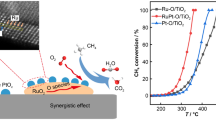
Abstract
The performance of a set of bimetallic Ru-Co catalysts dispersed on the TiO2 anatase phase, synthesized by the co-deposition–precipitation with urea method, was tested in the CO2 production by the entire oxidation of C3H8. The presence of properly dispersed ruthenium nanoparticles on a Co/TiO2-anatase support was notably favorable for enhancing the C3H8 activity/selectivity. Likewise, an increase in the combined acidity (Brönsted and Lewis) was also observed, and the interaction between Ru0-CoOx species stabilized on TiO2 prompted the C3H8 oxidation at low temperature. The performance of bimetallic Ru-Co/TiO2 catalysts was enhanced by increasing Ru0 species and loading, showing more efficient C3H8 oxidation by the combination of Ru0-CoOx, as revealed by DRIFTS, XPS, H2-TPR, and HAADF-STEM characterization outcomes. The higher acidity of the catalysts containing Ru (1.5 wt%) and Co (3 wt%) as well as the reducibility enhancement of the Ru and Co species led to the best capacity of these catalysts for C3H8 oxidation to CO2 under the experimental conditions employed in this study.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The emission of volatile organic compounds (VOCs), pollutants that are primarily released into the atmosphere, has become one of the main causes of air pollution by power generation systems using liquefied petroleum gases (LPG) and as component of automobile exhaust emissions [1, 2]. Catalytic oxidation is perhaps one of the most efficient methods of VOC abatement (light alkanes) and has proven to be a suitable solution for air treatment [3, 4]. Furthermore, bimetallic catalysts commonly stand out for their selectivity and anti-poisoning capacities, which are higher than in monometallic catalysts [5, 6]. On the other hand, supported ruthenium catalysts have been identified to be greatly active in total oxidation reactions, mainly for applications where low temperatures (around or even below 200 °C) are required [7, 8]. Ruthenium catalysts have increasingly enticed due to their high selectivity to long-chain hydrocarbons [9]. In contrast, Co catalysts added to oxides like TiO2 or Al2O3 are effective in the yield of ultraclean liquid fuels from syngas [10]. Therefore, cobalt oxide is an efficient material in oxidation processes due to its oxygen mobility in Co3+/Co2+ and redox ability, two factors that have proven to be important in the oxidation process [11]. Within Co catalysts for VOC oxidation, the spinel Co3O4 structure has stood out as an active species because of its great oxygen transfer ability stemming from the weak Co–O bond [12]. Likewise, the addition of noble metals such as Ag, Pt, Pd, Ru and Re in small loads to supported cobalt catalysts has shown to be effective at shifting the reduction temperature of cobalt oxides CoOx [13]. Therefore, the enhanced Co reducibility by noble-metal-promoted catalysts is broadly related to the interaction between noble metals and Co species in the bimetallic nanoparticles [14]. It has been proposed that the noble-metal-assisted reduction of CoOx oxide species that interact strongly with the support provokes high Co dispersion during the decomposition of the Co precursor, obtaining smaller crystallites [15].
Ruthenium and cobalt-based catalysts have been applied in different catalytic processes, highlighting R-NH2 oxidation, CO oxidation, and mainly long-chain VOC degradation [16, 17], displaying meaningful advantages in oxidation reactions. More recently, ruthenium nanoparticles have been employed in light alkane combustion [18, 19], where the metal-support interaction seems to be the major important factor that determines the catalytic properties of supported metal catalysts and that usually controls the oxidation step reaction [20]. However, the Ru-Co combination as a bimetallic catalyst has been hardly explored in the catalytic oxidation of short-chain VOCs. According to the foregoing, the main contribution of this work was to evaluate a series of Ru-Co catalysts supported on TiO2 anatase phase, by optimizing cobalt and ruthenium loads, in the efficient catalytic oxidation of C3H8 to CO2. Also, the catalytic activities of the synthesized Ru-Co during the C3H8 oxidation tests were correlated with the in-situ DRIFTS reaction and evolution of the physicochemical properties of the Ru-Co/TiO2 materials.
2 Experimental
2.1 Preparation of the TiO2 Anatase Phase
The TiO2 synthesis was performed by the sol–gel method, using titanium isopropoxide (Aldrich 99.99%) and 2-propanol (Baker 99%) heated up to 70 °C and adjusting the pH close to 3 with HNO3; then, 9 mL of deionized distilled water were added dropwise into the solution under vigorous stirring to carry out the hydrolysis of titanium isopropoxide, resulting in the formation of a gel material. Afterward, the mixture was evaporated in a rotary evaporator at 80 °C. The obtained sample was dried in an oven for 8 h at 80 °C and then annealed under static air at 500 °C for 5 h.
Ruthenium and cobalt were obtained by the deposition precipitation with urea (DPU) method [8]. The theoretical ruthenium loadings were mainly 1 and 1.5 wt% and the cobalt one was 3 wt%. The required amounts of ruthenium and cobalt precursors, RuCl3-3H2O (95%) and cobalt (II) nitrate hexahydrate (98%) as well as urea, were dissolved in distilled water. For the preparation of the bimetallic samples, a co-deposition–precipitation method was used for the simultaneous deposition of ruthenium and cobalt; to this end, an aqueous solution of RuCl3-3H2O and cobalt (II) nitrate hexahydrate at 98% with urea was added simultaneously into TiO2 and the reaction mixture was stirred at 80 ºC for 16 h; finally, the sample was washed and dried under vacuum for 2.5 h at 80 °C. After the DPU procedure, the bimetallic samples were centrifuged, washed four times with water at 50 °C, dried under vacuum for 2.5 h at 80 °C and activated in air or hydrogen as indicated below. The catalysts were labeled as XRu-YCo/TiO2, where X and Y represent the theoretical loading of metals.
