Abstract
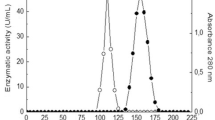
This study explores the purification and impact of metal ions on Aspergillus niger tannase, a metalloenzyme. Gel permeation chromatography achieved a 22.03-fold purification, yielding a specific activity of 106 U/mg. The enzyme, confirmed as a homodimer (100 kDa native, 50 kDa SDS PAGE), exhibited enhanced activity in the presence of calcium ions, reaching 163.46 U/mg, a 1.42-fold increase. UV absorption with 6 µM Ca ions indicated structural stability. Kinetic studies revealed a sigmoidal Michaelis–Menten graph with calcium ions, suggesting allosteric effects. The Lineweaver–Burk plot showed increased Km (14.04 to 36.39 mM) with mercuric ions, indicating competitive inhibition, while Vmax remained stable. Reactivation studies with DTT and different metal ions showed maximum reactivation with Hg (88.8%) and Ag (81.4%), implicating binding with cysteine residue in inactivation. EDTA-induced reactivation displayed maximum reactivation with Cu ions (67.6%) and minimum with Hg (22.5%), indicating Cu ions form complexes with amino acid groups. In contrast, Hg forms a coordination sphere with the thiol group of a cysteine residue. This comprehensive examination provides insights for optimizing tannase-based processes in various industries and expanding its biotechnological applications.
Graphical Abstract










Similar content being viewed by others
Abbreviations
- U :
-
Units
- Mg:
-
Milligrams
- kDa :
-
Kilodalton
- SDS :
-
Sodium dodecyl sulfate
- PAGE :
-
Polyacrylamide gel electrophoresis
- UV :
-
Ultraviolet
- Km :
-
Michaelis constant
- Vmax :
-
Maximum velocity
- mM :
-
Millimolar
- µM :
-
Micromolar
- EDTA :
-
Ethylenediamine tetraacetic acid
- EGTA :
-
Ethylene glycol-bis (β-aminoethyl ether)-N, N, N′, N′-tetraacetic acid
- DTT :
-
Dithiothreitol
- Ca :
-
Calcium
- Hg :
-
Mercury
- Cu :
-
Copper
- Cys :
-
Cysteine
- 3-D :
-
Three-dimensional
- TEMED :
-
N, N, N’, N’- Tetramethylethylenediamine
- BSA :
-
Bovine serum albumin
- NCCS :
-
National Centre for Cell Science
- NCBI :
-
National Center for Biotechnology Information
- SmF :
-
Submerged fermentation
- rpm :
-
Revolutions per minute
- NaCl :
-
Sodium chloride
- µl :
-
Microliter
- M :
-
Molar
- nm :
-
Nanometre
- DEAE :
-
Diethyl aminoethyl
References
Lekha PK, Lonsane BK (1997) Production and application of tannin acyl hydrolase: state of the art. Adv Appl Microbiol. https://doi.org/10.1016/S0065-2164(08)70463-5
Zhang YN, Yin JF, Chen JX, Wang QZ, Jiang YW, Xu YQ (2016) Improving the sweet aftertaste of green tea infusion with tannase. Food Chem. https://doi.org/10.1016/j.foodchem.2015.07.046
Ong C, Annuar MSM (2021) Cross-linked tannase-carbon nanotubes composite in elevating antioxidative potential of green tea extract. J Food Biochem. https://doi.org/10.1111/jfbc.13924
Kundu D, Karmakar S, Banerjee R (2022) Simultaneous debittering and clarification of enzyme mediated mixed citrus juice production. Appl Food Res. https://doi.org/10.1016/j.afres.2021.100031
Balakrishnan A, Kanchinadham SBK, Kalyanaraman C (2021) Studies on the effect of bacterial tannase supplementation to biodegradation of tannins in tannery wastewater. Ind Eng Chem Res. https://doi.org/10.1021/acs.iecr.1c02987
Sutaoney P, Akhand A, Meshram M, Sinha S, Joshi V, Shahadat M (2024) Tannase production using green biotechnology and its applications: a review. Biochem Eng J. https://doi.org/10.1016/j.bej.2023.109163
Ren B, Wu M, Wang Q, Peng X, Wen H, McKinstry WJ, Chen Q (2013) Crystal structure of tannase from Lactobacillus plantarum. J Mol Biol. https://doi.org/10.1016/j.jmb.2013.04.032
