72.7 kJ/mol and 79 kJ/mol, respectively. These results were in line with the experimental data showing that the menthol formation requires a relatively high temperature in comparison to hydrogenation. Analogous results were obtained in [33] in citronellal transformation over 5 wt% Ir/Beta in the temperature range of 25–80 °C under 1.5 MPa H2 in cyclohexane. Although selectivity to menthols increased at a higher temperature, formation of 3,7-dimethyloctanol was also slightly increased. This result indicates that 5 wt% Ir/Beta catalyst was active for both citronellal cyclisation and hydrogenation.
3.3 Pressure
The effect of hydrogen pressure in one-pot transformation of citronellal to menthols has been investigated in some extent [24, 28, 33]. A negligible pressure effect on menthol yields was obtained in citronellal transformation over Pt/H-Beta at 80 °C in the pressure range of 0.2–2 MPa in 1,4-dioxane [24], while selectivity to menthols increased from 17 to 62% in citronellal transformation over 2 wt% Ru/H-Beta-150 in n-hexane at 100 °C when the pressure was varied in a broader range of 1.5–4.5 MPa [28]. It should also be pointed out here that hydrogen solubility is higher in hexane in comparison to 1,4-dioxane facilitating a higher pressure effect in hexane than in 1,4-dioxane [28]. Furthermore, turnover frequency for pulegol hydrogenation to menthols increased by a factor of ca. 6 when increasing the hydrogen pressure from 15 to 49 bar at 100 °C [28]. However, a substantial pressure effect in citronellal transformation was obtained at 80 °C over Ir/Beta in the pressure range of 0.5–1.5 MPa in cyclohexane [33] and over 5 wt% Ir/Beta in cyclohexane at 80 °C [36]. In the former case selectivity to menthols increased over 3 wt% Ir/Beta from 32 to 88% when conversion increased from 94 to 100% [33].
The effect of hydrogen pressure in one-pot citral transformation in a batch reactor was investigated in [10]. A higher pressure was beneficial in citral transformations to menthols over Ni/Al-MCM-41 at 70 °C in toluene [10], i.e. at 0.5 MPa 89% yield to menthols was obtained while the corresponding value at 2 MPa was 93%. The initial citral transformation rate over 3 wt% Ni/Al-MCM-41 increased by a factor of 2 when increasing the hydrogen pressure from 0.5 to 2 MPa [10]. Analogously, the initial citral conversion was showing a square root dependence on hydrogen pressure in the pressure range of 0.5 to 5.5 MPa over 15 wt% Ni/H-MCM-41 catalyst [27]. This dependence is in accordance with Langmuir–Hinshelwood model following dissociative hydrogen adsorption on nickel sites. However, a low hydrogen pressure minimized formation of the undesired hydrogenation products.
4 Menthol Production in a Continuous Reactor
Menthol production has been investigated intensively in continuous reactors in a one-pot mode [13,14,15,16, 20, 32, 37] or in a sequential manner in a continuous mode [40]. A trickle bed reactor with either citronellal or citral as reactants has been recently utilized [13,14,15,16, 20, 32, 37] using as catalysts extrudates composed of a zeolite or a mesoporous material as well as a binder, which can be silica or a clay. The maximum yields of menthols are reported for different extrudates in Table 2. Catalyst deactivation was clearly visible in citronellal transformations over Ru–H-MCM-41-Bindzil composite catalysts (Fig. 5a) [32]. In that study four different catalyst preparation methods were applied, e.g. Ru was loaded first on H-MCM-41 or alternatively only on a silica binder, Bindzil, thereafter extruded with the other component. Another method was to deposit Ru on both H-MCM-41 and Bindzil followed by extrusion. Finally first Bindzil-H-MCM-41 extrudates were prepared and thereafter loaded with Ru giving an egg-shell type extrudates. The maximum yield of menthols from citronellal was 47% obtained over a catalyst when Ru was deposited on H-MCM-41 followed by extrusion with Bindzil (Fig. 5b, Table 2, entry 1). This catalyst exhibited the lowest BAS/LAS ratio among four catalysts and it gave also low amounts of defunctionalized products. However, the liquid phase mass balance closure was low for Ru-extrudates. This is due to formation of oligomeric products, which are not visible in gas chromatographic analysis, and strong adsorption of reactants and products on the catalyst surface. The results of [32] clearly illustrate that the location of Ru has a significant effect on catalytic performance.