2.2 Catalytic Reaction Study
To study the catalytic activity of the synthesized materials, 80 mg of sample were placed in a tubular quartz reactor (ID = 1 cm) inside an electric oven that allows heating at a programmed temperature. A porous quartz frit placed in the middle of the tube supported the catalyst. Prior to catalytic tests, the samples were calcined in-situ at 500 °C for 4 h with air or H2 flow rate of 80 mL min−1. The heating ramp was 3 °C per min. After heating, the samples were cooled down to room temperature at the same gas flow. Flow mass controllers were used to set the flow of a gas mixture at 5000 ppm of C3H8 and 2% of O2 in N2 with a total flow rate of 100 mL min−1. The space velocity, defined as the ratio of catalyst volume to the total flow of reaction gas, was around 40,000 h−1. CO2 concentration in the effluent gas was monitored online using a gas chromatograph (GC) Agilent Technologies 6890 network GC system equipped with a methanizer. H2O was delivered to the reactant mixture by a syringe pump, and it was vaporized in heated lines at 120 °C before reaching the reactor; the feed stream composition was 5000 ppm of C3H8 and 2% of O2 in N2; when water was added to the reaction mixture, the concentration was fixed at 5 vol. % and the total flow was maintained at 100 mL min−1. During the catalytic tests, the reactor temperature was increased from room temperature to 500 °C at a rate of 3 °C per min. The propane conversion (%) was defined as (([C3H8]in–[C3H8]out)/[C3H8]in) * 100, where [C3H8]in and [C3H8]out are the concentrations of propane at the inlet and outlet of the reactor, respectively.
2.3 Characterization of Catalysts
X-ray diffraction (XRD) patterns were collected at room temperature with Cu Kα radiation on a Bruker Advance D-8 diffractometer having a theta–theta configuration and a graphite secondary-beam monochromator. The data were collected for scattering angles (2θ) ranging from 5° to 80° with a step size of 0.01° for 2 s per point.
The samples were examined by transmission electron microscopy on a Jeol-2010 FasTem analytical microscope equipped with a Z-contrast annular detector and an EDS detector. The average size of metal particles and the histograms of particle sizes were established from the measurement of 200–300 particles. The textural properties were obtained by means of an ASAP-2000 analyzer from Micromeritics. The specific surface area SBET was calculated from the Brunauer–Emmett–Teller (BET) equation from N2 physisorption at 77 K.
H2-TPR profiles were performed on a Micromeritics AutoChem II 2920 automated catalyst characterization system under flow of a 10% H2/Ar gas mixture (25 mL•min−1) with a heating rate of 10 °C min−1 from room temperature to 600 °C.
Pyridine thermo-desorption was recorded on a Nicolet 8700 spectrophotometer with a resolution of 4 cm−1, accumulating 50 scans. In the cell, all the samples were treated under vacuum at 400 °C for 1 h and then, pyridine was admitted and adsorbed; evacuations were performed from room temperature to 400 °C. The acidity per surface area unit for Lewis and Brönsted sites was calculated following a previously reported procedure [21]. Diffuse Reflection Infrared Fourier Transform Spectroscopy (in-situ DRIFTS) was collected on a Nicolet 8700 spectrophotometer equipped with a Praying Mantis diffuse reflection cell and high-temperature reaction chamber.
The XPS analyses were performed with a Thermo VG Scientific Escalab 250 spectrometer equipped with a hemispherical electron analyzer and an Al Kα radiation source (1486.6 eV) powered at 20 kV and 30 mA. The binding energy was determined by using carbon C (1s) as reference line (284.6 eV). The spectrometer was operated at pass energy of 23.5 eV, and the base pressure in the analysis chamber was maintained in the order of 3 × 10−8 mbar. Peak fitting was done by using XPSPEAK 41 with Shirley background.
3 Results and Discussion
The X-Ray Diffraction (XRD) patterns for the monometallic Ru, Co and bimetallic Ru-Co catalysts supported on TiO2 are shown in Fig. 1. The X-ray diffraction pattern displayed peaks mostly representative of the TiO2 anatase phase at 2θ = 25.2°, 37.8°, 48°, 55°, 56°, 62.1°, 68.3°, 70.3°, 75°, JPDSC card no. 21-1272, and scarcely of the TiO2 brookite phase at 2θ = 30.8°, JCPDS card no. 29-1360. Likewise, no distinctive peaks of Ru and Co species were detected in the monometallic Ru, Co and Ru-Co bimetallic catalysts due to the low Ru and Co loadings and/or the good-dispersion of Ru (JPDSC card no. 06-0663) or Co3O4 (JPDSC card no. 06-0663) on TiO2 anatase in agreement with [22]. The average TiO2 crystallite sizes are given in Table 1, which were determined by integrated intensity of the 25.2° (101) reflection of the anatase phase; the average TiO2 crystallite size ranged in the order of 1.5Ru/TiO2 (17 nm), 3Co/TiO2 (18 nm), and 1.5Ru-3Co/TiO2 (17 nm), while the crystallite size of bare TiO2 was 18 nm. The nitrogen physisorption outcomes including only BET specific surface area for monometallic 3Co/TiO2, 1.5Ru/TiO2 and bimetallic 1.5Ru-3Co/TiO2 catalysts are shown in Table 2. The BET specific surface area of the 1.5Ru/TiO2 and 1.5Ru-3Co/TiO2 catalysts displayed surface area that was higher than that of the 3Co/TiO2 catalyst, which was associated with the highly dispersed ruthenium particles and slightly smaller TiO2 crystallite size. This behavior pattern could be due to a partial obstruction of TiO2 pores by the metal particles of cobalt oxides and ruthenium, which led to a slight reduction of the surface area, accompanied by a decrease in the volume of the mesopores (data not shown).