Dong L, McKinstry WJ, Pan L, Newman J, Ren B (2021) Crystal structure of fungal tannase from Aspergillus niger. Acta Crystallogr D: Struct Biol. https://doi.org/10.1107/S2059798320016484
Mahmudov KT, Gurbanov AV, Guseinov FI, Guedes da Silva MFC (2019) Noncovalent interactions in metal complex catalysis. Coord Chem Rev. https://doi.org/10.1016/j.ccr.2019.02.011
Neel AJ, Hilton MJ, Sigman MS, Toste FD (2017) Exploiting non-covalent π interactions for catalyst design. Nature. https://doi.org/10.1038/nature21701
Zhao Q, Chen C, Wen J, Dong XQ, Zhang X (2020) Noncovalent interaction-assisted ferrocenyl phosphine ligands in asymmetric catalysis. Acc Chem Res. https://doi.org/10.1021/acs.accounts.0c00347
Gurbanov AV, Mahmudov KT, Kopylovich MN, Guedes da Silva FM, Sutradhar M, Guseinov FI, Zubkov FI, Maharramov AM, Pombeiro AJL (2017) Molecular switching through cooperative ionic interactions and charge assisted hydrogen bonding. Dyes Pigm. https://doi.org/10.1016/j.dyepig.2016.11.029
Bulut H, Valjakka J, Yuksel B, Yilmazer B, Turunen O, Binay B (2020) Effect of metal ions on the activity of ten nad-dependent formate dehydrogenases. Protein J. https://doi.org/10.1007/s10930-020-09924-x
Prejano M, Alberto ME, Russo N, Toscano M, Marino T (2020) The effects of the metal ion substitution into the active site of metalloenzymes: a theoretical insight on some selected cases. Catalysts. https://doi.org/10.3390/catal10091038
Kluska K, Adamczyk J, Krezel A (2018) Metal binding properties, stability and reactivity of zinc fingers. Coord Chem Rev. https://doi.org/10.1016/j.ccr.2018.04.009
Garza-Lombo C, Posadas Y, Quintanar L, Gonsebatt ME, Franco R (2018) Neurotoxicity linked to dysfunctional metal ion homeostasis and xenobiotic metal exposure: redox signaling and oxidative stress. Antioxid Redox Signal. https://doi.org/10.1089/ars.2017.7272
Lu S, Shen Q, Zhang J (2019) Allosteric methods and their applications: facilitating the discovery of allosteric drugs and the investigation of allosteric mechanisms. Acc Chem Res. https://doi.org/10.1021/acs.accounts.8b00570
Mehrabi P, Di PC, Kim TH, Sljoka A, Taverner K, Ing C, Kruglyak N, Pomes R, Pai EF, Prosser RS (2019) Substrate-based allosteric regulation of a homodimeric enzyme. J Am Chem Soc. https://doi.org/10.1021/jacs.9b03703
Bajpai B, Patil S (2008) A new approach to microbial production of gallic acid. Braz J Microbiol. https://doi.org/10.1590/s1517-83822008000400021
Sharma S, Bhat TK, Dawra RK (2000) A spectrophotometric method for assay of tannase using rhodanine. Anal Biochem. https://doi.org/10.1006/abio.1999.4405
Scopes RK (1995) Strategies for protein purification. Curr Protoc Protein Sci. https://doi.org/10.1002/0471140864.ps0102s00
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. https://doi.org/10.1006/abio.1976.9999
Cummins PM, Rochfort KD, O’Connor BF (2017) Ion-exchange chromatography: basic principles and application. In: Walls D, Loughran S (eds) Protein chromatography. Methods in molecular biology, vol 1485. Humana Press, New York. https://doi.org/10.1007/978-1-4939-6412-3_11
Loughran ST, Milne JJ (2023) Protein chromatography. Methods in molecular biology, 3rd edn. Springer, US. https://doi.org/10.1007/978-1-0716-3362-5
Jan S, Khan S, Rabbani M, Khurshid H, Ibrahim M, Adil M, Ilyas M (2017) Comparison of electrophoretic protein profiles of Brassica rapa sub-species brown sarson through SDS-PAGE method. Genetika. https://doi.org/10.2298/gensr1701095j
Kumar M, Mehra R, Yogi R, Singh N, Salar RK, Saxena G, Rustagi S (2023) A novel tannase from Klebsiella pneumoniae KP715242 reduces haze and improves the quality of fruit juice and beverages through detannification. Front Sustain Food Syst. https://doi.org/10.3389/fsufs.2023.1173611