a Conversion of citronellal and b yield of menthols in citronellal transformation to menthols over Ru–H-MCM-41—Bindzil composite catalysts at 70 °C using initial concentration of citronellal 0.086 mol/l in cyclohexane. Notation: (green circle) (Ru/H-MCM-41) + Bindzil, (red triangle) (Ru/Bindzil) + H-MCM-41, (light blue square) Ru/(H-MCM-41 + Bindzil) post synthesis and (dark blue diamond) Ru/(H-MCM-41 + Bindzil) in situ synthesis [32] (Color figure online)
Analogously to [32] several different methods were used for synthesizing Pt- and Ru- extrudates with H-Beta-25 and bentonite as a binder [20]. The menthol yields from citronellal decreased with increasing Brønsted acidity of Pt/H-Beta-25-bentonite extrudates in a trickle bed reactor under presence of mass transfer limitations (Fig. 6) [20]. At the same time the yields of acyclic hydrogenation products increased in comparison to cyclic products (Fig. 7). The amount of acyclic hydrogenation products was high due to the presence of small Pt particles, which exhibit high hydrogenation activity (Table 2, entry 3) [20]. The highest amount of Brønsted acid sites was present in 2 wt% [Pt/(H-beta-25 + bentonite) when the metal was deposited on extrudates giving also the highest concentration of acyclic products, while the lowest Brønsted acid concentration was found in 2 wt% [Pt/(H-Beta-25) + bentonite]. In [20] an optimum Pt particle size of 7 nm was obtained in 2 wt% Pt/(H-Beta-25 + bentonite). The metal location is crucial for maximizing the menthol yield and the best result was obtained over Pt supported first on H-Beta-25 followed by its extrusion with bentonite. On the other hand, if the metal was loaded via the post-synthesis method on the extrudates, i.e. the metal was at the outer surface, a high turnover frequency (0.053 s−1) for citral transformation was obtained. This catalyst was, however, more selective to acyclic hydrogenation products than for menthols. Interestingly for Ru/H-Beta-bentonite extrudates the menthol yield was nearly constant for different sizes of Ru. It should also be pointed out here that due to mild reaction conditions only minor catalyst deactivation occurred with increasing time on stream.
Menthol yield (Y) after 2 h as a function of Brønsted acid sites (BAS) in citronellal transformation to menthol over Pt–H-Beta-25-bentonite and Ru-Beta-25 bentonite extrudates in a trickle bed reactor at 30 °C under 10 bar [20]
Concentration of acyclic hydrogenation products as a function of cyclization products in citronellal transformation to menthol in a trickle bed reactor cyclohexane, 1.0 MPa H2, 35 °C, c0 = 0.22 mol/l, mcal/mcat = 10 wt/wt. Notation: (o) 2 wt% [Pt/(H-Beta-25 + bentonite) post synthesis, (filled ball) 2 wt% [Pt/(H-Beta-25 + bentonite) in situ], (filled rectangular) 2 wt% [Pt/(bentonite) + H-Beta-25], (triangle) 2 wt% [Pt/(H-Beta-25 + bentonite] [20]. Notation: ACP acyclic hydrogenation products, CP cyclic products
Menthol has also been synthesised from citronellal in a fixed bed reactor, with two consecutive columns containing iron modified scrap automotive converter catalysts. In the first step pulegols were formed being further hydrogenated to menthols downstream [40]. This method gave a high menthol yield of 91% (Table 2, entry 4, Fig. 8). The catalyst was washed between two cycles with toluene and it can be observed that the yields and selectivity to (±)-menthol were recovered.
Menthol yield and selectivity as a function of time-on-stream in a two-step menthol process over catalyst made from iron loaded scrap automotive catalytic converter. Step 1: 100 °C, step 2: 140 °C, 0.5 MPa H2. Notation: (■) menthols yield, (o) selectivity and (Δ) yield to (±)-menthols. Adapted from [40]
In one-pot citral transformations to menthols in a trickle bed reactor several Ru- and Ni- extrudates were applied [13,14,15,16, 37]. The menthol yield was maximally 75% obtained over 5 wt% Ni/mesoporous silica (Table 2, entry 5, Figs. 9, 10). The second highest yield of menthols of 49% was achieved over 5 wt% Ni/H-Beta-38—sepiolite composite catalysts (Fig. 11, Table 2, entry 6) followed by Ni supported on H-Beta and extruded with attapulgite clay (Table 2, entry 7) [37]. The latter result was promising, because attapulgite clay contains more impurities in comparison to sepiolite clay [37]. On the other hand, Ru containing extrudates with a high BAS/LAS ratio exhibited low selectivity to menthols (Table 2, entries 8–9). It should, however, be pointed out here that a rapid catalyst deactivation occurred for Ni supported on mesoporous alumino-silicate (MAS) and at the same time selectivity to acyclic hydrogenation products and pulegols increased indicating a decrease of acidity with increasing time-on-stream by blocking of the acid sites (Fig. 10). The main side reaction is dehydration of menthols to menthatrienes, which are further hydrogenated to menthanes. Their formation is promoted on especially strong acid sites present in Ni–H-Beta-38-sepiolite composite catalyst (Fig. 11b) [14], while the Ni/mesoporous aluminosilicate-sepiolite composite giving a higher menthol yield did not contain any strong Brønsted acid sites [13]. Over the former extrudates with a higher acidity, the main products were initially defunctionalized menthatrienes, however, their yield decreased with increasing time on stream due to deactivation of the most acidic sites (Fig. 11b).