The morphology of the monometallic 3Co/TiO2 and bimetallic 1Ru-3Co/TiO2 (1 wt%) and 1.5Ru-3Co/TiO2 (1.5 wt%) catalysts was measured by HAADF–STEM, and the obtained results are shown in Fig. 2. The HAADF-STEM image confirmed that the ruthenium particles were uniformly dispersed, displaying an average size of metal particles close to 1.8, 1.6 and 2 nm for 1.5Ru/TiO2, 1Ru-3Co/TiO2, 1.5Ru-3Co/TiO2, respectively, see Figs. 2B, C and D. On the other hand, the 1.5Ru-Co/TiO2 catalysts thermally treated under air atmosphere (1.5Ru-Co/TiO2-air) displayed an average metal particle size close to 3 nm. It is noted that the atmosphere used for the thermal treatment of the bimetallic sample (air instead of hydrogen) significantly impacted the size of the metal particle, see supplementary data Fig. S1. As for the EDS studies, the 3Co/TiO2 catalyst revealed the presence of cobalt species (Fig. 2A). These data ratified the presence of highly dispersed Co species in that catalyst. Likewise, when a loading of 1.5 wt% of ruthenium was added to the sample, HAADF images confirmed the presence of ruthenium-rich nanoparticles in different regions of the catalysts thermally treated under air or hydrogen, see Figs. 2D and S1 for the catalysts thermally treated in air. On the other hand, distinct lattice fringes that could be associated with ruthenium or cobalt species were observed in HRTEM images, see supplementary data Fig. S2, however, it was not possible to assign them unambiguously due to very close distances for Ru (100) 0.23 nm and Co3O4 (311) 0.24 nm, according to Ru (JPDSC card no. 06-0663) and Co3O4 (JPDSC card no. 06-0663).
The H2-TPR profiles, presented in Fig. 3, were used to observe the reducibility as well as the interplay among Ru and Co supported on TiO2. The H2-TPR outcomes for the 1.5Ru/TiO2 and 1.5Ru-3Co/TiO2 catalysts presented a pair of reduction peaks: the first one at 70–110 °C, which can be allocated to the reduction of RuOx toward Ru in metallic state (Ru0) [23, 24], and the second reduction peak, observed in the 150–250 °C interval, can be ascribed to Ru species interacting with TiO2 anatase as Ru-TiOx sites, in agreement with earlier works [25]. Additionally, the wide reduction peak observed at 400–600 °C corresponded to the reduction of surface oxygen of TiO2 anatase. Likewise, the reduction peak of RuOx to Ru0 may be related to the existence of distinct Ru ion species [26]. The 3Co/TiO2 catalysts presented one peak at 354 °C, accompanied by a small shoulder at 410 °C associated with a two-stage reduction: Co3O4 → CoO → Co0 [27]. The peak at 354 °C was ascribed to the reduction of Co3O4 toward CoO, followed by a subsequent reduction of CoO to Co0 at 410 °C. Furthermore, the peaks located at 540 °C have been assigned to the reduction of Co species interacting with TiO2 [28]. The lowest reduction temperature observed for cobalt species in the 1.5Ru-3Co/TiO2 catalyst with regard to 3Co/TiO2 may be due to the fact that ruthenium exerted a promotional effect on Co that prompted its reduction to lower temperature via H2 spillover, in agreement with earlier reports [28].
3.1 Surface Acidity
The existence of Brönsted and Lewis acid sites was measured by thermo-desorption of pyridine for TiO2, 3Co/TiO2, 1.5Ru/TiO2, and 1.5Ru-3Co/TiO2 catalysts, Figs. 4(A–D). The quantitative distribution of acidity in the catalysts was determined by infrared spectroscopy, calculating the concentration of Brönsted (B) and Lewis (L) acid centers in the catalyst, based on the results shown in Fig. 4. The bands at 1450 cm−1 and 1596 cm−1 are attributed to Lewis acid sites, while the bands at 1540 cm−1 and 1650 cm−1 are ascribed to Brönsted acid sites. Additional bands observed at 1575, 1602, and 1613 cm−1 are related to pyridine ring vibrations; the signal at 1575 cm−1 represents physically adsorbed pyridine, whereas hydrogen bound pyridine with surface OH groups was revealed by the characteristic signals at 1602 and 1613 cm−1, which were observed in all the catalysts at low temperature and gradually disappeared as the temperature increased, being more intense in the Ru/TiO2 and Ru-Co/TiO2 catalysts, since they were the materials that presented higher quantity of Brönsted acid sites [29, 30]. Keeping in mind that propane oxidation markedly depends on the kind of catalyst acidity, the bare TiO2 anatase showed higher amount of Lewis acid sites, and the addition of Co to TiO2 mildly increased Lewis acid sites. In contrast, the presence of ruthenium nanoparticles in the 1.5Ru/TiO2 and 1.5Ru-3Co/TiO2 catalysts promoted the appearance of Brönsted acid sites, modifying the total acid sites, which are summarized in Table 2 in the interval ranging from 100 to 300 °C. On the other hand, it is important to emphasize that the 1.5Ru/TiO2 and 1.5Ru-3Co/TiO2 catalysts presented superior selectivity to CO2 in the catalytic oxidation of propane, which was corroborated by in-situ FTIR (see results below), and that may be associated with the acid sites on these samples. Likewise, the joint action between Lewis and Brönsted acid sites has been proposed as a determining factor for propane oxidation at low temperature [31], as observed for the 1.5Ru/TiO2 and 1.5Ru-3Co/TiO2 catalysts. In this sense, based on the findings related to these catalysts and earlier literature [31, 32], the Brönsted acid sites contribute to the removal of the -OH group, and the Lewis acid sites foster the elimination of the O-CH3 group, and then a proper increase in total acidity could lead to an efficient catalytic performance for the oxidation of C3H8 to CO2. The acidity measurement demonstrated that the increase in the total acidity and Brönsted acid sites in the catalysts is necessary for the scission of the C-O bonds in C3H8 at low temperature [32].