Ahmed AI, Abou-Taleb KAA, Abd-Elhalim BT (2023) Characterization and application of tannase and gallic acid produced by co-fungi of Aspergillus niger and Trichoderma viride utilizing agro-residues substrates. Sci Rep. https://doi.org/10.1038/s41598-023-43955-5
Farag AM, Hassan SW, El-Says AM, Ghanem KM (2018) Purification, characterization and application of tannase enzyme isolated from marine Aspergillus nomius GWA5. J Pure Appl Microbiol. https://doi.org/10.22207/jpam.12.4.30
Selwal KK, Selwal MK (2023) Purification and characterization of extracellular tannase from Aspergillus fumigatus MA using Syzigium cumini leaves under solid state fermentation. Prep Biochem Biotechnol. https://doi.org/10.1080/10826068.2023.2279106
Zhong X, Peng L, Zheng S, Sun Z, Ren Y, Dong M, Xu A (2004) Secretion, purification, and characterization of a recombinant Aspergillus oryzae tannase in Pichia pastoris. Protein Expr Purif. https://doi.org/10.1016/j.pep.2004.04.016
Bagga J, Pramanik SK, Pandey V (2015) Production and purification of tannase from Aspergillus aculeatus using plant derived raw tannin. Int J Eng Sci Technol. https://doi.org/10.17950/ijset/v4s2/205
Mahendran B, Raman N, Kim DJ (2006) Purification and characterization of tannase from Paecilomyces variotii: hydrolysis of tannic acid using immobilized tannase. Appl Microbiol Biotechnol. https://doi.org/10.1007/s00253-005-0082-y
Rk G, Krishnamurthy M, Neelamegam R, Shyu DJH, Muthukalingan K, Nagarajan K (2019) Purification, structural characterization and biotechnological potential of tannase enzyme produced by Enterobacter cloacae strain 41. Process Biochem. https://doi.org/10.1016/j.procbio.2018.10.013
Al-Mraai STY, Al-Fekaiki DF, Abd Al-Manhel AJ (2019) Purification and characterization of tannase from the local isolate of Aspergillus niger. J Appl Biol Biotechnol. https://doi.org/10.7324/jabb.2019.70106
Abdal A K, Al-jubori SS, Muslim SN (2020) Screening, extraction and purification for tannase produced from Iraqi Klebsiella pneumonia isolates and molecular detection of tanA gene. EurAsian J Biosci. https://doi.org/10.36295/asro.2020.2369
Lekshmi R, Arif NS, Thirumalai VP, Kaleeswaran B (2021) A comprehensive review on tannase: Microbes associated production of tannase exploiting tannin rich agro-industrial wastes with special reference to its potential environmental and industrial applications. Environ Res. https://doi.org/10.1016/j.envres.2021.111625
Costa AM, Ribeiro WX, Kato E, Monteiro ARG, Peralta RM (2008) Production of tannase by Aspergillus tamarii in submerged cultures. Braz Arch Biol Technol. https://doi.org/10.1590/s1516-89132008000200021
Dimarogona M, Topakas E, Christakopoulos P, Chrysina ED (2020) The crystal structure of a Fusarium oxysporum feruloyl esterase that belongs to the tannase family. FEBS Lett. https://doi.org/10.1002/1873-3468.13776
Borah A, Selvaraj S, Murty VR (2023) Production of gallic acid from Swietenia macrophylla using tannase from Bacillus Gottheilii M2S2 in semi-solid state fermentation. Waste Biomass Valorization. https://doi.org/10.1007/s12649-022-02023-1
Guan L, Wang K, Gao Y, Li J, Yan S, Ji N, Ren C, Wang J, Zhou Y, Li B, Lu S (2021) Biochemical and structural characterization of a novel bacterial tannase from Lachnospiraceae bacterium in ruminant gastrointestinal tract. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2021.806788
Kielkopf CL, Bauer W, Urbatsch IL (2020) Methods for measuring the concentrations of proteins. Cold Spring Harb Protoc. https://doi.org/10.1101/pdb.top102277