Selectivity to different products as a function of conversion in citral transformation over Ni/MAS (mesoporous silica) at 70 °C under 1 MPa H2 [13]. Note that conversion is decreasing with increasing time-on-stream. S selectivity, ACP acyclic hydrogenation products, IP isopulegols, ME menthols, DFP defunctionalized products, DM dimeric products
Effect of pressure on a) conversion/liquid phase mass balance and b) the product yields in citral transformation to menthols over Ni/(H-Beta-38 + Sep.) composite catalyst [14]. Notation: X is conversion, MB is liquid phase mass balance closure, the reactant and product peaks visible in GC analysis, SS denotes diastereoselectivity to (−)-menthol, ACP acyclic hydrogenation products, Me menthols, DFP defunctionalized products
Quantitative analysis of reaction kinetics was performed in [14], in which citral was transformed to menthol in a trickle bed reactor. The novelty in this modelling was to take into account effectiveness factor, which changes for non first-order reactions when concentration dependent Thiele modulus changes. Thus in [14] in addition to applying a noncompetitive adsorption of organic compound and hydrogen, also the rate constants were lumped with the effectiveness factor for each reaction step and temperature dependency was also taken into account. Furthermore, deactivation of both metal and acid sites was incorporated in the model. The results demonstrated that it was possible to describe the data including step changes, i.e. changes of temperature and pressure (Figure 12).
From an industrial point of view, it is also interesting to compare performance of powder and extrudate catalysts. The results in [16] from a comparative study for citral transformation over powder and extrudate Ru–H-MCM-41-Bindzil catalysts confirmed the presence of diffusional limitations when calculating the effectiveness factor, which was in the range of 0.18–0.29 over different Ru–H-MCM-41-Bindzil composite catalysts [16]. Furthermore, it is important to note that the product distribution, for example yields of different menthols were not same in batch and continuous reactors with the same type of catalyst. The highest menthol yield was obtained in a batch reactor over Ru/(Bindzil) + H-MCM-41, while the same extrudate catalyst in a trickle bed reactor gave the lowest menthol yields among the studied catalysts (Fig. 13a, b). It can be thus concluded that mass transfer limitations can have a large impact on product distribution, which should be obviously considered during catalyst development and further scale-up (Fig. 13).
Modelling of the concentration of a citral, b isopulegols and menthol and c menthols as a function of time on stream. Dots represent experimental data points [14]
Comparison of the menthol yields obtained from citral a in a batch reactor and b in a trickle bed reactor as a function of time on stream. Notation: Batch reactor: Ru/(Bindzil) + H-MCM-41 (square), Ru/(H-MCM-41 + Bindzil) (open circle), Ru/(H-MCM-41) + Bindzil (triangle), trickle bed reactor: post synthesis Ru/(H-MCM-41 + Bindzil) (diamond). Conditions: 70 °C, 1 MPa H2, residence time 12.5 min in a trickle bed reactor, initial citral concentration 0.086 mol/l. Adapted from [16]
5 Conclusions and Future Outlook
One-pot synthesis of menthol from natural sources, such as citral and its hydrogenated product citronellal can be a prominent route for future practical implementations, because very high selectivities have been obtained both over noble and transition metal supported catalysts, such as Pd supported on heteropolyacid, Ir-H-Beta and a mixture of Zr–H-Beta and Ni–H-MCM-41. When rationalizing the published catalytic results for one-pot synthesis of menthol the most important parameters are the metal particle size and acidity. Analysis of the comparative data from one-pot synthesis of menthol over Pt–H-Beta-14 showed that for Pt the optimum particle size was ca. 7 nm giving the highest menthol yield of ca. 90%, while for Ru supported on H-Beta zeolite with different Si/Al ratios no optimum particle size was observed giving menthol yield between 60 and 75%. Furthermore, Ni supported catalysts with Ni size of 8–25 nm afforded ca. 68–72% menthol selectivity starting from citral. In addition, not only metal selection, but also catalyst acidity is also crucial obtaining high menthol yields. Typically, rather mild acidity and a suitable Lewis to Brønsted acid ratio promotes one-pot synthesis of menthol. Lewis acid sites are required for cyclisation of citronellal, which is a rate determining step. However, some Brønsted acidity is also beneficial for menthol synthesis, presence of especially strong Brønsted acid sites promotes dehydration of menthol as well as catalyst deactivation. Based on the analysis of available data, it is recommended to generate systematic experimental data only changing one parameter at a time and determining the metal particle size and acidity of the catalyst in order to facilitate a rational catalyst design in the future for this type of complex reaction systems with a bifunctional catalyst.