3.2 XPS Analysis
XPS experiments were conducted to examine the electronic configuration and to observe the interaction between Ru-Co and the metal species present in the 3Co/TiO2, 1.5Ru/TiO2 and bimetallic Ru-Co/TiO2 catalysts, see Fig. 5. In this sense, the C 1s spectra were deconvoluted and C–C (284.8 eV) and C–OH (286.2 eV) bonds were mainly found [33]. With respect to ruthenium species, it is difficult to assign them with certainty because they overlap carbon species, however, a binding energy of 280.0 eV was related to Ru0 3d5/2 in the 1.5Ru/TiO2 and 1.5Ru-3Co/TiO2 catalysts [34]. In the presence of cobalt, the binding energy of Ru0 3d5/2 at 280.0 eV shifted by 0.3 eV, suggesting the interaction and electron transfer from Ru-Co species, see Fig. 5. As for the Co samples, the deconvolution was carried out for Co2p spectra (Fig. 5), which displayed mainly two peaks at binding energies of 782.1 eV and 796.3 eV corresponding to Co 2p3/2 and Co 2p1/2 species; likewise, shake-up satellites were found at 787.5 eV and 802.8 eV for the Co/TiO2 and 1.5Ru-3Co/TiO2 catalysts, severally. Therefore, the presence of Co 2p3/2 and Co 2p1/2 shake-up satellites revealed the presence of Co2+ species in the monometallic Co and bimetallic Ru-Co catalysts [34]; in this way, it is important to mention that Co metallic species are easy to be oxidized by environmental oxygen. The XPS Co peaks were shifted to lower binding energies in the 1.5Ru-3Co/TiO2 sample with regard to 3Co/TiO2, indicating an interaction between Ru-Co species; these electronic effect outcomes suggest that in Ru-Co/TiO2, Ru could be electron deficient, causing Co electron enrichment [35]. In this sense, an earlier work reported that the d-band center allows the adsorption properties, which would result in a different catalytic performance [36]. The O 1s in Fig. 5 is deconvoluted by three components occurring at 530.7, 533.2 and 532.9 eV, which are assigned to oxygen and OH-like species and adsorbed H2O molecules [33, 34].
3.3 Propane Oxidation by in-situ DRIFTS
The C3H8 oxidation for the 3Co/TiO2, 1.5Ru/TiO2 and 1.5Ru-3Co/TiO2 catalysts was carried out using in-situ FTIR spectroscopy. In these tests, DRIFT spectra were obtained at chosen temperatures, following the same composition used during the catalytic test: 5000 ppm of C3H8 and 2% of O2 in N2 with a total flow rate of 100 mL min−1, heating up to reach total oxidation to CO2. The bands observed in all the catalysts at 3000–2920 cm−1 are assigned to asymmetric vibrations and those at 2980 and 2966 cm−1 to symmetric ones; the band observed at 2958 cm−1 is ascribed to C-H stretching in CH3 groups [37] and the one at 2966 cm−1 may be related to propane from the gas phase.
Regarding the region between 1700 and 1250 cm−1, bands at 1640 and 1544 cm−1 are due to carboxylate and carbonate species [38], severally, which were observed in all the catalysts. It should be pointed out that adsorbed steam may have contributed to the band situated at 1640 cm−1. With regard to the intensity observed in these latter bands, it gradually decreased with the temperature increase and disappeared at above 250 °C; likewise, bands at 1544 and 1375 cm−1 related to formate species on TiO2 anatase [39] along with a band at 1486 cm−1 due to carbonate species were observed [38, 39]. On the other hand, two peaks at 2065 and 2040 cm−1, which stemmed from CO adsorbed linearly on reduced Ru sites (Ru0), and a lower intensity peak at 1800 cm−1, characteristic of bridged bound CO, were observed [40]. Therefore, according to earlier studies, the band at ~ 2005 cm−1 may be related to linearly adsorbed carbonyls or to the existence of hydrogen adsorbed on ruthenium sites [41]. It is also feasible that this band at 2005 cm−1 could be due to the formation of Ru carbonyl hydride, according to previous studies [40, 41]. Also, based on earlier literature, the bands at 1632 cm−1 and 3336 cm−1 are assigned to δ (H–O–H) and ν(O–H) of physisorbed water, respectively. In this sense, the oxidation of C3H8 to CO2 starts to be observable in all the catalysts between 170 and 200 °C, whereas the activity tests showed that propane conversion in the 1.5Ru-3Co/TiO2 catalyst reached the total oxidation at 300 °C (see catalytic activity for C3H8 oxidation below). According to the DRIFT spectra, propane already interacts with the catalysts surface at 150 °C, appearing bands related to the formation of Ru0 and to the Ru0-CoOx interaction. This finding is important to elucidate the active sites present during the C3H8 oxidation, which may be connected with the highly interaction between the metals (Ru0 and CoOx) and the support (TiO2). According to the obtained results, the Co interaction with the support in the monometallic 3Co/TiO2 catalyst, under these reaction conditions, is lower than that of the catalysts containing ruthenium, 1.5Ru/TiO2 and 1.5Ru-3Co/TiO2, where ruthenium Ru0 species clearly interact with TiO2, playing an important role during the transformation process of C3H8 to CO2 and becoming evident between 150 and 320 °C, where the catalysts showed activity to the propane oxidation; unlike when the catalyst was treated with air, it did not show the presence of Ru0, displaying oxidation towards CO at higher temperature as shown in the supplementary data, Fig. S3. In general, the main highlights for all the catalysts are the presence of bands at 1544 and 1486 cm−1 that are mainly related to the νas (COO) and νs (COO) of acetate species, suggesting that propane was promptly oxidized into acetate species on the 1.5Ru/TiO2 and 1.5Ru-3Co/TiO2 catalysts. Furthermore, the presence of bands at 1640, 1437 and 1358 cm−1 was related to the νs (C = O) of aldehyde, νas (COO) and νs (COO) of acetate species, respectively. Additionally, in the 1.5Ru/TiO2 and 1.5Ru-3Co/TiO2 catalysts, two bands at 2065 and 2005 cm−1 were observed, which stemmed from CO adsorbed linearly on reduced Ru sites (Ru0) and the interaction with CoOx. The band in the region ~ 2005–2040 cm−1 may be associated either with linearly adsorbed carbonyls or with the existence of CoO, which looked more intense in the bimetallic Ru-Co catalyst.
3.4 C3H8 Oxidation Reaction Outcomes
The C3H8 oxidation activities of the monometallic Co/TiO2 catalysts and the corresponding TiO2 support were tested under the same conditions. As shown in Fig. 7A, TiO2 was inactive in the C3H8 oxidation at temperatures less than 300 °C. Therefore, after loading Co (1, 2, 3 and 4 wt%), all the catalysts exhibited activity in the C3H8 oxidation between 200 and 400 °C. The catalyst with 3 wt% of cobalt displayed the best activity, reaching complete conversion of C3H8 at approximately 360 °C see, Fig. 7A. In this way, according to T90 (temperature to reach 90% C3H8 conversion), the activities followed the order: 3Co/TiO2 (360 °C) > 4Co/TiO2 (380 °C) > 2Co/TiO2 (400 °C) > 1Co/TiO2 (480 °C). In this sense, the production of CO2 was nearly equivalent to the conversion of C3H8, indicating almost the total mineralization of C3H8. Only traces of other products equivalent to 6 ppm were observed from 200 to 300 °C.