Mostafa HS (2022) Potato peels for tannase production from Penicillium commune HS2, a high tannin-tolerant strain, and its optimization using response surface methodology. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-021-02205-2
Dainese E, Oddi S, Simonetti M, Sabatucci A, Angelucci CB, Ballone A, Dufrusine B, Fezza F, De Fabritiis G, Maccarrone M (2020) The endocannabinoid hydrolase FAAH is an allosteric enzyme. Sci Rep. https://doi.org/10.1038/s41598-020-59120-1
Sun P, Liu Y, Ma T, Ding J (2020) Structure and allosteric regulation of human NAD-dependent isocitrate dehydrogenase. Cell Discov. https://doi.org/10.1038/s41421-020-00220-7
Fan Y, Cross PJ, Jameson GB, Parker EJ (2018) Exploring modular allostery via interchangeable regulatory domains. Proc Natl Acad Sci.https://doi.org/10.1073/pnas.1717621115
Jimenez N, Esteban-Torres M, Mancheno JM, de las RB, Munoz R (2014) Tannin degradation by a novel tannase enzyme present in some Lactobacillus plantarum strains. Appl Environ Microbiol.https://doi.org/10.1128/aem.00324-14
Piscopo M, Notariale R, Tortora F, Lettieri G, Palumbo G, Manna C (2020) Novel insights into mercury effects on hemoglobin and membrane proteins in human erythrocytes. Molecules. https://doi.org/10.3390/molecules25143278
Dutta N, Miraz SM, Khan MU, Karekar SC, Usman M, Khan SM, Amin U, Rebezov M, Shariati MA, Thiruvengadam M (2021) Heterologous expression and biophysical characterization of a mesophilic tannase following manganese nanoparticle immobilization. Colloids Surf B. https://doi.org/10.1016/j.colsurfb.2021.112011
Barton CC (2014) EDTA (ethylenediaminetetraacetic acid). Encyclopedia of toxicology, 3rd edn. Elsevier. https://doi.org/10.1016/b978-0-12-824315-2.00692-8
Arkhypova V, Soldatkin O, Soldatkin A, Dzyadevych S (2023) Electrochemical biosensors based on enzyme inhibition effect. Chem Rec. https://doi.org/10.1002/tcr.202300214
Sun S, Xu W, Zhou H, Zhang Y, Zhang J, Li X, Li B, Ma K, Xu J (2021) Efficient purification of selenoprotein thioredoxin reductase 1 by using chelating reagents to protect the affinity resins and rescue the enzyme activities. Process Biochem. https://doi.org/10.1016/j.procbio.2020.11.019
Acknowledgements
The authors are highly grateful to the Department of Biotechnology, Himachal Pradesh University, Shimla, India, for providing the laboratory and chemical facilities during the study. The authors also gratefully acknowledge ICMR, New Delhi, India for providing the financial assistance as SRF (JRF-2019/ HRD-008, 20546) and necessary facilities for the completion of this research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they do not have conflicts of interest among themselves.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. Examining the impact of various metal ions, including alkali, alkali earth, and transition metals, on the structural attributes of purified tannase sourced from Aspergillus niger.
2. Performing kinetic analyses to understand the mechanisms that activate or inhibit tannase function.
3. Exploring methods to reactivate inhibited tannase by employing metal ion chelators.
4. Suggesting a potential regulatory role of Ca ions and identifying tannase as an allosteric enzyme.
5. Investigating the impact of metal ion inactivation on tannase using EDTA and DTT, highlighting interactions with specific thiol and non-thiol groups within the enzyme.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alka, K., Kaushal, L., Arti et al. Impact of Metal Ions on Catalytic Kinetics, Stability, and Reactivation of Purified Tannase from Aspergillus niger. Catal Lett (2024). https://doi.org/10.1007/s10562-024-04664-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10562-024-04664-4