Reaction conditions affect very much the menthol synthesis. An increased temperature has typically a positive effect on formation of menthol, which was also confirmed by kinetic modelling and determination of an activation energy for cyclisation of citronellal in comparison to hydrogenation for formation of citronellol and dihydrocitronellal. On the other hand, analysis of the data on the hydrogen pressure dependence shows either a minimal effect or an increase of menthol selectivity depending on the type of catalyst. In addition, hydrophobic solvents promote menthol formation.
Recently continuous one-pot menthol synthesis has been intensively studied with several metal supported extrudates in which clays have been used as binders. In addition, the metal location has been systematically varied by selecting different catalyst synthesis method. The results have shown that both acidity and metal particle size can be tuned by varying the catalyst synthesis method. The best results have been obtained with the extrudates, in which the metal is loaded on both the support and the binder and only on the support. On the other hand, if the metal is primarily loaded on a binder, it is not very selective for menthol formation. Noteworthy from the industrial point of view is that the product distribution obtained under diffusional limitations is different than the one obtained with the same catalyst in a batch reactor. Modelling of the continuous reactor data obtained from citral transformation to menthols in a trickle bed was performed taking into account the temperature dependence, changes in catalyst effectiveness factor due to changes in concentration as well as catalyst deactivation at two different sites, the acid and the metal. It was possible to successfully fit the experimental results obtained when the step changes in experimental conditions were introduced.
References
Kamatou GPP, Vermaak I, Viljoen AM, Lawrence PM (2013) Phytochemistry 96:15–25
Neaţu F, Coman S, Pârvulescu VI, Poncelet G, De Vos D, Jacobs P (2009) Topics Catal 52:1292–1300
Davis JC (1978) Chem Eng 85:62–63
Emura MH (2014) Chem Biodivers 11:1688–1699
Misono M, Nojiri N (1990) Appl Catal 64:1–30
Dylong D, Hausoul PJ, Palkovits R, Eisenacher M (2022) Synthesis of (−)-menthol: industrial synthesis routes and recent development. Flavour Fragr J. https://doi.org/10.1002/ffj.3699
Muraza O, Rebrov EV, Mäki-Arvela P, Kumar N, de Croon MHJM, Murzin DY, Schouten JC (2010) Sci Central Asia 1:30–38
Negoi A, Teinz K, Kemnitz E, Wuttke S, Parvulescu VI, Coman SM (2012) Topics Catal 55(7):680–687
Mäki-Arvela P, Kumar N, Kubička D, Nasir A, Heikkilä T, Lehto VP, Sjöholm R, Salmi T, Murzin DY (2005) J Mol Catal A 240:72–81
Trasarti AF, Marchi AJ, Apesteguía CR (2007) J Catal 247:155–165
Nie Y, Jaenicke S, Chuah GK (2009) Chemistry 15:1991–1999
Shah AK, Maitlo G, Shah AA, Channa IA, Kandhro GA, Maitlo HA, Bhatti UH, Shah A, Memon AQ, Jatoi AS, Park YH (2019) React Kinet Mech Catal 128:917–934
Simakova IL, Vajglová Z, Mäki-Arvela P, Eränen K, Hupa L, Peurla M, Mäkilä EM, Wärnå J, Murzin DY (2022) Org Proc Res Dev 26(2):387–403
Simakova I, Mäki-Arvela P, Martinez-Klimov M, Muller J, Vajglova Z, Peurla M, Eränen K, Murzin DY (2022) Appl Catal A 636:118586
Vajglová Z, Navas M, Mäki-Arvela P, Eränen K, Kumar N, Peurla M, Murzin DY (2022) Chem Eng J 429:132190
Vajglová Z, Mäki-Arvela P, Eränen K, Kumar N, Peurla M, Murzin DY (2021) Catal Sci Technol 11:2873–2884