The outcomes of C3H8 oxidation light-off tests for the 3Co/TiO2, 1.5Ru/TiO2 and 1.5Ru-3Co/TiO2 catalysts treated in hydrogen at 500 °C are shown in Fig. 7B. The total C3H8 conversion for the 3Co/TiO2 catalyst was reached at 400 °C. In comparison, in the light-off curves for Ru/TiO2, displaced to lower temperatures, it can be seen that the total C3H8 oxidation was achieved at 220 °C, while for 1.5Ru-3Co/TiO2, the light-off curves shifted to even lower temperatures, reaching total C3H8 oxidation at 200 °C. In this sense, T50 and T90 were also employed to differentiate the catalytic C3H8 behavior; hence, the T50 and T90 values of 3Co/TiO2 were 260 and 300 °C, while the values of the 1.5Ru/TiO2 catalysts were 170 and 200 °C, and the ones for 1.5Ru-3Co/TiO2 were even lower, 150 and 170 °C, respectively. The 1.5Ru-3Co/TiO2 catalysts showed superior activity, as it is also shown by the reaction rates and turn over frequencies presented in Table 1. In another way, the evaluation of the 3Co/TiO2, 1.5Ru/TiO2 and 1.5Ru-3Co/TiO2 catalysts in the propane oxidation when air was used instead of hydrogen as the atmosphere for the thermal treatment are displayed in Fig. 6C. The C3H8 conversion of the 3Co/TiO2 catalyst was enhanced with rising reaction temperature, reaching C3H8 oxidation at 350 °C. For 1.5Ru/TiO2, the light-off curves also shifted to high temperatures regarding to the same sample thermally treated in hydrogen, reaching total C3H8 oxidation at 320 °C, and in the case of 1.5Ru-3Co/TiO2, the light-off curves shifted again to higher temperatures, reaching total C3H8 oxidation at 300 °C. The tendency was the same as the one observed in the samples treated under hydrogen atmosphere, but at higher reaction temperatures when the catalysts were thermally treated in air; then, it is better to pretreat the samples in hydrogen than in air.
Moreover, Fig. 7D shows the catalytic behavior of the 1.5Ru-3Co/TiO2 catalysts annealed at distinct temperatures in hydrogen in the total oxidation of C3H8. A remarkable difference in the catalytic performance of the 1.5Ru-3Co/TiO2 catalyst annealed at 650 °C, compared with the same catalysts annealed at 400 and 500 °C, was observed. 1.5Ru-3Co/TiO2 annealed at 500 °C showed superior performance and the T50 value increased in the following order: 650 °C > 400 °C > 500 °C.
A C3H8 conversions as a function of the reaction temperature for the Co/TiO2 catalysts treated under H2 atmosphere at 500 °C with different loads, B C3H8 conversions as a function of the reaction temperature for the 3Co/TiO2, 1.5Ru/TiO2 and 1.5Ru-3Co/TiO2 catalysts treated under H2 atmosphere at 500 °C, C C3H8 conversions as a function of the reaction temperature for the 3Co/TiO2, 1.5Ru/TiO2 and 1.5Ru-3Co/TiO2 catalysts treated under air atmosphere at 500 °C, D C3H8 conversions for 1.5Ru-3Co/TiO2 catalysts thermally treated at different temperatures under hydrogen, E C3H8 conversions for different Ru/Co ratios in the catalysts, F temporal stability of C3H8 conversions for the 3Co/TiO2, 1.5Ru/TiO2 and 1.5Ru-3Co/TiO2 catalysts thermally treated in H2 at 500 °C under dry conditions and G temporal stability of C3H8 conversions for the 1.5Ru-3Co/TiO2 catalyst with presence of 5 vol.% of water
The Ru-Co/TiO2 catalyst was evaluated in the C3H8 oxidation with different ruthenium loadings, maintaining the same amount of cobalt (3 wt%), as shown in Fig. 7(E). The 1Ru-3Co/TiO2 catalyst presented T50 of 210 °C, reaching 100% of conversion at 280 °C, while the catalyst 1.5Ru-3Co/TiO2 presented T50 of 185 °C, achieving 100% of conversion at 260 °C. Both catalysts significantly boosted the catalytic activity for propane oxidation, where the 1.5Ru-3Co/TiO2 catalyst turned out to be better. It was observed that the catalytic activity in the C3H8 oxidation displayed by ruthenium incorporation into 3Co/TiO2 depends on the metal load, temperature and atmosphere used during the thermal treatment, showing that the well-dispersed ruthenium particles in interaction with CoOx produced very active catalysts and that the conversion increased as the ruthenium loading grew when the samples were thermally treated in hydrogen, also see Supplementary Data S4.
On the other hand, the long-term test for the 3Co/TiO2, 1.5Ru/TiO2, and 1.5Ru-3Co/TiO2 catalysts was investigated in the C3H8 oxidation under dry conditions, see Fig. 7F. In continuous operation at 200 °C for 1.5Ru-3Co/TiO2 and 1.5Ru/TiO2, and at 260 °C for Co/TiO2, the C3H8 conversion to CO2 firstly declined from 77% to 74% in 2 h and then turned stable at around 73% in the later 3 h for the 1.5Ru-3Co/TiO2 catalyst, while for the 1.5Ru/TiO2 catalyst, the C3H8 conversion firstly declined quickly from 64% to 50% during the first 2 h and then got relatively stable at around 49% in the subsequent 3 h; as for the Co/TiO2 catalyst, the C3H8 conversion to CO2 firstly decayed from 64% to 59% in 2 h and then, it got stable at around 56% in the subsequent 3 h. These experiments demonstrated that the Ru-Co catalyst was more stable under dry conditions than its monometallic counterparts. Earlier works have reported that when the formation rate of reactive coke was faster than the reaction rate, the formed compounds would be quickly oxidized to induce self-heating of the catalyst, thus causing the conversion of VOCs into CO2 [42]. For the Ru-Co/TiO2 catalysts, the introduction of water (5 vol. %) into the reaction mixture showed a decrease in the conversion during the first 2 h of the reaction test, then it remained stable at around 60% for the next 4 h, as shown in Fig. 7G. After this catalytic test, the catalyst was recovered and thermally treated again at 500 °C under hydrogen; a new catalytic test was carried out in the presence of water, showing that the catalyst was completely regenerated, maintaining the same conversion during a 5-h test, which indicates that the deactivation observed during the initial test was reversible. Similar behavior occurred when the test was repeated, evidencing the reproducibility of the experimental result.
For comparison purposes, Table 3 summarizes the results obtained for propane oxidation in the literature and they are compared with the 3Co/TiO2, 1.5Ru/TiO2, and 1.5Ru-3Co/TiO2 catalysts studied in this work. The 1.5Ru-3Co/TiO2 catalyst displayed lower light-off temperature and higher T50 and T90 activity in propane oxidation under moderate GHSV. As shown in Table 1, the apparent activation energy of propane oxidation on 3Co/TiO2 was observed to be 87 kJ/mol, and this value is close to 77.4 kJ/mol reported for Co3O4/TiO2 catalysts [43] and lower than 87.9 kJ/mol for Co3O4 [43]. Meanwhile, the apparent activation energy on the 1.5Ru/TiO2 catalyst was found to be 79 kJ/mol, which is slightly lower than the literature value of 79.9 kJ/mol [7, 8], while for 1.5Ru-3Co/TiO2, it was 74 kJ/mol, and this value is lower than 79.7 kJ/mol, reported for Co3O4/ZSM [43]. In the study here reported, 1.5Ru-3Co/TiO2 exhibited lower apparent activation energy than the 1.5Ru/TiO2 and 3Co/TiO2 catalysts, Table 1. As it can be observed in Table 3, the 1.5Ru-3Co/TiO2 catalyst displayed the lowest activation energy values, using much lower mass of catalyst than with other Ru-Co catalysts supported on pure or mixed oxides that have been previously reported, although this comparison should be taken with care, since most of the evaluation conditions are different. It is conjectured that the lowest T50 and T90 values shown by the 1.5Ru-3Co/TiO2 catalyst could be correlated to a larger number of active sites because of the interaction between Ru and Co in the particles, promoting lower values of the pre-exponential factor and activation energy.
3.5 XRD Patterns of Spent Catalysts
The 3Co/TiO2, 1.5Ru/TiO2 and 1.5Ru-3Co/TiO2 catalysts were analyzed after the C3H8 oxidation test by X-ray diffraction and the results are shown in Fig. 8. The X-ray diffraction patterns revealed some very low intensity peaks ascribed to ruthenium in the 1.5Ru/TiO2 and 1.5Ru-3Co/TiO2 samples, while diffraction peaks of cobalt oxides were not observed after the propane oxidation reaction test, compared to the same samples treated at 500 °C before the catalytic test, where ruthenium and cobalt peaks were not observed (Fig. 1). These results indicate a slight growth of ruthenium metal particles while anatase remained as the main TiO2 phase, see Fig. 8. Likewise, in all the samples, a small peak of brookite phase was observed at 2θ = 30.8° (1 2 1), being more visible in the 1.5Ru/TiO2 catalyst. Through X-ray diffraction patterns, the presence of Ru0 after the propane oxidation test was confirmed as well as the slight increase in ruthenium particle size by HAADF-STEM from 1.6 nm in the fresh catalyst to 2.6 nm in the spent one. The samples tested in the C3H8 oxidation reaction were also analyzed by H2/TPR analysis, observing a pair of reduction peaks: the first one at 78–180 °C was related to the reduction of RuOx toward Ru in metallic state (Ru0) in the 1.5Ru/TiO2 catalyst; also, the 3Co/TiO2 catalyst presented one peak at 260 °C, accompanied by a small shoulder at 330 °C associated with a two-stage reduction: Co3O4 → CoO → Co0. The 1.5Ru-3Co/TiO2 catalyst showed a notable change in the reduction profile, displaying two main peaks at 250 and 379 °C, which were mainly ascribed to the reduction of CoOx species; the shift in the reduction temperature of CoOx species suggests their interaction with Ru0, which remained metallic during the propane oxidation, and the presence of cobalt oxide may help to delay the sintering of ruthenium during the thermal treatment at 500 °C under hydrogen atmosphere. Moreover, the combination of Ru and CoOx and the anatase phase may provide higher stability in the oxidation of propane to the bimetallic catalysts than in the monometallic Ru and Co ones.
4 Conclusions
In summary, monometallic Co/TiO2 and Ru/TiO2 and bimetallic Ru-Co/TiO2 catalysts were prepared by the deposition–precipitation with urea method. Co/TiO2-based catalysts exhibited activity toward propane oxidation in the middle reaction temperature (300–400 °C) interval; it was also found that the addition of ruthenium (1 and 1.5 wt%) strongly influenced the catalytic behavior of Co/TiO2 catalysts. Notably, bimetallic Ru-Co/TiO2 catalysts displayed superior activity in the oxidation of propane to CO2, which may be related to both the smallest size of the Ru particles and to the best reducibility of Co species. The Ru-Co/TiO2 system was the most suitable catalyst for the C3H8 oxidation, displaying excellent catalytic results, showing T50 propane conversion at only 150 °C. This performance could be assigned to the different interactions involving Ru and CoOX and those between TiO2 and Ru nanoparticles. The bimetallic Ru-Co catalysts also presented not only the best acid and redox properties, but also the highest oxygen mobility, thus exhibiting thermal stability and excellent deactivation resistance at least for 30 h under the selected reaction conditions.