Virtanen P, Karhu H, Toth G, Kordas K, Mikkola JP (2009) J Catal 263:209–219
Trasarti AF, Marchi AJ, Apesteguía CR (2004) J Catal 224:484–488
Adilina IB, Pertiwi R, Sulaswatty A (2015) Biopropal Ind 6:1–6
Azkaar M, Mäki-Arvela P, Vajglová Z, Fedorov V, Kumar N, Hupa L, Hemming J, Peurla M, Aho A, Murzin DY (2019) React Chem Eng 4:2156–2169
Balu AM, Campelo JM, Luque R, Romero AA (2010) Org Biomol Chem 8:2845–2849
Cirujano FG, Xamena F, Corma A (2012) Dalton Trans 41:4249–4254
Cortés CB, Galván VT, Pedro SS, García TV (2011) Catal Today 172:21–26
Mertens P, Verpoort F, Parvulescu AN, De Vos D (2006) J Catal 243:7–13
Milone C, Gangemi C, Neri G, Pistone A, Galvagno S (2000) Appl Catal A 199:239–244
Nie Y, Niah W, Jaenicke S, Chuah GK (2007) J Catal 248:1–10
Nie Y, Chuah GK, Jaenicke S (2006) Chem Commun 790–792
Plößer J, Lucas M, Claus P (2014) J Catal 320:189–197
Plößer J, Lucas M, Wärnå J, Salmi T, Murzin DY, Claus P (2016) Org Proc Res Dev 20:1647–1653
Ravasio N, Poli N, Psaro R, Saba M, Zaccheria F (2000) Topics Catal 13(3):195–199
da Silva Rocha KA, Robles-Dutenhefner PA, Sousa EMB, Kozhevnikova EF, Kozhevnikov IV, Gusevskaya EV (2007) Appl Catal A 317:171–174
Vajglová Z, Kumar N, Peurla M, Eränen K, Mäki-Arvela P, Murzin DY (2020) Catal Sci Technol 10:8108–8119
Negoi A, Wuttke S, Kemnitz E, Macovei D, Parvulescu VI, Teodorescu CM, Coman SM (2010) Angew Chem Int Ed 49:8134–8138
Neaţu F, Coman S, Pârvulescu VI, Poncelet G, De Vos D, Jacobs P (2009) Topics Catal 52(9):1292–1300
Moreno-Marrodan C, Liguori F, Barbaro P, Caporali S, Merlo L, Oldani C (2017) ChemCatChem 9:4256–4267
Iosif F, Coman S, Pârvulescu V, Grange P, Delsarte S, De Vos D, Jacobs P (2004) Chem Commun 11:1292–1293
Simakova IL, Mäki Arvela P, Martínez-Klimov M, Muller J, Vajglová Z, Peurla M, Eränen K, Murzin DY (2022). Ind Eng Chem Res. 61 (35): 12988–13010
Chuah GK, Liu SH, Jaenicke S, Harrison LJ (2001) J Catal 200:352–359
Nakatani Y, Kawashima K (1978) Synthesis 8:147–148
Zuliani A, Cova CM, Manno R, Sebastian V, Romero AA, Luque R (2020) Green Chem 22:379–387
Itoh H, Maeda H, Yamada S, Hori Y, Mino T, Sakamoto M (2014) Org Chem Front 1(9):1107–1115
Ten Dam J, Ramanathan A, Djanashvili K, Kapteijn F, Hanefeld U (2017) RSC Adv 7:12041–12053
Chauvel A, Delmon B, Hölderich WF (1994) Appl Catal A 115:173–217
Allakhverdiev AI, Kul’kova NV, Murzin DY (1994) Catal Lett 29(1):57–67
Boronat M, Corma A, Renz M (2006) J Phys Chem B 110(42):21168–21174
Acknowledgements
The authors are grateful to Academy of Finland for funding through the project: Synthesis of spatially controlled catalysts with superior performance. IS is grateful for the support from the Ministry of Science and Higher Education of the Russian Federation under the governmental order for Boreskov Institute of Catalysis (Project AAAA- A21-121011390055-8).
Funding
Open access funding provided by Abo Akademi University (ABO).
Author information
Authors and Affiliations
Contributions
PMA wrote the first draft of the manuscript. All authors reviewed the manuscript
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mäki-Arvela, P., Simakova, I., Vajglová, Z. et al. One-Pot Synthesis of Menthol from Citral and Citronellal Over Heterogeneous Catalysts. Catal Surv Asia 27, 2–19 (2023). https://doi.org/10.1007/s10563-022-09376-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10563-022-09376-6