References
Bari A, Kindzierski WB (2018) Ambient volatile organic compounds (VOCs) in Calgary, Alberta: sources and screening health risk assessment. Sci Total Environ 631–632:627–640
Schauer JJ, Kleeman MJ, Cass GR, Simoneit BRT (2002) Measurement of emissions from air pollution sources. 5. C-1−C-32 organic compounds from gasoline-powered motor vehicles. Environ Sci Technol 36(6):1169–1180
He C, Cheng J, Zhang X, Douthwaite M, Pattisson S, Hao Z (2019) Recent advances in the catalytic oxidation of volatile organic compounds: a review based on pollutant sorts and sources. Chem Rev 119:4471–4568
Ma R, Geng L, Chen Y, Yan X, Li N, Schwank JW (2020) Reaction mechanism of propane oxidation over Co3O4 nanorods as rivals of platinum catalysts. Chem Eng J 402:125911
Sreethawong T, Sukjit D, Ouraipryvan P, Schwank JW, Chavadej S (2010) Oxidation of oxygenated volatile organic compound over monometallic and bimetallic Ru-Au catalysts. Catal Lett 138:160–170
Aouad S, Saab E, Abi-Aad E, Aboukaïs A (2007) Study of the Ru/Ce system in the oxidation of carbon black and volatile organic compounds. Kinet Catal 48:835–840
Debecker DP, Farin B, Gaigneaux EM, Sanchez C, Sassoye C (2014) Total oxidation of propane with a nano-RuO2/TiO2 catalyst. Appl Catal A Gen 481:11–18
Camposeco R, Miguel O, Torres AE, Armas DE, Zanella R (2023) Highly active Ru/TiO2 nanostructures for total catalytic oxidation of propane. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-023-29153-w
Qadir K, Sang HJ, Mun BS, Butcher DR, Renzas JR, Aksoy F, Zhi L, Somorjai GA, Park JY (2012) Intrinsic relation between catalytic activity of CO oxidation on Ru nanoparticles and Ru oxides uncovered with ambient pressure XPS. Nano Lett 12:5761–5768
Khodakov AY, Chu W, Fongarland P (2007) Advances in the development of novel cobalt Fischer-Tropsch catalysts for synthesis of long-chain hydrocarbons and clean fuels. Chem Rev 107(5):1692–1744
Xunxun L, Yaru W, Jiaqin H, Jun X, Wanjun X, Dongyun C, Najun L, Qingfeng X, Hua L, Jianmei L (2023) Combination of porous covalent triazine frameworks with spinel for highly improved photothermal catalytic oxidation of toluene. Appl Catal B Environ 56:122690
Baijun F, Heng Y, Xiao X, Qianru Z, Xugang Z, Wei Y (2023) Metal-organic frameworks-derived hollow Co3O4 nanotubes for efficient detection of toluene vapor. J Alloys Compd 937:168535
Cook KM, Perez HD, Bartholomew CH, Hecker WC (2014) Effect of promoter deposition order on platinum-, ruthenium-, or rhenium-promoted cobalt Fischer-Tropsch catalysts. Appl Catal A 482:275–286
Eschemann TO, Oenema J, de Jong KP (2016) Effects of noble metal promotion for Co/TiO2 Fischer-Tropsch catalysts. Catal Today 261:60–66
Hong J, Marceau E, Khodakov AY, Gaberová L, Griboval-Constant A, Girardon JS, Fontaine CL, Briois V (2015) Speciation of ruthenium as a reduction promoter of silica-supported Co catalysts: A. Time-resolved in situ XAS investigation. ACS Catal 5(2):1273–1282
Okal J, Zawadzki M, Tylus W (2011) Microstructure characterization and propane oxidation over supported Ru nanoparticles synthesized by the microwave-polyol method. Appl Catal B Environ 101:548–559
Li C, Vijay N, Jens A, Martin M (2006) Controlled synthesis of supported ruthenium catalysts for CO oxidation by organometallicchemical vapor deposition. Studies in Surface Science and Catalysis. Elsevier 162:473–480
Baranowska K, Okal J (2015) Bimetallic Ru-Re/γ-Al2O3 catalysts for the catalytic combustion of propane: effect of the Re addition. Appl Catal A Gen 499:158–167
Beamson G, Papworth AJ, Philipps CA, Smith M, Whyman R (2010) Selective hydrogenation of amides using ruthenium/molybdenum catalysts. Adv Synth Catal 352:869–883
Tauster SJ, Fung SC, Garten RL (1978) Strong metal-support interactions. Group 8 noble metals supported on titanium dioxide. J Am Chem Soc 100:170–175
Selli E, Forni L (1999) Comparison between the surface acidity of solid catalysts determined by TPD and FTIR analysis of pre-adsorbed pyridine. Microporous Mesoporous Materials. 31:129–140
Niu T, Liu GL, Liu Y (2014) Preparation of Ru/graphene-meso-macroporous SiO2 composite and their application to the preferential oxidation of CO in H2-rich gases. Appl Catal B 154–155:82–92
Fontana J, Vignado C, Jordao E, Figueiredo FCA, Carvalho WA (2011) Evaluation of some supports to RuSn catalysts applied to dimethyl adipate hydrogenation. Catal Today 172(1):27–33
Upadhyay PR, Srivastava V (2016) Selective hydrogenation of CO2 gas to formic acid over nanostructured Ru-TiO2 catalysts. RSC Adv 6(48):42297–42306
Mishra DK, Lee JM, Chang JS, Hwang JS (2012) Liquid phase hydrogenation of d-glucose to d-sorbitol over the catalyst (Ru/NiO–TiO2) of ruthenium on a NiO-modified TiO2 support. Catal Today 185(1):104–108
Kalala J (2016) Fischer-Tropsch synthesis over Co/TiO2 catalyst: effect of catalyst activation by CO compared to H2. Catal Commun 74:71–74
Liu Y, Wang Y, Wang H, Wu Z (2011) Catalytic oxidation of gas-phase mercury over Co/TiO2 catalysts prepared by sol–gel method. Catal Commun 12(14):1291–1294
Fiorenza R, Scirè S, Venezia AM (2018) Carbon supported bimetallic Ru-Co catalysts for H2 production through NaBH4 and NH3BH3 hydrolysis. Int J Energy Res 42(3):1183–1195
Busca G, Lietti L, Ramis G, Berti F (1998) Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: A review. Appl Catal B: Environ 18:1–36
González J, Wang JA, Chen LF, Manríquez ME, Dominguez JM (2017) Structural defects, lewis acidity, and catalysis properties of mesostructured WO3/SBA-15 nanocatalysts. J Phys Chem C 121(43):23988–23999
Busca G (1998) Spectroscopic characterization of the acid properties of metal oxide catalysts. Catal Today 41:191–206
Bordiga S, Lamberti C, Bonino F, Travert A, Thibault-Starzyk F (2015) Probing zeolites by vibrational spectroscopies. Chem Soc Rev 44(20):7262–7341
Moulder J, Stickle WF, Sobol PEBK (1992) Handbook of X-ray photoelectron spectroscopy. Perkin Elmer Corporation, Minnesota
NIST X-ray Photoelectron Spectroscopy Database, NIST Standard Reference Database (2012) National Institute of Standards and Technology, Gaithersburg MD, 20899
Bock C, Paquet C, Couillard M, Botton GA, MacDougall BR (2004) Size-Selected Synthesis of PtRu Nano-Catalysts: Reaction and Size Control Mechanism. J Am Chem So 126:8028–8037. https://doi.org/10.1021/ja0495819
Okal J, Zawadzki M (2009) Influence of Catalyst Pretreatments on Propane Oxidation Over Ru/γ-Al2O3. Catal Lett 132:225–234
Faria WL, Perez CA, César DV, Dieguez LC, Schmal M (2009) Appl Catal B 92:217–224
Saracco GF, Baldi G (1999) Appl Catal B 20:277–288
Tang CW, Hsu LC, Yu SW, Wang CB, Chien SH (2013) Vib Spectrosc 65:110–115
Finocchio E, Busca G, Lorenzelli V, Willey RJ (1994) J Chem Soc Faraday Trans 90:3347–3356
Petkov VS, Shan P, Chupas J, Yin L, Yang J, Zhong L, Zhong CJ (2013) Nanoscale 5:7379–7387
Chesters MA, Cruz CDL, Gardner P, McCash EM, Pudney P, Shahid G, Sheppard N (1990) Infrared spectroscopic comparison of the chemisorbed species from ethane, propene, but-1-ene and cis- and trans-but-2-ene on Pt(111) and on a platinum silica catalyst. J Chem Soc Faraday Trans. https://doi.org/10.1039/ft9908602757
Wang B, Wu X, Ran R, Si Z, Weng D (2012) IR characterization of propane oxidation on Pt/CeO2–ZrO2: The reaction mechanism and the role of Pt. J Mol Catal A Chem 356:100–105
Tsou J, Magnoux P, Guisnet M, Órfão JJM, Figueiredo JL (2004) Oscillations in the catalytic oxidation of volatile organic compounds. J Catal 225:147–154
Chai G, Zhang W, Liotta LF, Li M, Guo Y, Giroir-Fendler A (2021) Total oxidation of propane over Co3O4-based catalysts: Elucidating the influence of Zr dopant. Appl Catal B Environ 298:120606
Zhu W, Chen X, Jin J, Di X, Liang C, Liu Z (2020) Insight into catalytic properties of Co3O4-CeO2 binary oxides for propane total oxidation. Chin J Catal 41(4):679–690
Hu Z, Wang Z, Guo Y, Wang L, Guo Y, Zhang J, Zhan W (2018) Total oxidation of propane over a Ru/CeO2 catalyst at low temperature. Environ Sci Tech 52(16):9531–9541
Okal J, Zawadzki M (2011) Combustion of propane over novel zinc aluminate-supported ruthenium catalysts. Appl Catal B Environ 105(1–2):182–190
Ledwa KA, Kępiński L, Ptak M, Szukiewicz R (2020) Ru0.05Ce0.95O2-y deposited on functionalized alumina as a smart catalyst for propane oxidation. Appl Catal B Environ 274:119090
Wang M, Li G, Wang S, Liu X, Wang A, Cao H, Zhang C (2023) Catalytic oxidation of propane over nanorod-like TiO2 supported Ru catalysts: structure-activity dependence and mechanistic insights. Chem Eng J 48:148344
Liu CF, He LC, Wang XF, Chen J, Lu JQ, Luo MF (2022) Tailoring Co3O4 active species to promote propane combustion over Co3O4/ZSM-5 catalyst. Mole Catal 524:112297
Zha K, Liu H, Xue L, Huang Z, Xu H, Shen W (2021) Co3O4 nanoparticle-decorated SiO2 nanotube catalysts for propane oxidation. ACS Appl Nano Mater 4(9):8937–8949
Wu S, Yuan C, Huang Z, Xu H, Shen W (2024) Engineering Mn-O strength in manganese oxide catalyst to enhance propane catalytic oxidation. Chem Eng J 479:147928
Liu Z, Cheng L, Zeng J, Hu X, Zhangxue S, Yuan S, Jiang Y (2021) Boosting catalytic oxidation of propane over mixed-phase CoO–Co3O4 nanoparticles: effect of CoO. Chem Phys 540:110984
Cai T, Deng W, Xu P, Yuan J, Liu Z, Zhao K, He D (2020) Great activity enhancement of Co3O4/γ-Al2O3 catalyst for propane combustion by structural modulation. Chem Eng J 395:125071
Albilali R (2024) Total oxidation of propane using titania-supported platinum nanoparticles prepared through sol-immobilization. Arab J Basic Appl Sci 31(1):53–64
Huang LL, Xu LL, Gao BW, Ma YK, Jia AP, Wang Y, Lu JQ (2023) Deep oxidation of propane over PtIr/TiO2 bimetallic catalysts: Mechanistic investigation of promoting roles of Ir species. Appl Surf Sci 638:158149
Ruth K, Hayes M, Burch R, Tsubota S, Haruta M (2000) The effects of SO2 on the oxidation of CO and propane on supported Pt and Au catalysts. Appl Catal B Environ 24(3–4):L133–L138
Acknowledgements
The authors want to thank the financial support provided by the Consejo Nacional de Humanidades Ciencias y Tecnologías (CONAHCYT) through the CB A1-S-18269 grant, Dirección General de Asuntos del Personal Académico-UNAM through the PAPIIT IN104022 grant. R. Camposeco gratefully acknowledges CONAHCYT for the postdoctoral scholarship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Camposeco, R., Gómora-Herrera, D. & Zanella, R. Insights into the Performance of Bimetallic Ru-Co/TiO2 Catalysts Carrying out a Low Temperature C3H8 Oxidation Reaction. Catal Lett (2024). https://doi.org/10.1007/s10562-024-04626-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10562-024-04626-w